Abstract
Plant‐derived polysaccharides have emerged as sustainable biopolymers for fabricating nanoparticles (polysaccharide‐based nanomaterials [PS‐NPs]), presenting unique opportunities to enhance food functionality and human health. PS‐NPs exhibit exceptional biocompatibility, biodegradability, and structural versatility, enabling their integration into functional foods to positively influence gut microbiota. This review explores the mechanisms of PS‐NPs interaction with gut microbiota, highlighting their ability to promote beneficial microbial populations, such as Lactobacilli and Bifidobacteria, and stimulate the production of short‐chain fatty acids. Key synthesis and stabilization methods of PS‐NPs are discussed, focusing on their role in improving bioavailability, stability, and gastrointestinal delivery of bioactive compounds in food systems. The potential of PS‐NPs to address challenges in food science, including enhancing nutrient absorption, mitigating intestinal dysbiosis, and supporting sustainable food production through innovative nanotechnology, is critically evaluated. Barriers such as enzymatic degradation and physicochemical stability are analyzed, alongside strategies to optimize their functionality within complex food matrices. The integration of PS‐NPs in food systems offers a novel approach to modulate gut microbiota, improve intestinal health, and drive the development of next‐generation functional foods. Future research should focus on bridging knowledge gaps in metagenomic and metabolomic profiling of PS‐NPs, optimizing their design for diverse applications, and advancing their role in sustainable and health‐promoting food innovations.
Keywords: functional foods, gut microbiota, nanotechnology, short‐chain fatty acids, sustainable technology
1. INTRODUCTION
Different regions of the human body harbor various commensal microbes, known as microbiota; however, the gut microbiota remains the most extensively studied due to its diversity, complex interactions, and high population density—10 times greater than bacterial species in human eukaryotic cells (Almeida et al., 2019; Ladaycia et al., 2021). The gut microbiota is a complex and diverse group of microbes, including bacteria, fungi, viruses, and archaea, predominantly found in the colonic regions of the human gut (Corrie et al., 2022). This microbial community comprises over 1000 unique strains, with Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes as the predominant phyla (Guan et al., 2020). These microbes benefit gut and body functions by maintaining gut homeostasis (El Aidy et al., 2013), regulating blood sugar, preserving xenobiotic‐controlled metabolic pathways (Gérard & Vidal, 2019), supporting immunomodulation and immunoregulation (Corrie et al., 2022), maintaining gut integrity and barrier functions (Chelakkot et al., 2018), and exhibiting antimicrobial effects (Pilmis et al., 2020).
As a key component of the gastrointestinal tract (GIT) from birth, gut microbiota also plays an essential role in preserving gut physiology, synthesizing vitamin B12 (Danchin & Braham, 2017), fermenting endogenous mucus, and digesting large indigestible polymers like polysaccharides (PS) to produce short‐chain fatty acids (SCFAs) for energy (Ladaycia et al., 2021; Shang et al., 2018), thereby promoting overall gut and body health. Alterations in gut microbiota composition can have substantial local and systemic effects, often leading to gut dysbiosis (Walker & Lawley, 2013). In addition to the GIT, the absence of gut microbiota also affects various systemic dysregulations. For example, germ‐free rats exhibited a notable decrease in cardiovascular regulation, leading to significant changes in blood circulation (Gordon et al., 1963). Similarly, cohort germ‐free mice displayed altered behavioral responses to stress due to disturbances in the hypothalamic–pituitary–adrenal axis (Sudo, 2006; Sudo et al., 2004).
The use of pharmaceuticals to modulate gut microbiota often leads to adverse effects, including kidney and liver damage (Feng et al., 2021), highlighting the need for a non‐toxic, effective alternative. Recently, nanoparticles (NPs) have gained attention for their potential to modulate colonic microflora as innovative drug delivery systems. NPs offer several advantageous properties, such as enhanced solubility, localization, drug protection, and controlled release. Typically, NPs are solid particles with uniform components, measuring between 10 and 100 nm (Lima et al., 2022). Advances in nanobiotechnology and nanomedicine have led to the incorporation of NPs in various commercial products, including foods (as flavor enhancers and packaging agents) (Calderón‐Jiménez et al., 2017; Dahiya et al., 2018), pharmaceuticals (as supplements) (Tulve et al., 2015), and medical applications (as drug delivery systems, antimicrobial agents, and treatments for colonic diseases) (Tulve et al., 2015), as well as in consumer goods like toothbrushes and pacifiers (Mackevica et al., 2017). The addition of 2% silver–copper NPs in fish skin gelatin‐based bio‐composite films has been shown to improve thermo‐mechanical, rheological, structural, and antimicrobial properties against Listeria monocytogenes and Staphylococcus typhimurium (Arfat et al., 2017). In a similar vein, Hashem et al. (2018) noted that incorporating 5% nano‐SiO2 into gelatin‐k‐carrageenan biocomposites significantly boosts tensile strength and Young's modulus while reducing water vapor and oxygen permeability, as well as delaying UV light‐induced reactions by four times compared to biocomposites lacking nano‐SiO2. Furthermore, exposing Plasmodium falciparum and Leishmania infantum to artemisinin‐based silica NPs at concentrations of 50 and 1.43 µg/mL, respectively, demonstrated remarkable delivery of antiplasmodial and antileishmanial effects compared to traditional artemisinin (Tsamesidis et al., 2021).
However, oral administration of these NPs presents limitations, raising concerns among researchers and leading to an interest in structural modifications to enhance stability in the harsh gastrointestinal environment (Hua et al., 2023) and improve localization to target sites (Moonwiriyakit et al., 2023). One promising approach involves using biopolymeric materials, such as PS, proteins, and polyphenols, as capping agents for NPs. This facilitates drug transportation to the upper colon with an extended residence time. Among these, PS has received notable attention due to its multi‐functional effects and resistance to upper gut digestion.
Bioactive PS are well distributed in plants, animals, and microorganisms with diverse structures and intriguing bioactivities. They are well regarded in the fields of nanomedicine, nanobiotechnology, and biomedical engineering. They have shown considerable promise in gene transport and therapy, tissue engineering, wound healing, and drug delivery (Manivasagan & Oh, 2016). Structurally, PS are complex biopolymeric substances and are sometimes bound to lipids, proteins, and nucleic acids (Laurienzo, 2010). Numerous PS have been reported to offer prebiotic actions to the host by improving the colonic environment, modulating gut microbiota, preventing the activities of pertinent metabolic disorders, preserving mucosal immune balance, and controlling intestinal permeability. For example, date pomace and date seed PS were found to influence gut microbiota and enhance SCFA production (Bamigbade, Subhash, Al‐Ramadi, et al., 2024; Bamigbade, Subhash, Tarique, et al., 2024; Jayasree Subhash et al., 2024; Subhash et al., 2024). Likewise, PS derived from probiotic lactic acid bacteria (LAB) displayed notable prebiotic, antimicrobial, and gut‐modulating properties (Tarique et al., 2023, 2024).
