Abstract

The Haber–Bosch process, which synthesizes ammonia (NH3) from nitrogen (N2) and hydrogen (H2), consumes approximately 2% of the global energy supply. A sustainable alternative is the direct electrochemical conversion of N2 to NH3. The selectivity and activity of the electrocatalysts for this process are assessed by quantifying the NH3 present in the electrolyte. Compared with other analytical methods, 1H NMR offers a straightforward approach for detecting NH3 (by analyzing NH4+). 1H NMR method can also definitely confirm that the detected ammonia originates from the electroreduction of N2 by comparing results obtained from isotopically labeled 15N2 and regular 14N2 gases. This capability is unique to the 1H NMR method, as no alternative approaches offer this level of specificity. However, this method suffers from low sensitivity when measuring NH4+ of low concentration of such as at μM or lower. To address this issue, we developed a novel approach that improves sensitivity by ∼3-fold through the introduction of 14N decoupling during the 1H NMR data acquisition. Recently [Kolen M.et al. ACS Omega 2021, 6, 5698–5704], demonstrated a ∼3.5-fold increase in sensitivity by using a 1 mM concentration of the paramagnetic relaxation agent Gd3+. By combining our 14N decoupling technique with the relaxation agent Gd3+, we achieved a synergistic enhancement in sensitivity, resulting in an overall ∼10.9-fold sensitivity increase for the 1H NMR detection of 14NH4+. This translates to a reduction in NMR detection time by a factor of ∼119 (10.92). This significant advancement enables the fast detection of ammonia at μM concentration or lower. 1H NMR of 15NH4+ with 15N decoupling was also demonstrated.
Introduction
Ammonia (NH3) is an efficient hydrogen carrier and has numerous industrial applications, including the production of fertilizers and explosives.1−3 The Haber–Bosch process, which has been used for over 100 years, synthesizes NH3 from nitrogen (N2) and hydrogen (H2) using an iron catalyst at high temperature and pressure. This process, however, consumes ∼2% of global energy,4,5 drives the search for alternative, more sustainable methods. One promising approach is electrocatalytic nitrogen reduction (e-NRR), which produces NH3 from nitrogen and water using an applied voltage. This method is considered a green and sustainable alternative for NH3 synthesis. However, the current yield from electrocatalytic nitrogen reduction remains at the micromolar (μM) level,6−9 making quantitative measurement of NH3, new catalyst development, and reaction mechanism analysis of e-NRR challenging. In addition, the e-NRR system is highly prone to false-positive results caused by the electroreduction of impurities (e.g., NO2–/NO3–) present in the electrolytic cell, which can potentially overshadow the true signal entirely.10,11 Therefore, it is crucial to provide definitive proof that any detected ammonia originates from the intended electroreduction process.12
Various analytical techniques have been developed to quantify NH3, such as ion chromatography,13 fluorescence,14 laser absorption spectroscopy,15 colorimetric methods,16 and 1H NMR.17−271H NMR is unique in its ability to provide both accurate quantification of NH3 and N isotope tracing.28,29 Specifically, it can definitively confirm that the detected ammonia originates from the electroreduction of N2 by utilizing isotopically labeled 15N2 gas—a capability unmatched by any alternative approach.17 However, this method suffers from low sensitivity, especially at low NH4+ concentrations, due to interference from the large water 1H NMR signal and a few other factors. Several approaches have been developed to address these issues. Nielander et al. used a pulsed field gradient spin echo (PGSE) pulse sequence with a selective 180° shaped pulse to target the NH4+ signal while avoiding excitation of the water signal.18 Other methods, such as excitation sculpting (ES)25 or water suppression by gradient tailored excitation (WATERGATE)26 pulse sequences, have also been employed for water signal suppression. Assafiri et al. reported enhanced sensitivity by using a 3 mm NMR tube in a 5 mm NMR probe due to radiation damping reduction with a 3 mm NMR tube.27 More recently, Kolen et al. demonstrated a ∼3.5-fold sensitivity increase by using the paramagnetic relaxation agent Gd3+.25
In all these studies, 1H NMR signals of NH4+ were measured directly, resulting in a triplet for 14NH4+ and a doublet for 15NH4+, respectively.7,9,17−27 Here we introduce a new approach that increases 1H NMR sensitivity by ∼3-fold through 14N decoupling during 1H NMR acquisition. By combining this technique with the use of Gd3+, we achieved a synergistic effect, enhancing sensitivity by ∼10.9-fold for 1H NMR of 14NH4+. This corresponds to a reduction in NMR detection time by a factor of ∼119 (10.92). 1H NMR of ammonia with 15N decoupling was also demonstrated. This advancement allows for the detection of much lower concentration of NH3.
Experimental Section
14NH4Cl (99.995%), 15NH4Cl (≥98 atom %, 15N ≥ 99% CP), maleic acid (≥99%), and H2SO4 (≥97.5%) were purchased from Sigma-Aldrich. Gadolinium(III) nitrate hexahydrate (99.9%) was obtained from Fisher Scientific. DMSO-d6 (99.9% D) was obtained from Cambridge Isotope Laboratories. NMR was conducted with a 400 MHz Bruker Avance III NMR spectrometer with a 5 mm broadband observe (BBO) or a 10 mm BBO probe at 25 °C. 1H NMR transmitter frequency was set on water chemical shift, 14N and 15N decoupling frequencies were set on-resonance on the 14N NMR signal of 14NH4+ and 15N NMR signal of 15NH4+, respectively. Detailed experimental parameters are listed in the Results and Discussion section and Supporting Information (SI).
Results and Discussion
We began our experiments using a 400 MHz 5 mm BBO probe, where the inner coil is the X-coil and the outer coil is the 1H-coil. To start, we measured the 14N 90° pulse using a sample containing 500 μL of 10 wt % NH4Cl and 50 μL of DMSO-d6. On our spectrometer, the 14N 90° pulse is 19.25 μs at 120 W radio frequency (RF) power. The power level for a 200 μs composite decoupling (CPD) pulse was then calculated using Bruker’s “edprosol” program. For the continuous wave (CW) decoupling portion, we applied an additional −6 dB of power (i.e., increasing power by 2-fold) when employing the bi-Waltz65–256pl method for 14N decoupling, based on our previous studies.30,31
As the WATERGATE method is associated with baseline roll and signal phasing issues32 and the excitation sculpting (ES) NMR pulse sequence is well-known for its effective water suppression for the NMR analysis of NH4+ signals,25 we evaluated four ES pulse sequences in the studies:
-
(1)
Bruker “zgesgp”: A standard ES pulse sequence for water suppression.
-
(2)
“zgesgpig”: An enhanced version of “zgesgp” that includes inverse gated decoupling on the f2 channel.
-
(3)
“zgesfpgp”: This pulse sequence incorporates a 90° water flip-back pulse at the start of the “zgesgp” sequence on the f1 channel. The first two pulses (90°flip-back and hard 90°) will store most of the water magnetization along +z (since 90°flip back pulse is applied on −x, while hard 90°pulse is on +x, so effectively 0° rotation). The remaining water magnetization in the transverse plane (caused by radiation damping) will then be defocused by the following excitation sculpting element. These can be advantageous when using a short relaxation delay (D1). It also reduces radiation damping, which can broaden the NH4+ signals and decrease the signal-to-noise ratio (S/N). Note that the radiation damping becomes a significant issue with a cryoprobe on a high field NMR spectrometer.27
-
(4)
“zgesfpgpig”: This combines the features of “zgesfpgp” with inverse gated decoupling on the f2 channel.
The new pulse sequences “zgesgpig” and “zgesfpgpig” are shown in Figure 1 (detailed Bruker pulse sequence codes and parameters are provided in the Supporting Information).
Figure 1.
New pulse sequences of zgesgpig and zgesfpgpig. Blue rectangles are hard, blue bullets are shaped and black bullets are pulsed field gradient pulses, respectively.
Sample without Gd3+ in a 5 mm NMR Tube with a 5 mm BBO Probe
To evaluate the pulse sequences and different decoupling methods, we first tested a 32 mM NH4+ high-concentration sample. According to previous reports, the 1H spin–lattice relaxation time (T1) of NH4+ at 25 °C is ∼2.2 s.25 Based on this, we used a relaxation delay (D1) of 10.36 s and an acquisition time (AQ) of 0.64 s in our experiments (D1 + AQ = 5 × T1). Figure 2 shows the 1H NMR spectra of the 32 mM NH4+ sample acquired with the four ES pulse sequences discussed previously. When using the “zgesgp” and “zgesfpgp” pulse sequences (without 14N decoupling), typical 1H triplet signals of NH4+ were observed. The triplet pattern arises from the coupling between 1H and 14N nuclei of the NH4+. In contrast, when using “zgesgpig” and “zgesfpgpig” sequences (with 14N decoupling), the 1H–14N heteronuclear coupling pattern of the NH4+ signal was eliminated, resulting in a singlet signal. The bi-Waltz65–256pl decoupling method was employed for 14N decoupling in Figure 2, as the NH4+ signal is strong at this concentration. Using conventional Waltz65 decoupling30,31 at such a high NH4+ concentration produced decoupling artifacts (Figure S1).