Recently, polysaccharide‐based nanomaterials (PS‐NPs) (nanohydrogels, NPs, nanofibers) have garnered significant attention in nanomedicine and biomedical and food research owing to their excellent properties including low cost, bioavailability, biodegradability, biocompatibility, and non‐toxicity (Raveendran et al., 2013). These properties are sought after by nanotechnologists for developing bionanomaterials. PS‐NPs are regarded as nano‐carriers that combine different intermolecular forces (electrostatic, covalent, hydrophobic, and ammonia bonds) between their amphiphilic regions to encapsulate and enhance the stability and target delivery of the desired therapeutics. Commonly studied PS for NP synthesis in ascending order include chitosan, alginate, starch, cellulose, and chitin (Ghaffarian et al., 2016). Over the years, PS‐NPs synthesized from metals, metal oxides, polymers, dendrimers, liposomes, and micelles have been actively explored for various biomedical applications including the treatment of inflammatory bowel disease (IBD), cancer therapy, gene therapy, drug and gene delivery, and more recently for regulating gut microbiota activities (Jayakumar et al., 2010; Manivasagan & Oh, 2016). The uprise in this field is attributed to the physicochemical properties of PS‐NPs, including stability in extreme GIT conditions, specificity, improved bioavailability, and nano‐size which cumulatively influence their bioactivities.
For instance, Zhou et al. (2022) successfully synthesized chitosan–quercetin NPs utilizing a self‐assembly technique, achieving a quercetin sequestration rate of 83% and demonstrating excellent colonic target delivery. Furthermore, the capping of ferulic acid and resveratrol NPs with chitosan PS has been shown to enhance storage stability, improve adsorption, increase adhesiveness, and extend the residency period within the intestinal mucous membranes (Senthil Kumar et al., 2020). The complexation of gum Arabic and chitosan has also been identified as effective in enhancing the synthesis of NPs with exceptional characteristics, such as excellent hydrophobicity, uniform particle size, and enhanced stability, alongside facilitating the release of curcumin within the GIT (Novickij et al., 2020). Moreover, hamamelitin loaded within NPs at a higher chitosan concentration exhibited a more significant intestinal antioxidant effect compared to those loaded at a higher alginate concentration with NPs (Han et al., 2020).
Researchers suggest that functional foods provide more than just satisfaction of hunger and nutritional needs, as they include both refined and crude biological agents that can enhance disease prevention, treatment, and consumers' overall health (Ahmad et al., 2022; Karimzadeh et al., 2018). Recent advancements in nanobiotechnology and nano‐chemistry have laid the groundwork for integrating PS‐NPs as functional ingredients in functional foods. In food systems, PS‐NPs serve as energy reservoir adjuvants, modifiers, flavor agents, and enhancers, improving texture and structure to boost the bioactivity and health benefits of these foods (Shen et al., 2020; Zarrintaj et al., 2022; Zheng et al., 2020). Importantly, PS‐NPs have been added to specially crafted nutritional products such as processed chocolates (Rabadan‐Chavez & Lugo‐Cervantes, 2018), spreads (Perumal et al., 2019), and hamburgers (Q. Li & Liu, 2021) to lower calorie content. The extraction of dietary fibers for PS‐NP synthesis and their use in functional foods have been highlighted as key principles of a circular economy, promoting valorization and sustainable waste management solutions for food waste (Datta et al., 2020).
However, few studies in nanomedicine and nanotechnology have reported the significant role of NPs as a functional food ingredient and modulating gut microbiota by inhibiting the growth of pathogenic and detrimental gut microflora and elevating the richness of beneficial Lactobacillus and Bifidobacteria (Gangadoo et al., 2018; Yausheva et al., 2018). Furthermore, some narrative reviews have been published on the topic, including those by Lamas and Osti (2020), Plucinski et al. (2021), Ahmad et al. (2022), Y. Zhang et al. (2020), and Bouwmeester et al. (2018); however, comprehensive analysis of PS‐NPs as modulators of the gut microbiome is still poorly reported. To our knowledge, the impact of PS‐NPs on the gut's metagenomics and metabolomics has not been thoroughly studied. Bioactive PS exhibits unique properties such as biocompatibility, biodegradability, low toxicity, and reduced immunogenicity, making them promising candidates as nanocarriers for enhanced bioactivities. To fully exploit the nanotechnological potential of bioactive PS conjugated with NPs, it is crucial to conduct a thorough structure‐function analysis related to their bioactivities, particularly in modulating gut microbiota. This review evaluates various NMs and their conjugation with PS from multiple sources used as capping agents to create PS‐NPs. Additionally, this review offers a detailed examination of the relationship between different PS‐NPs structures and their gut microbiota‐modulating functions, mechanisms of action, and assessment techniques, while placing a specific emphasis on recent developments in metagenomics, metabolomics, predictive analysis, and synthesis SCFAs. Additionally, the review covers different factors that facilitate the nano‐delivery system in the gut, efficacy of the nano‐formulations, and provides strategies to enhance the bioavailability for the full utilization of the PS‐NPs. Finally, we addressed the current challenges facing modern nanotechnological approaches toward developing PS‐NPs as well as potential future directions. Overall, this review aims to advance the development and application of nanotechnology in maintaining the intestinal environment and treating intestinal disorders. Figure 1 provides a general framework of the thematic areas covered in this review.
FIGURE 1.

General review framework.
2. NANOPARTICLES: A BRIEF OVERVIEW OF METHODS AND FEATURES
NPs are synthesized through two primary approaches: bottom‐up and top‐down. The latter process involves reducing bulk materials into NPs through techniques such as grinding, laser ablation, and sputtering (Figure 2) (Barhoum et al., 2022; Mekuye & Abera, 2023). Although effective, this method is limited by high costs and is more suitable for laboratory experiments than large‐scale production (Patil et al., 2021). In contrast, the bottom‐up approach is constructive, forming NPs from atomic or molecular precursors through techniques such as sol–gel synthesis, hydrothermal methods, and pyrolysis. This method is widely used in industrial applications due to its scalability and cost‐effectiveness (Bokov et al., 2021; Devatha & Thalla, 2018).
FIGURE 2.

Nanoparticle synthesis approaches.