Figure 2.

1H NMR spectra of 32 mM NH4+ with different NMR pulse sequences. The decoupling method used in “zgesgpig” and “zgesfpgpig” is bi-Waltz65–256pl. The sample used was 525 μL of 41.1 mM NH4Cl, 50 μL of 0.5 M H2SO4, 50 μL of DMSO-d6, 25 μL of 12.5 mM maleic acid and 25 μL of H2O.
Table 1 summarizes the detailed results for the 32 mM NH4+ sample, with the S/N values representing the average of three experiments. Notably, a ∼ 3-fold increase in sensitivity was achieved with both “zgesgpig” and “zgesfpgpig” pulse sequences.
Table 1. 1H NMR S/N Ratio Results for a 32 mM NH4+ Solution Obtained with Different NMR Pulse Sequences and Decoupling Methods by Using a 5 mm NMR Tube in a 5 mm BBO Probea.
| pulse sequence | decoupling method | S/N | S/N gain with decoupling |
|---|---|---|---|
| zgesgp | no decoupling | 901 | |
| zgesgpig | waltz65 | 2685 | 3.0 |
| zgesgpig | bi-waltz65–256pl | 2647 | 2.9 |
| zgesfpgpig | waltz65 | 2511 | 2.8 |
| zgesfpgpig | bi-waltz65–256pl | 2571 | 2.9 |
The S/N ratio represents the average of 3 experiments. Sample: 525 μL of 41.1 mM NH4Cl, 50 μL of 0.5 M H2SO4, 50 μL of DMSO-d6, 25 μL of 12.5 mM maleic acid, and 25 μL of H2O.
Sample without Gd3+ in a 3 mm NMR Tube with a 5 mm BBO Probe
As mentioned in the introduction, Assafiri et al. reported that using a 3 mm NMR tube in a 5 mm probe could enhance S/N, even with the same sample concentration. The improvement was attributed to the reduction in radiation damping.27 We re-evaluated this claim by analyzing the same 32 mM NH4+ sample, previously tested in a 5 mm NMR tube with a 5 mm BBO probe, in a 3 mm NMR tube with the 5 mm BBO probe. The results, averaged from three experiments, are shown in Table 2. The findings reveal three key points:
-
1.
A ∼3-fold increase in sensitivity was achieved with both the “zgesgpig” and “zgesfpgpig” pulse sequences, consistent with our previous results.
-
2.
The “zgesfpgpig” pulse sequence performed similarly to the “zgesgpig” sequence under these experimental conditions.
-
3.
Contrary to the expectation, using a 3 mm NMR tube in the 5 mm BBO probe resulted in ∼72% loss of sensitivity, rather than a gain, compared to the S/N values listed in Table 1.
Table 2. 1H NMR S/N Results Obtained on a 32 mM NH4+ Solution with Different NMR Pulse Sequences and Decoupling Methods by Using a 3 mm NMR Tube with a 5 mm BBO Probea.
| pulse sequence | decoupling method | S/N | S/N gain with decoupling |
|---|---|---|---|
| zgesgp | no decoupling | 249 | |
| zgesgpig | waltz65 | 721 | 2.9 |
| zgesgpig | bi-waltz65–256pl | 728 | 2.9 |
| zgesfpgpig | waltz65 | 720 | 2.9 |
| zgesfpgpig | bi-waltz65–256pl | 711 | 2.9 |
S/N represents the average of three experiments. Sample: 525 μL of 41.1 mM NH4Cl, 50 μL of 0.5 M H2SO4, 50 μL of DMSO-d6, 25 μL of 12.5 mM maleic acid, and 25 μL of H2O.
A 3 mm NMR tube has 28% of the sample volume of a 5 mm NMR tube. Note that the thin wall thickness of the glass tube is 0.38 mm, and the sample diameters of 3.0 and 5.0 mm tubes are 2.24 (3.0–2 × 0.38) and 4.24 (5.0–2 × 0.38) mm, respectively. Thus, the ratio of the NMR detection area is 2.242/4.242 = 0.28. Therefore, the 72% loss of sensitivity using a 3 mm NMR tube compared with a 5 mm NMR tube is consistent with the amount of sample volume reduction.
Our results contradict the findings of Assafiri et al.27 One possible explanation is that the radiation damping is not a significant issue with our 5 mm 400 MHz BBO probe, whereas Assafiri et al. used a 5 mm 600 MHz triple channel inverse (TCI) cryoprobe,27 which has much higher sensitivity and may be more prone to radiation damping effects. Additionally, while our “zgesfpgpig” pulse sequence was designed to mitigate radiation damping and enhance S/N, it did not outperform the “zgesgpig” pulse sequence in our experiments (see Tables 1 and 2). This further supports the conclusion that radiation damping is not a significant concern with our 5 mm BBO probe setup.
Sample with Gd3+ in a 5 mm NMR Tube with a 5 mm BBO Probe
Kolen et al. reported that maleic acid is an effective internal standard for NH4+ quantification, and Gd3+ is an excellent relaxation agent. By using 1 mM Gd3+, they were able to reduce 1H T1 of maleic acid from 2.1 to 0.13 s and NH4+ from 2.2 to ∼0.14 s, respectively.25 Consequently, the quantitative NMR acquisition interdelay (D1 + AQ = 5 × T1) can be shortened from ∼11.0 to ∼0.75 s with 1 mM Gd3+. This approach increased sensitivity by approximately 3.5-fold,25 allowing Kolen et al. to reduce the NH4+ NMR detection time by a factor of 3.52 = 14.
Inspired by this finding, we investigated the combined use of 14N decoupling and the Gd3+ relaxation agent to achieve a synergistic sensitivity enhancement. We adopted Kolen et al’s sample preparation method,25 which included
525 μL of 56 μM NH4Cl
50 μL of 0.5 M H2SO4
50 μL of DMSO-d6
25 μL of 12.5 mM maleic acid
25 μL of 27 mM Gd3+
This mixture resulted in a final concentration of 43.6 μM NH4+ and 1 mM Gd3+. We then conducted our experiments in a 5 mm NMR tube using a 5 mm 400 MHz Bruker BBO probe, with relaxation delay (D1) of 10.34 s and acquisition time (AQ) of 0.64 s without Gd3+, and with D1 = 0.11 s and AQ = 0.64 s with Gd3+. The Waltz65 decoupling was employed, as decoupling artifacts were not an issue with the weak 1H signal at low NH4+ concentration.
The results are
shown in Figure 3,
with the S/N values averaged from two experiments.
Upon adding Gd3+, the sensitivity increased by a factor
of 3.8 (6.4/1.7), consistent with the expected value derived from
the reduction in acquisition time 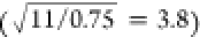 . Notably, our S/N values, both with and
without Gd3+, were lower than those reported by Kolen et
al, despite using the same 400 MHz NMR spectrometer.25 This difference may be attributed to their use of a 5 mm
Agilent OneNMR probe, which is optimized for 1H detection
and offers excellent salt tolerance.33
. Notably, our S/N values, both with and
without Gd3+, were lower than those reported by Kolen et
al, despite using the same 400 MHz NMR spectrometer.25 This difference may be attributed to their use of a 5 mm
Agilent OneNMR probe, which is optimized for 1H detection
and offers excellent salt tolerance.33
Figure 3.

1H NMR spectra of 43.6 μM NH4+ (56 μM NH4+ was diluted in NMR tube). The S/N values shown represent the average of two experiments. Bottom: without Gd3+ (D1 = 10.34 s, AQ = 0.64 s, with zgesfpgp pulse sequence). Middle: with 1 mM Gd3+ (D1 = 0.11 s, AQ = 0.64 s, with zgesfpgp pulse sequence). Top: with 1 mM Gd3+ (D1 = 0.11s, AQ = 0.64 s, with zgesfpgpig pulse sequence and Waltz65 decoupling).