Synthesis methods for NPs can be categorized into physical, chemical, and biological techniques. Physical methods, including thermal decomposition, mechanical milling, sputtering, and laser ablation, are frequently employed to produce high‐purity NPs (M. Kim et al., 2017). The thermal decomposition technique has been recognized as a commercially viable and low‐cost method for synthesizing various metallic nanoparticles (MNPs). For instance, Hosseinpour‐Mashkani and Ramezani (2014) successfully synthesized Ag2O and Ag‐NPs (40–50 nm) using solid‐state thermal decomposition in the presence of Ag(Hsal) complex precursor. Similarly, Cu and Cu2O NPs with diameters of 8–10 nm were synthesized using thermal decomposition in the presence of Cu(sal)2 precursor (Salavati‐Niasari & Davar, 2009). Mechanical milling, another common top‐down technique, is particularly useful for metal‐based nanoalloys and is considered environmentally friendly (Mekuye & Abera, 2023). Arbain et al. (2011) synthesized hematite (iron oxide) NPs while varying milling time and mill rotational speed across three levels. The authors confirmed a superior mechanochemical effect, identifying a minimum particle size of 76.6 nm, minimum crystallite size of 17.1 nm, and a minimum degree of crystallinity of 9.37% when the hematite was ground at 600 rpm for 10 h. In sputtering, the NPs are deposited on a thin layer through annealing. The size and shape of the synthesized NPs are influenced by temperature, annealing time, layer thickness, and substrate (Ijaz et al., 2020). Monoclinic phase CuO NPs with high‐purity and crystalline characteristics were successfully synthesized by Verma et al. (2018) using the magnetron sputtering technique at a range of sputtering pressures (10, 20, 30, 40, and 50 mTorr). Meanwhile, laser ablation has been shown to be effective for producing quantum dots and carbon nanotubes (M. Kim et al., 2017).
Chemical synthesis methods involve processes such as sol–gel, co‐precipitation, and chemical vapor deposition. These techniques allow precise control over particle size and shape. The sol–gel method, for example, is widely used to create metal oxide NPs due to its simplicity. The synthesis of ZnO NPs via the sol–gel method in the presence of a precursor (zinc acetate dehydrate), solvent (ethanol), and medium (sodium hydroxide and distilled water) was reported by Hasnidawani et al. (2016). The authors confirmed the synthesized NPs exhibited nanosize (81.28–84.98 nm), rod‐like morphology, and good crystallinity. In another study, amorphous SiO2 NPs (∼25 nm) were successfully synthesized using the sol–gel method with tetraethylorthosilicate as the precursor and polyvinylpyrrolidone as a surfactant (Dubey et al., 2015).
Similarly, co‐precipitation is an efficient technique for synthesizing superparamagnetic iron oxide NPs through the simultaneous precipitation of multiple compounds (Kandpal et al., 2014). Magnetite NPs with various biocompatible coatings were synthesized via co‐precipitation methods by Mohammadi et al. (2021). In this study, it was established that the synthesized NPs coated with silica and oleic acid had lower cytotoxicity and improved surface modification. In the chemical vapor deposition method of synthesis, a gaseous reactant is deposited onto a heated substrate, allowing interaction with the gas to form a thin film. The chemical vapor deposition method is suitable for large‐scale synthesis of carbon NMs with high purity (Ijaz et al., 2020). However, some chemical methods produce toxic byproducts, limiting their environmental compatibility (Ijaz et al., 2020).
Biological synthesis, often referred to as green synthesis, utilizes eco‐friendly sources such as plant extracts, microorganisms, and biomolecules. This method is especially significant for biomedical applications, as it avoids the toxicity associated with chemical synthesis. For instance, plant extracts containing phenolic compounds and terpenoids can reduce metal ions to form NPs under mild conditions (Mittal et al., 2013). Microorganisms, including bacteria and fungi, are also effective in producing NPs with tailored properties. Genetically modified microorganisms offer additional advantages by enabling precise control over particle size and bioactivity (Jha et al., 2009; Kato & Suzuki, 2020). A comparative study of chemically synthesized and Staphylococcus aureus synthesized Ag NPs by Kato and Suzuki (2020) confirms the superior stability and reduced toxicity of the microbial‐based Ag NPs. Similarly, Ag NPs were successfully synthesized from 22 Lactobacillus strains and tested against both Gram‐positive and Gram‐negative bacterial pathogens (Garmasheva et al., 2016).
NPs are classified based on their origin, structure, composition, and physical state (Figure 3a,b). Natural NPs are formed through geochemical processes, while anthropogenic NPs are either incidental or engineered (Hochella et al., 2019). Structurally, NPs are categorized as zero‐dimensional (e.g., quantum dots), one‐dimensional (e.g., nanowires), two‐dimensional (e.g., thin films), or three‐dimensional (e.g., nanotubes and polycrystals) (Mekuye & Abera, 2023; Saleh, 2020). Compositionally, NPs may be organic (e.g., polymers), inorganic (e.g., metallic and ceramic), or carbon‐based (e.g., fullerenes and graphene). These classifications are essential for tailoring NPs to specific applications, such as wastewater treatment and drug delivery (Barhoum et al., 2022; Plucinski et al., 2021).
FIGURE 3.

Types of nanomaterials (a) and classification of nanomaterials (b).
The size, shape, surface charge, and crystallinity of NPs (Figure 4) play a crucial role in their functionality. NPs are characterized by unique magnetic, optical, and mechanical traits that differ significantly from their bulk counterparts (Gatoo et al., 2014). For biomedical applications, smaller NPs exhibit enhanced cellular uptake and biodistribution compared to larger particles (Desai et al., 1997). Spherical NPs demonstrate higher cellular uptake than other shapes, making them suitable for drug delivery and cancer therapy (Y. Li et al., 2015). Surface properties, such as polarity and zeta potential, influence solubility, aggregation, and interaction with biomolecules. For example, hydrophilic NPs like chitosan and alginate are widely used in gene therapy and tissue engineering (Abedini et al., 2018; Montes et al., 2019).
FIGURE 4.

Physicochemical properties of nanomaterials.
Crystallography is another critical property, as NPs can exist in amorphous, crystalline, or pseudo‐close‐packed forms. Crystalline structures, analyzed through techniques like X‐ray diffraction, significantly impact material functionality (Giannini et al., 2016; Shevchenko & Madison, 2002). Furthermore, the high surface energy of NPs makes them highly reactive, enhancing their interaction with proteins and other biomolecules (Saptarshi et al., 2013). Advances in green synthesis and surface chemistry have broadened the applications of NPs in fields ranging from energy and agriculture to medicine. Eco‐friendly synthesis methods, coupled with precise control over NP properties, are crucial for developing sustainable and efficient NMs (Barhoum et al., 2022; Nyabadza et al., 2023).