Additionally, the 14N decoupling provided a further 2.9-fold increase in sensitivity (S/N ratio of 18.6/6.4). The combined effect of Gd3+ and 14N decoupling resulted in a 10.9-fold increase in sensitivity (18.6/1.7). In other words, the NMR acquisition time can be reduced by a factor of 10.92 = 119, more than 2 orders of magnitude.
Kolen et al. proposed a simple and convenient method to calculate NH4+ concentration by using internal reference maleic acid, with the following eq 1(25)
| 1 |
where I, N, and C are the integral area, number of nuclei, and concentration of NH4+ and standard, respectively. However, many researchers still prefer to use a calibration curve to quantify NH4+ concentration. To facilitate this, we prepared four samples with NH4+ concentrations ranging from 7.5 to 18.9 μM, each containing 1 mM Gd3+, following Kolen et al’s sample preparation method.25 We then acquired 1H NMR spectra using the zgesfpgpig pulse sequence with Waltz65 decoupling. The NH4+ concentrations were calculated using eq 1, and the resulting data were used to plot a calibration curve (Figure 4). The integral of the maleic acid was set to 100. Researchers can use this calibration curve (Figure 4) to quickly estimate the NH4+ concentration in their samples.
Figure 4.
1H NMR of 525 μL solutions of 7.5 to 18.9 μM NH4Cl, 50 μL of 0.5 M H2SO4, 50 μL of DMSO-d6, 25 μL of 12.5 mM maleic acid, and 25 μL of 27 mM Gd3+. NMR was carried out with zgesfpgpig pulse sequence and Waltz65 decoupling (D1 = 0.11 s, AQ = 0.64 s).
To confirm the origin of the detected NH4+, researchers sometimes use 15N2 gas to generate 15NH3 with their catalyst and process.17 To explore the effect of 15N decoupling, we briefly tested its performance using a 5 mm NMR tube with a 5 mm BBO probe. A 10 wt % of 15NH4Cl sample was used to measure the 90° pulse, which was 19.35 μs at 70 W power on our spectrometer. The corresponding CPD and CW decoupling parameters were calculated with Bruker’s “edprosol” program.
A 16.7 mM 15NH4+ sample was tested with and without decoupling, the results are shown in Figure 5. As expected, the 1H NMR sensitivity gain of 15N decoupling was approximately 2-fold. The best performance was observed with the zgesfpgpig pulse sequence with Waltz65 decoupling method. Additionally, representative 1H NMR spectra of a blend containing 8.3 mM 15NH4+ and 8.3 mM 14NH4+, both with and without 14N or 15N decoupling, are shown in Figure S2. As seen in Figure S2, 15N decoupling resulted in a singlet that overlapped with the central peak of the triplet caused by 14N heteronuclear coupling at the 400 MHz field strength (9.40 T). With an ultrahigh field 3-channel NMR spectrometer, it might be possible to simultaneously decouple 14N and 15N, allowing for the observation of two distinct 1H NMR peaks for 14NH4+ and 15NH4+ in future studies.
Figure 5.

1H NMR spectra of a sample of 500 μL of 18.7 mM 15NH4Cl, 50 μL of DMSO-d6, and 10 μL of 1 M H2SO4 with and without 15N decoupling. The S/N values shown are the average of two experiments. D1 = 3 s, AQ = 1 s, dummy scans = 8 and number of scans = 16.
Finally, we explored the analysis with a 10 mm 400 MHz BBO probe. To begin, a sample of 10 wt % of NH4Cl was used to measure the 14N 90° pulse, which was 36 μs at 99 W power on our spectrometer. The decoupling parameters were calculated with Bruker’s “edprosol” program. The experiments were conducted with a sample of a mixture of 2 mL of 18.7 mM NH4Cl, 40 μL of 1 M H2SO4, and 200 μL of DMSO-d6. The NMR parameters are D1 = 3 s, AQ = 1 s, dummy scans = 16 and number of scans = 32. The bi-Waltz-256pl decoupling method was applied for 14N decoupling. The results are summarized in Table 3 (NMR spectra are shown in Figure S3). It was observed that “zgesfpgpig” pulse sequence performed better than the “zgesgpig” pulse sequence, potentially due to its capability to reduce the radiation damping effects. We plan to further investigate the radiation-damping effects in the future when a cryoprobe becomes available. To date, most studies have focused on using a 5 mm NMR probe.7,9,17−27 However, it may be worthwhile to attempt detecting very low concentrations of NH4+ with a 10 mm NMR probe using 1 mM Gd3+ and 14N decoupling, provided that good probe tuning/matching and efficient water suppression can be achieved.