3. POLYSACCHARIDES AS NANOPARTICLE‐CAPPING AGENTS
Particles ranging in size from ∼1 to 100 nm, which exhibit unique surface properties compared to their bulk counterparts, are known as NPs (Javed et al., 2020). The bioactivities of these particles are directly linked to their size, making size an important consideration in NP synthesis. Due to their extensive applications across various sectors (Pedroso‐Santana & Fleitas‐Salazar, 2023; Ponce et al., 2023; Sidhu et al., 2022; Yosri et al., 2021), large quantities of these uncapped particles are produced on a commercial scale. However, from a sustainability perspective, these aggregated NPs pose environmental hazards when released. The use of capping agents or moieties as stabilizers has become integral to NP synthesis. These stabilizers, which are binding molecules, allow precise control over the surface chemistry, morphology, and size distribution of NPs (Restrepo & Villa, 2021; Sidhu et al., 2022). Capping agents are amphiphilic molecules comprising a polar head and a non‐polar tail (Figure 5), which confer specific functionalities to the capped NPs (Javed et al., 2020).
FIGURE 5.

Nanoparticle interaction with different biogenic capping agents.
3.1. Role of polysaccharides as capping agents
Composite evidence from available data demonstrates that capping agents, including organic ligands, PS, polymers, polyphenols, and others, are essential for synthesizing stable metal NPs with controlled growth, particle size, and distinct shapes (Phan & Nguyen, 2017). The primary roles of these agents include shielding NPs from agglomeration and enhancing reduction kinetics by forming complex structures with metallic ions from precursor salts. The covalent bonding between the chains of capping ligands and the NPs surface induces steric hindrance, contributing to the stability of the nanocomposite (Pedroso‐Santana & Fleitas‐Salazar, 2023). Capping agents stabilize NPs, addressing challenges like overgrowth, aggregation, and recyclability (Priya et al., 2021). Depending on the makeup of the metal–ligand interface formed between the stabilizer and the NPs, these capping agents can occasionally act as either “poison” or “promoter” (Campisi et al., 2016). An excessive concentration of capping agents may lead to a “bridge effect,” where the charge on the NPs surface is neutralized, resulting in agglomeration (B. Cao et al., 2021).
Metallic NPs, renowned for their strength, elasticity, optical and electrical properties, and enhanced biological functionality, are widely utilized in biomedical and therapeutic applications. Extensive research has focused on metal NPs such as gold, silver, copper, nickel, and platinum NPs; bimetallic NPs combining two metals (e.g., FeCo, FeNi, and FePt NPs); and metal oxide NPs, including FeO and SiO₂ (Bharathi et al., 2019). Despite their high surface energy, these NPs tend to aggregate, making capping agents necessary to prevent oxidation in biological media, control particle size, and delay aggregation. Figure 6 provides a brief overview of the role of capping agents in NP synthesis and stabilization. Capping agents stabilize NPs through various mechanisms, including steric, electrostatic, hydration, and van der Waals forces (Pedroso‐Santana & Fleitas‐Salazar, 2023).
FIGURE 6.
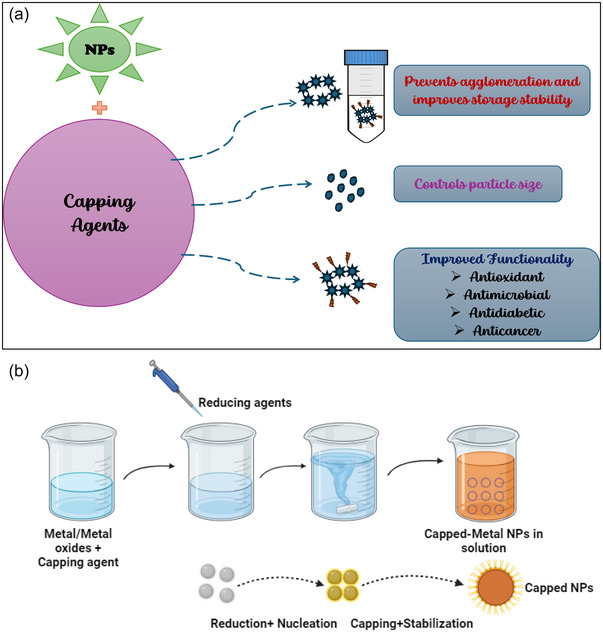
Role of capping agents in nanoparticles (NPs) synthesize and stabilization (a) and steps in the synthesis of NPs stabilized by capping agents (b).
Various methods are employed to generate NPs; however, traditional chemical and physical methods for synthesizing MNPs have significant drawbacks, including environmental hazards. Consequently, several eco‐friendly techniques have been developed to produce metallic NPs using plants, microbes, and biological extracts, which are considered less harmful to the environment and avoid the use of hazardous chemical reagents (Priya et al., 2021; Skiba et al., 2020; Sood et al., 2016). Figure 6a,b provides an overview of PS–NPs synthesis and stabilization.
Reducing agents, such as ascorbic acid, ferrous sulfate, and nitrophenols, mediate NP synthesis by forming core–shell structures. In some cases, ascorbic acid functions as both a reducing and stabilizing agent, as observed by Sood et al. (2016), where simultaneous redox reactions produced dehydroascorbic acid that capped gold‐coated iron oxide nanocomposites. The formation of an orange‐red solution typically confirms NPs synthesis in these reactions. In contrast, when 4‐nitrophenol was used as a reducing agent in palladium NPs synthesis, catalytic reduction was observed, causing the solution to change color from light yellow to bright yellow as 4‐nitrophenol (4‐NP) was protonated to release 4‐nitrophenolate ions (Nagaraja et al., 2023). In physical synthesis, green methods utilizing irradiation, such as gamma, UV, microwave, and ultrasonic waves, have proven successful as reducing agents in NP synthesis (Elsupikhe et al., 2015).
Traditional methods utilize various chemical capping agents, such as surfactants, citrates, ethylenediamine tetraacetic acid (EDTA), quaternary ammonium compounds with halides, hexadecyltrimethylammonium bromide (CTAB), and thiols, as effective stabilizers (Arulmozhi & Mythili, 2013; Niu & Li, 2014; Phan & Nguyen, 2017; Rahal et al., 2017; Salassi et al., 2021). However, these synthetic moieties are not biodegradable and may cause toxicity and genotoxicity even at low concentrations, raising concerns about their compatibility in biological systems and prompting the development of green synthesis methods (Hong et al., 2021).
Green synthesis harnesses naturally occurring biomolecules, including PS (Y. Xiao et al., 2017; Z. Yang, Hu, et al., 2023; Yosri et al., 2021; W. Zhang, Zhang, et al., 2018; J. Zhang, Yang, et al., 2022), plant extracts (dos Santos Corrêa et al., 2018; Pirsaheb et al., 2024), lipids (Bhattacharya & Srivastava, 2003; Mensah et al., 2018), chitosan‐based compounds (Cinteza et al., 2018; Franconetti et al., 2019), and proteins (JyothiKumar et al., 2019; S. Zhao et al., 2019). These biomolecules, being biodegradable, bio‐soluble, and biocompatible, serve to reduce, stabilize, or cap NPs, enhancing their applicability in living systems. Such biogenic capping agents have enabled the production of nontoxic, surface‐functionalized, and monodispersed NPs (Restrepo & Villa, 2021; Sidhu et al., 2022). Sidhu et al. (2022) and Restrepo and Villa (2021) critically reviewed various capping agents used in NPs synthesis and their therapeutic potential.
Some studies have reported the effective use of deep eutectic solvents (DES) combined with PS as capping agents and their influence on NPs stabilization (Hong et al., 2021; Ponce et al., 2023). DES provides an effective medium for polymers such as carrageenan to act as capping and stabilizing agents, modifying reduction potentials, neutralizing surface charges, and enabling specific crystal growth patterns (Hong et al., 2021). This section of the review discusses the role of PS extracted from natural sources through green extraction as potential reducing and capping agents in NPs synthesis.
The high hydroxyl group content and complex branched structure of PS, along with their natural, safe, and eco‐friendly properties, make them appealing candidates as dispersants or capping agents in NPs synthesis (B. Cao et al., 2021; Kong et al., 2014). PS functionalities, combined with their abundance, ease of synthesis from diverse sources, renewability, biodegradability, and biocompatibility, underscore their suitability as capping agents (Sidhu et al., 2022). PS molecules cap or adsorb to the NPs surface through their specific chemical structures, inhibiting aggregation and precipitation. Through capping, PS can synergize with metallic NPs to enhance their inherent bioactivities, including anti‐inflammatory, antioxidant, radical scavenging, antibacterial, anticancer, and gut microbiota modulation properties (Ashraf et al., 2023; Feng et al., 2021). Additionally, NPs have broad applications in the pharmaceutical industry, offering novel therapeutic options for IBD. Natural PS, known for their prebiotic and other physiological activities beneficial for IBD remission, have been utilized in loaded NPs synthesis, supporting therapeutic applications in this area (Cui et al., 2021; Feng et al., 2021; Ye et al., 2023).
PS used as decorators for selenium nanoparticles (Se‐NPs) improve colloidal stability and solubility (H. Li et al., 2019). For instance, Astragalus PS, when utilized as a capping agent for Se‐NPs, resulted in enhanced Se bioavailability—which is crucial for maintaining health and mitigating various chronic degenerative diseases—and increased water solubility of the PS (Y. Meng et al., 2018). Numerous studies have demonstrated that the structural properties of PS impact the characteristics and biological activities of Se‐NPs (B. Cao et al., 2021; Y. Liu, Huang, et al., 2021). For example, 1,6‐α‐d‐glucan from Castanea mollissima Blume, used as a capping agent for Se‐NPs, produced spherical CPA‐SeNPs, which exhibited significant effects on the proliferation of cancer cells, specifically HeLa cells (H. Li et al., 2019). Starch, with reducing groups in the C6 position of glucose units, has also been employed in silver NP synthesis as both a reducing and capping agent (Restrepo & Villa, 2021).
Other studies have explored the correlation between the molecular weight of PS and the particle size and stability of NPs. PS from Oudemansiella radicata, namely ORP1 and ORP2 with molecular weights of 100,096 and 13,903 Da, respectively, were used as capping agents for Se‐NPs. The results indicated that ORP1‐capped Se‐NPs had a particle size of 109.75 nm, whereas ORP2‐capped NPs measured 115.97 nm, suggesting that the molecular size of PS can influence the dispersion and stability of Se‐NPs (H. F. Liu, Pan, et al., 2021). This variation in size distribution may be attributed to the PS backbone structure and monomeric units. Additionally, the molecular weight distribution of PS affects the variability in size, morphology, and bioactivity of NPs stabilized by the same PS (C. Zhang et al., 2015).
Ye et al. (2023) illustrated the synthesis of Se‐NPs using Eucommia ulmoides polysaccharide (EUP) as the primary reducing and capping agent, resulting in the formation of EUP‐SeNPs. The synthesized SeNPs were spherical in shape with a diameter of 170 nm and were evaluated for their effects on induced colitis in mice. EUP‐SeNPs significantly altered colon‐intestinal histology, gut microbiota composition, and inflammatory cytokine levels in colitis‐afflicted mice. The gut microflora patterns in mice treated orally with EUP‐SeNPs indicated a reduction in pathogenic bacteria such as Campylobacterota, Clostridia, Oscillospirales, Desulfovibria, and Ruminococcaceae, suggesting their potential as a therapeutic option for IBD (Ye et al., 2023).
In a similar study, Ulva lactuca PS supplementation resulted in Se‐NPs that displayed notable anti‐inflammatory properties in mice suffering from acute colitis. These functionalized NPs helped reduce both body weight loss and colon inflammation in the treated mice (C. Zhu et al., 2017). Meanwhile, Singh and Dutta (2020) employed metal oxide, particularly zinc oxide (ZnO), as a precursor for NP synthesis, using a chitin–glucan complex as the capping agent. The chitin–glucan capped ZnO NPs (ChGC‐ZnONPs) were found to be under 40 nm in size and exhibited radical scavenging capabilities resulting from the electron‐donating attributes of the oxygen atoms in the metal oxide. Additionally, curcumin‐loaded NPs created from this mixture demonstrated improved biological and antimicrobial properties.
Chitosan is utilized as a capping agent to create nanocomposites from laminarin‐embedded ZnO nanoparticles (Lm‐ZnO NPs). This approach employs two sequential PS: laminarin for the synthesis of ZnO NPs and chitosan for the formation of Lm‐ZnO nanocomposites. The resulting nanocomposites display a reduced particle size compared to the stand‐alone NPs, underscoring chitosan's role as an effective reducing and stabilizing agent. Cytotoxicity was noted for both NPs and nanocomposites when tested on human dermal fibroblasts (HDF) and human colon cancer (HT‐29) cells (Vijayakumar et al., 2022). Alginate, a natural anionic PS, combined with glucose, acted as a supporting matrix for the synthesis and stabilization of silver nanoparticles (Ag NPs) (X. Zhao et al., 2014). This stabilized colloidal mixture was later used to create alginate–AgNP composite fibers, which exhibited antimicrobial properties against S. aureus and Escherichia coli. In a similar fashion, sodium alginate (Na‐Alg) functioned both as a reducing and stabilizing agent, together with ascorbic acid, to synthesize spherical Ag NPs ∼50 nm in size. These stabilized NPs showed antibacterial activity against both Gram‐positive and Gram‐negative strains (X. Zhao et al., 2017).