Table 3. 1H NMR Results of a Sample of 2 mL of 18.7 mM NH4Cl, 40 μL of 1M H2SO4, and 200 μL of DMSO-d6 with Different Pulse Sequences and bi-Waltz65-256pl Decoupling by Using a 10 mm BBO Probe.
| NMR tube size | pluse sequence | decoupling method | S/N | S/N gain |
|---|---|---|---|---|
| 10 mm | zgesgp | No | 3347 | |
| 10 mm | zgesgpig | bi-Waltz65–256pl | 8974 | 2.7 |
| 10 mm | zgesfpgpig | bi-Waltz65–256pl | 10,831 | 3.2 |
Conclusions
This study presents a significant advancement in the detection of aqueous ammonia using 1H NMR, enhanced by the integration of 14N decoupling and the paramagnetic relaxation agent Gd3+. This innovative technique achieves a remarkable 10.9-fold increase in sensitivity, significantly reducing the NMR detection time by a factor of 119. The enhanced sensitivity facilitates the rapid detection of ammonia at micromolar concentrations, making it a valuable tool for sustainable ammonia synthesis research. It may also be used in ammonia analysis in industrial wastewater. Additionally, this improvement not only allows for the fast detection of ammonia but also reduces the environmental impact by minimizing the usage of NMR superconducting magnets, thereby lowering the consumption of liquid nitrogen and helium, which are associated with greenhouse gas emissions. The method’s ability to detect ammonia originating from the electroreduction of nitrogen using isotopically labeled 15N2 gas further underscores its potential for applications in sustainable ammonia synthesis research. For analyzing the low concentration of 14NH4+, it is recommended to use either the zgesgpig or zeesfpgpig pulse sequence with a Waltz65 scheme for 14N decoupling.
Acknowledgments
We would like to thank Dr. Jihong Chen for carefully reading through the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c00337.
zgesgpig pulse sequence; zgesfpgpig pulse sequence; 1H NMR spectra of a 32 mM NH4+ solution acquired with different pulse sequences and Waltz65 decoupling, decoupling artifacts are shown (Figure S1); 1H NMR spectra of a blend of 8.3 mM 15NH4+ and 8.3 mM 14NH4+ with and without decoupling (Figure S2); 1H NMR spectra of a sample of 2 mL of 18.7 mM NH4Cl, 40 μL 1 M H2SO4, and 200 μL of DMSO-d6 acquired with different pulse sequences and bi-Waltz65–256pl decoupling by using a 10 mm BBO probe (Figure S3) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cui X.; Tang C.; Zhang Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 2018, 8, 1800369 10.1002/aenm.201800369. [DOI] [Google Scholar]
- Wang B.; Li T.; Gong F.; Othman M.; Xiao R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell - a comprehensive review. Fuel Process. Technol. 2022, 235, 107380 10.1016/j.fuproc.2022.107380. [DOI] [Google Scholar]
- Milton R. D.; Cai R.; Abdellaoui S.; Leech D.; De Lacey A. L.; Pita M.; Minteer S. D. Bioelectrochemical Haber–Bosch process: An ammonia-P\producing H2/N2 fuel cell. Angew. Chem., Int. Ed. 2017, 56, 2680–2683. 10.1002/anie.201612500. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xia M.; Wang H.; Huang K.; Qian C.; Maravelias C. T.; Ozin G. A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. 10.1016/j.joule.2018.04.017. [DOI] [Google Scholar]
- Martín A. J.; Shinagawa T.; Pérez-Ramírez J. Electrocatalytic reduction of nitrogen: from Haber-Bosch to ammonia artificial leaf. Chem 2019, 5, 263–283. 10.1016/j.chempr.2018.10.010. [DOI] [Google Scholar]
- Qing G.; Ghazfar R.; Jackowski S. T.; Habibzadeh F.; Ashtiani M. M.; Chen C. P.; Smith M. R.; Hamann T. W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020, 120, 5437–5516. 10.1021/acs.chemrev.9b00659. [DOI] [PubMed] [Google Scholar]