A study on mice with Alzheimer's disease examined the effects of surface‐modified SeNPs (L. Yang et al., 2022). To improve the stability of SeNPs, dihydromyricetin (DMY) and chitosan were utilized as coatings. Furthermore, a peptide designed to target the blood–brain barrier was incorporated to aid the NPs' passage through the membrane. The modified SeNPs triggered the release of inflammatory cytokines, successfully penetrated the blood–brain barrier, and influenced gut microflora by regulating the growth of Bifidobacterium and Dubosiella, while enhancing the relative abundance of certain beneficial bacteria Gordonibacter. Table 1 summarizes recent studies on PS as capping agents for NP synthesis and their influence on functional properties. Based on consolidated evidence from various studies, it can be concluded that capping agents enhance the stability of metallic NPs by promoting nucleation and preventing aggregation. This stabilization occurs through the formation of hydrogen bonds between the carbonyl and hydroxyl groups of the capping agents and the NPs surfaces.
TABLE 1.
Polysaccharides as capping agents for nanoparticle (NP) synthesis and their influence on functional properties of NPs.
| Polysaccharide and source | Nanoparticle type | Particle size (nm) | Characteristics and functionality | References |
|---|---|---|---|---|
| Astralagus polysaccharide (APS) | Selenium | 478.1 | Uniform ellipsoid shape, minimal weight loss, high IC50 for radical scavenging. | Y. Meng et al. (2018) |
| Exopolysaccharide from Bacillus sp. S3 (EPS) | Selenium | 100–200 | Amorphous, stable, moderate radical scavenging compared to PS and sodium selenite. | Ran et al. (2024) |
|
Grateloupia livida polysaccharides (GLPs) |
Selenium | 115.54 | Stable (30 days), high radical scavenging, selective cytotoxicity toward cancer cells. | B. Cao et al. (2021) |
|
Gracilaria lemaneiformis polysaccharides (GLPs) |
Selenium | 83.6 | Stable (42 days), spherical, antioxidant potential against DPPH, ABTS, α‐amylase, and glucosidase. | Tang et al. (2021) |
| 1,6‐α‐d‐Glucan from Castanea mollissima Blume. | Selenium | 53.7 | Spherical, inhibited HeLa cells proliferation (anti‐cancer). | H. Li et al. (2019) |
| Lycium barbarum polysaccharide (LBP) | Selenium | 105.4 | Maintained morphology, improved digestion stability and bioavailability. | G. Liu, Yang, et al. (2021) |
|
Oudemansiella radicata polysaccharide (ORP1) |
Selenium | 106.28 | Monodispersed, stable (40 days), strong antioxidant and radical scavenging. | Y. Liu, Huang, et al. (2021) |
| Alkali lignin | Silver | <20 | Significant capping and stabilizing effects, reduced nanoparticle size distribution. | S. Hu and Hsieh (2016) |
| Wheat bran Xylan | Silver | 57.92 | Spherical, acts as a radical scavenger, stabilized NPs. | Harish et al. (2015) |
| Carboxymethyl starch (CMS) | Selenium | 91 | Capping and reducing agent for selenium nanorods with trigonal phase. | Vishakha et al. (2023) |
| Gellan gum‐dopamine (GG‐DA) | Silver | 13 | Spherical, radical scavenging, antibacterial, incorporated into injectable hydrogels. | Biscari et al. (2024) |
|
Galactomannan from seeds of Astragalus gombiformis Pomel (Fabaceae) |
Silver | 144.4 | Reducing and capping agent, antimicrobial and antioxidant properties. | Bouziane et al. (2024) |
| Strychnos potatorum polysaccharide | Palladium | 51.08 | Stable, spherical, narrow size distribution, catalytic activity. | Nagaraja et al. (2023) |
| Fungal (Phlebopus portentosus) polysaccharide | Silver | 64.5 | Stable, spherical, antioxidant, antidiabetic, anticancer, and antimicrobial properties. | H. F. Li, Pan, et al. (2024) |
| Ulva lactuca polysaccharide | Selenium | 130 | Spherical, anti‐inflammatory, minimized colitis symptoms. | C. Zhu et al. (2017) |
|
Chitin‐glucan complex (ChGC) from Agaricus bisporus PS |
Zinc oxide | 33.01 | Hexagonal‐shaped, antibacterial and antioxidant activities increased with concentration. | Singh and Dutta (2020) |
| κ‐Carrageenan | Silver | 4.2‐56.36 | Spherical, stable, smaller size with increased κ‐carrageenan concentration. | Elsupikhe et al. (2015) |
| Carrageenan oligosaccharide | Gold | 35 | Ellipsoidal, stable, cytotoxic against human colon and breast cancer cells. | Chen et al. (2018) |
| Arabinogalactane | Selenium | 94 | Spherical, bacteriostatic, and antibiofilm action against Clavibacter sepedonicus. | Lesnichaya et al. (2022) |
| Pectin | Selenium | 41 | Stable, spherical, strong antioxidant, lower cytotoxicity in cancer and normal cells. | W. Y. Qiu et al. (2018) |
3.2. Mechanisms of polysaccharide‐based nanoparticle stabilization
PS‐NPs affect the gut microbiota through their prebiotic properties, selective microbial adherence, and the delivery of bioactive compounds. They impact microbial metabolism, enhance immune responses, strengthen the gut barrier, and promote microbial diversity and overall gut health. These NPs support gut health and prevent dysbiosis, offering valuable therapeutic and dietary strategies. Interest in PS‐NPs for modulating the gut microbiome has grown due to their biocompatibility, ease of absorption, and ability to deliver substances to specific sites. Their influence on the gut microbiome arises from various interactions with gut bacteria, both direct and indirect, resulting in beneficial changes in microbial composition, metabolites, and overall gut well‐being (L. Yang, Wang, et al., 2023; Y. Zhao et al., 2022).
One mechanism of action was investigated, revealing that the anthraquinone compound, naturally integrated into several PS‐NP delivery systems such as chitosan and fucoidan, enabled RH‐F/C‐NPs to effectively reduce DSS‐induced inflammation. This reduction occurred by inhibiting the TLR4/NF‐κB anti‐inflammatory pathway, activating the Nrf2/HO‐1 antioxidant pathway, restoring the colonic mucosal barrier, and adjusting intestinal microflora through pH and reactive oxygen species (ROS) (Qi et al., 2022). Pharmacokinetic studies further indicated that F/C‐NPs enhance RH levels in the colon by increasing plasma concentration.
Another study demonstrated that NPs loaded with quercetin, synthesized from PS extracted from Hohenbuehelia serotina mushrooms (QC‐HSP NPs), can boost immune function and improve intestinal health.
The administration of QC‐HSP NPs resulted in higher fecal moisture, shorter defecation time, and improved intestinal peristalsis. Furthermore, QC‐HSP NPs facilitated various metabolic functions of gut microbiota, such as replication, recombination, repair, translation, ribosomal structure and biogenesis, carbohydrate transport and metabolism, lipid transport and metabolism, amino acid transport and metabolism, and signal transduction mechanisms (Zhou et al., 2022). The structure–function analysis, mechanisms of action, evaluation models, and assessment techniques for efficacy are summarized in Table 2.
TABLE 2.
Polysaccharide‐based nanoparticles function, mechanism of action, and modulation of gut microbiota.
| Structure function analysis | Mechanism of action | Models of evaluation | Assessment efficacy | Reference |
|---|---|---|---|---|
|
Quercetin‐loaded nanoparticles based Hohenbuehelia serotina polysaccharides |
Boosts immunity, improves intestinal health by increasing fecal moisture, enhancing peristalsis, and reducing defecation time. | In vivo | Immune organ index, colon index and colon length, colon histology, colonic fecal moisture content, intestinal peristalsis, defecation time, pH and SCFAs contents, sequence clustering | Zhou et al. (2022) |
|
Polysaccharide curcumin‐based nanoemulsifying delivery system |
Provides colon‐targeted drug release, protects in the upper GI tract, bursts release in colon, supports microbiota delivery. | In vitro |
Quantitative reverse transcription‐polymerase chain reaction (RT‐qPCR),16s metagenomic analysis, dissolution testing of polysaccharide‐based oral colon‐targeted delivery systems |
Corrie et al. (2022) |
|
Berberine‐loaded carboxylmethyl chitosan nanoparticles |
Reduces colitis by regulating IL‐6 expression and remodeling gut microbiota. | In vivo |
Intestinal mucosal injury index analysis, histological analysis, 16SrNA analysis, α‐diversity estimated by ACE, Shannon index, PCA score and PCoA score plots, LDA score, relative abundance |
L. Zhao et al. (2021) |
| Chitosan derivative‐based NPs encapsulating quercetin | Restores gut microbiota altered by 5‐Fu‐induced mucositis by decreasing Bacteroides abundance. | In vivo | Model of 5‐Fu‐induced intestinal mucositis, PCA analysis, α‐diversity indicated by the Chao index, relative abundance | L. Yan et al. (2021) |
| Peptide‐functionalized chitosan nanoparticles | Regulates oxidative stress‐related gut microbiota, including Helicobacter and Bifidobacterium. | In vivo | Principal coordinates analysis, LDA analysis, Shannon index, Chao1 index and Sob index, LDA effect size (LEfSe), Spearman's correlation analysis | L. Yang, Wang, et al. (2023) |
| Retrograded starch/pectin in the delivery of chitosan NPs | Shortens transit time in GIT, enhances PS complex protection, and targets colon release via microbiota degradability. | In vivo | Kinetic analysis of drug release, In vivo biodistribution of PS‐ complex microparticles by small animal optical imaging | dos Santos et al. (2021) |
| Spirulina maxima‐derived modified pectin and modified pectin NPs | Modulates gut microbiota by increasing Bacteroidetes and reducing Firmicutes; improves gut permeability gene expression. | In vivo | Metagenomics analysis, sequencing reads, operational taxonomic units count and diversity indices, taxonomic analysis, comparison of relative abundance of metagenomics | Chandrarathna et al. (2020) |
| Date seed polysaccharide‐capped selenium NPs | Modulation of gut microbiota via increased SCFAs production, microbial richness and diverse metabolic pathways. | In vitro | Fecal fermentation properties, metagenomics, and metabolomics | Subhash et al. (2025) |
Abbreviations: ACE, angiotensin‐converting enzyme; GIT, gastrointestinal tract; LDA, linear discriminant analysis; NPs, nanoparticles; PCA, principal component analysis; PCoA, principal coordinate analysis; PS, polysaccharides; SCFAs, short‐chain fatty acids.
4. APPLICATIONS OF POLYSACCHARIDE‐BASED NANOPARTICLES
4.1. Polysaccharide‐based nanoparticles in gut microbiota modulation
Natural PS are ideal for large intestinal targeting due to their abundance. These PS are stable, affordable, and available in various grades, reflecting differences in their physicochemical, functional, and structural properties. They are safe, non‐toxic, water‐binding, and can be naturally degraded by microbiota. Drug release is prevented in the upper GIT and only occurs in the presence of simulated colonic content through the enzymatic fermentation of PS, which is dependent on their structure and function (Azehaf et al., 2023). The arrangement of NPs coated with PS is crucial in determining their impact on gut microbiota by affecting the interaction between microbiota and the host. These complex carbohydrates serve as a food source for certain bacteria, thus modulating the microbiota and supporting gut health.
Different PS, classified as polymers with complex structures and specific functions, have evolved naturally. Some of these compounds consist of amino acids, nucleobases, monosaccharides, and disaccharides. Based on the type of heteroatom in the main chain, natural PS can be modified for targeting or cross‐linking NPs (De Anda‐Flores et al., 2021). PS‐NPs are versatile tools for influencing the gut microbiota due to their unique structural features, including size, charge, and the capacity to carry and release prebiotics and probiotics. Beyond merely providing nutrients or drugs, they contribute to shaping microbial communities, boosting immune function, and enhancing overall intestinal health. Understanding the relationship between the structure of PS‐NPs and their functionality is essential for improving their efficacy in therapeutic and dietary applications (Q. Hu & Luo, 2018).
Many PS are intricate polymers that have naturally evolved with specific biological roles. This category includes compounds made up of amino acids, nucleobases, and mono‐ and disaccharides. Natural PS can be modified by functionalizing with targeting components or by cross‐linking NPs, due to the presence of heteroatoms in their main chain (De Anda‐Flores et al., 2021; Plucinski et al., 2021). The PS commonly used as NM capping agents include cellulose, chitosan, pectin, alginate, and xanthan gum. These PS stabilize various NPs, such as silver, gold, ZnO, and silica NPs. Each of these PS has distinct structural and functional properties that influence their interactions with gut microbiota, ultimately modulating microbial diversity and composition within the gut environment.