- Fu X.; Pedersen J. B.; Zhou Y.; Saccoccio M.; Li S.; Sažinas R.; Li K.; Andersen S. Z.; Yu A.; Deissler N. H.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707–712. 10.1126/science.adf4403. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhou Y.; Li K.; Saccoccio M.; Sažinas R.; Andersen S. Z.; Pedersen J. B.; Fu X.; Shadravan V.; Chakraborty D.; et al. Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid-electrolyte interphase. Joule 2022, 6, 2083–2101. 10.1016/j.joule.2022.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.; Sun K.; Liu X.; Yang W.; Li L.; Jiang H. Electronic state and microenvironment modulation of metal nanoparticles stabilized by MOFs for boosting electrocatalytic nitrogen reduction. Adv. Mater. 2023, 35, 2210669 10.1002/adma.202210669. [DOI] [PubMed] [Google Scholar]
- Li L.; Tang C.; Yao D.; Zheng Y.; Qiao S. Electrochemical nitrogen reduction: Identification and elimination of contamination in electrolyte. ACS Energy Lett. 2019, 4, 2111–2116. 10.1021/acsenergylett.9b01573. [DOI] [Google Scholar]
- Izelaar B.; Ripepi D.; Asperti S.; Dugulan A. I.; Hendrikx R. W. A.; Böttger A. J.; Mulder F. M.; Kortlever R. Revisiting the electrochemical nitrogen reduction on molybdenum and iron carbides: Promising catalysts or false positives?. ACS Catal. 2023, 13, 1649–1661. 10.1021/acscatal.2c04491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanto B. H. R.; Du H.; Wang D.; Chen J.; Simonov A. N.; MacFarlande D. R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. 10.1038/s41929-019-0252-4. [DOI] [Google Scholar]
- Thomas D. H.; Rey M.; Jackson P. E. Determination of inorganic cations and ammonium in environmental waters by ion chromatography with a high-capacity cation-exchange column. J. Chromatogr. A 2002, 956, 181–186. 10.1016/S0021-9673(02)00141-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Yuan D.; Lin H.; Zhou T. Determination of ammonium in seawater by purge-and-trap and flow injection with fluorescence detection. Anal. Lett. 2016, 49, 665–675. 10.1080/00032719.2015.1041027. [DOI] [Google Scholar]
- Yang W.; You K.; He Y.; Zhang Y.; Xin X.; Zhang X.; Zhu A. A laser absorption spectroscopy chamber system based on closed dynamic chamber method for multi-point synchronous monitoring ammonia emissions. Sci. Total Environ. 2023, 884, 163799 10.1016/j.scitotenv.2023.163799. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Shi R.; Bian X.; Zhou C.; Zhao Y.; Zhang S.; Wu F.; Waterhouse G. I. N.; Wu L. Z.; Tung C. H.; Zhang T. Ammonia detection methods in photocatalytic and electrocatalytic experiments: How to improve the reliability of NH3 production rates?. Adv. Sci. 2019, 6, 1802109 10.1002/advs.201802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. Z.; Čolić V.; Yang S.; Schwalbe J. A.; Nielander A. C.; McEnaney J. M.; Enemark-Rasmussen K.; Baker J. G.; Singh A. R.; Rohr B. A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. 10.1038/s41586-019-1260-x. [DOI] [PubMed] [Google Scholar]
- Nielander A. C.; McEnaney J. M.; Schwalbe J. A.; Baker J. G.; Blair S. J.; Wang L.; Pelton J. G.; Andersen S. Z.; Enemark-Rasmussen K.; et al. A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques. ACS Catal. 2019, 9, 5797–5802. 10.1021/acscatal.9b00358. [DOI] [Google Scholar]
- Yao Z.; Liu S.; Liu H.; Ruan Y.; Hong S.; Wu T.; Hao L.; Soo Y.; Xiong P.; Li M.; et al. Pre-adsorbed H-assisted N2 activation on single atom cadmium-O5 decorated In2O3 for efficient NH3 electrosynthesis. Adv. Funct. Mater. 2022, 33, 2209843 10.1002/adfm.202209843. [DOI] [Google Scholar]
- Feng X.; Liu J.; Chen L.; Kong Y.; Zhang Z.; Zhang Z.; Wang D.; Liu W.; Li S.; Tong L.; Zhang J. Hydrogen radical-induced electrocatalytic N2 reduction at a low potential. J. Am. Chem. Soc. 2023, 145, 10259–10267. 10.1021/jacs.3c01319. [DOI] [PubMed] [Google Scholar]
- Hodgetts R. Y.; Kiryutin A. S.; Nichols P.; Du H.; Bakker J. M.; Macfarlane D. R.; Simonov A. N. Refining universal procedures for ammonium quantification via rapid 1H NMR analysis for dinitrogen reduction studies. ACS Energy Lett. 2020, 5, 736–741. 10.1021/acsenergylett.9b02812. [DOI] [Google Scholar]
- Varghese A. P.; Neppolian B.; Lakhera S. K. Pitfalls of using Nessler’s reagent for ammonia detection in photocatalytic nitrogen fixation studies: Leveraging 1H NMR for enhanced accuracy and precision. Ind. Eng. Chem. Res. 2023, 62, 12530–12537. 10.1021/acs.iecr.3c01779. [DOI] [Google Scholar]
- Lv X.-W.; Liu X.; Suo Y.; Liu Y.; Yuan Z. Identifying the dominant role of pyridinic-N–Mo bonding in synergistic electrocatalysis for ambient nitrogen reduction. ACS Nano 2021, 15, 12109–12118. 10.1021/acsnano.1c03465. [DOI] [PubMed] [Google Scholar]
- Chu K.; Li X.; Li Q.; Guo Y.; Zhang H. Synergistic enhancement of electrocatalytic nitrogen reduction over boron nitride quantum dots decorated Nb2CTx-MXene. Small 2021, 17, 2102363 10.1002/smll.202102363. [DOI] [PubMed] [Google Scholar]
- Kolen M.; Smith W. A.; Mulder F. M. Accelerating 1H NMR detection of aqueous ammonia. ACS Omega 2021, 6, 5698–5704. 10.1021/acsomega.0c06130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A. J.; Veenstra F. L. P.; Lüthi J.; Verel R.; Pérez-Ramírez J. Toward reliable and accessible ammonia quantification in the electrocatalytic reduction of nitrogen. Chem. Catalysis 2021, 1, 1505–1518. 10.1016/j.checat.2021.10.002. [DOI] [Google Scholar]
- Assafiri A.; Jia C.; Thomas D. S.; Hibbert D. B.; Zhao C. Fast and sensitive detection of ammonia from electrochemical nitrogen reduction reactions by 1H NMR with radiation damping. Small Methods 2024, 8, 2301373 10.1002/smtd.202301373. [DOI] [PubMed] [Google Scholar]
- Yao Z.; Liu S.; Liu H.; Ruan Y.; Hong S.; Wu T.; Hao L.; Soo Y.; Xiong P.; Li M. Pre-adsorbed H-assisted N2 activation on single atom cadmium-O5 decorated In2O3 for efficient NH3 electrosynthesis. Adv. Funct. Mater. 2022, 2209843 10.1002/adfm.202209843. [DOI] [Google Scholar]
- Shin D.; Choi A.; Han D.; Bak G.; Yoo S.; Jeon Y.; Park S.; Hwang Y. Enhancing lithium-mediated nitrogen reduction with porous polymer fibers featuring lithium-ion affinity. Adv. Funct. Mater. 2025, 2416484 10.1002/adfm.202416484. [DOI] [Google Scholar]
- Zhou Z.; Kuemmerle R.; Qiu X.; Redwine D.; Cong R.; Taha A.; Baugh D.; Winniford B. A new decoupling method for accurate quantification of polyethylene copolymer composition and triad sequence distribution with 13C NMR. J. Magn. Reson. 2007, 187, 225–233. 10.1016/j.jmr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Kuemmerle R.; Rau N.; Eldred D.; Moreno A.; Czarniecki B.; Qiu X.; Cong R.; Gies A.; Fan L.; Auyeung E.; Beezer D.; Dau H.; Harth E. Polyolefin analyses with 10 mmmultinuclear NMR cryoprobe. Anal. Chem. 2020, 92, 15596–15603. 10.1021/acs.analchem.0c03753. [DOI] [PubMed] [Google Scholar]
- Berger S.; Braun S.. 200 and More NMR Experiments-A Practical Course; Wiley-Vch Verlag GmbH & Co. KGaA: Weinheim, 2004; pp 506–511. [Google Scholar]
- https://www.agilent.com/cs/library/usermanuals/public/G7446-21130_OneNP.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