A colon‐targeted drug delivery system called Polymeric colon drug delivery system (PCDDS) has been developed using prebiotic pectin and chitosan as a shell, with the drug sulfasalazine (SAS) loaded into a PLGA core to form SAS@PLGA‐chitosan‐pectin NPs. This complex provides effective pH‐responsive characteristics. The pectinase secreted by the gut microbiota initiates the breakdown of the pectin/chitosan shell, releasing pectin oligosaccharides with significant prebiotic benefits. The PCDDSs exhibit strong distribution and accumulation in colon tissue, rapid cellular absorption, effective therapeutic outcomes in vivo, and regulation of imbalanced gut microbiota, as demonstrated in a mouse colitis model (H. Li, Cheng, et al., 2024).
These PS consist of more than 10 monosaccharide units linked by O‐glycosidic bonds. Due to their high abundance of hydroxyl groups, PS can be modified by carboxylation, esterification, and amination, enhancing their functional properties as NP capping agents (Ding & Wu, 2020). Their hydrophilic and hydrophobic characteristics make PS ideal for use in drug delivery systems. However, using PS alone for coating in colon‐targeted drug delivery systems can lead to premature drug release due to their high solubility and swelling in water. To prevent this, hydrophobic polymers can be added to reduce swelling and delay drug release until reaching the colon. Additionally, PS must resist digestive enzymes in the upper GIT but be easily degraded by bacterial enzymes in the colon (Chiesa et al., 2018).
The selection and arrangement of PS and the method of NPs capping are essential for enhancing the gut microbiome. The choice of PS is significant, as different types offer unique benefits: cationic PS from chitin provides biocompatibility and antimicrobial properties, negatively charged PS from brown algae offers gel‐forming capacity, PS rich in galacturonic acid (commonly found in fruits) promotes prebiotic effects, and PS from plant sources ensures structural integrity and effective delivery systems (Behr & Ganesan, 2022; Y. Li et al., 2021). Additionally, examining the structure of NPs post‐capping reveals that NPs typically range in size from 10 to 100 nm, a size that facilitates easy absorption or interaction with gut bacteria. The surface charge also influences interactions, as cationic or anionic surfaces affect attachment to bacterial cell walls and mucosal surfaces in the gut. Furthermore, porous formations and interconnected systems can control the release and distribution of bioactive substances, such as medications or prebiotics, enhancing their functional impact on the gut microbiome (S. Zhang et al., 2024).
4.1.1. Lactobacilli impacted by PS‐NPs
PS‐NPs have a notable impact on gut microbiota, especially through altering the composition and functional activity of Lactobacillus species, potentially leading to significant health benefits. Through interactions with the gut microbiome, PS contributes to the production of SCFAs, vital metabolites that support gut health and sustain beneficial bacteria such as Lactobacillus. Specifically, NPs with PS coatings, such as hyaluronic acid‐gold NPs, have been developed to stabilize strains like Lactobacillus casei in an in vitro gut simulation study (W. Huang et al., 2022). The authors confirmed that these coatings modulated bacteriocin gene expression within L. casei, enhancing its probiotic functions and resilience within the gut microbiome, thereby promoting microbial balance and inhibiting pathogenic species (Q. Huang et al., 2024). Additionally, the synthesized NPs did not interfere in the immunomodulatory properties of L. casei in epithelial cells, making them medically relevant for immune signaling. The resistance of upper gut digestion and absence of hyaluronidase‐encoding gene responsible for hyaluronic acids biodegradation in L. casei have been reported as the major mechanism behind the observed gut modulation (W. Huang et al., 2022).
Prebiotic‐based PS‐NPs also show therapeutic potential, especially in managing conditions such as colitis. By selectively promoting the growth of beneficial microbes, including Lactobacillus, these NPs contribute to a balanced and diverse microbial ecosystem and help mitigate inflammatory responses in the intestinal tract. This capacity of PS‐capped NPs to support microbial diversity and reduce inflammation underscores their promise as a targeted intervention for gut health (W. Huang et al., 2022; H. Li, Cheng, et al., 2024; D. Zhang, Liu, et al., 2022). In a study examining copper‐loaded chitosan nanoparticles (CNP‐Cu) as a dietary supplement, the results showed a significant increase in Lactobacillus populations in both the jejunum and cecum, indicating that CNP‐Cu (100 mg/kg) supplementation supports growth, beneficial intestinal microflora, and gut morphology (C. Wang et al., 2011). These findings suggest that CNP‐Cu may serve as an effective alternative to chlortetracycline in the diets of weaned piglets. While the exact mechanism remains uncertain, the authors suggest that selectively inhibiting certain pathogens could encourage the growth of Lactobacillus and diminish competition, potentially providing a viable solution explanation. In a separate in vivo study on the functional properties of PS‐NPs, nanocrystalline cellulose was found to enhance Lactobacillus populations and alleviate constipation by modulating gut microbiota metabolism in constipated mice (M. Wang et al., 2022). This approach required only a small dose, minimizing impact on organs and intestines, and showing promise as a therapeutic alternative to traditional medications and dietary fiber for constipation. The authors have proposed the regulation of bile acid receptors (FXR and TGR5) and expression of bile acid‐encoding genes for biosynthesis, metabolism, and reabsorption to be possible mechanisms of action of nanocrystalline cellulose for Lactobacillus modulation
Another PS‐NPs structure, designed as a microsphere composed of an alginate‐based nano dietary fiber carrier, was developed to protect therapeutic agents from acidic and enzymatic environments in the GIT, ensuring targeted drug delivery to the colorectum. Upon reaching the target site, fermentation by gut bacteria utilizing non‐digestible fibers (NDFs) and proteins as carbon and nitrogen sources promotes drug release and exerts a probiotic effect. This system was particularly effective in modulating Lactobacillus murinus and Lactobacillus johnsonii, demonstrating the potential of PS‐NPs‐based microspheres to enhance gut microbiota health (L. Qiu et al., 2023; M.‐Q. Wang et al., 2012; M. Wang et al., 2022). However, plant‐derived nanovesicles in the form of PS‐NPs, such as those derived from Tartary Buckwheat flour, did not significantly alter Lactobacillus levels. Nonetheless, they contributed to greater diversity in fecal microbiota and increased SCFA levels, offering a novel nutritional approach as a functional food ingredient. This enhancement of microbial diversity and SCFA production highlights their potential role in promoting gut health from a dietary perspective (Y. Liu et al., 2022).
4.1.2. PS‐NPs and Bifidobacteria
Modulating Bifidobacteria within the gut microbiota is essential for enhancing host health due to their roles in immune modulation, metabolic activity, and pathogen inhibition. Bifidobacteria produce SCFAs, such as acetate, which help maintain a low intestinal pH, creating an environment unfavorable for pathogens and supporting overall gut health. Their presence has been linked to protective effects against infections and an enhanced immune response, particularly in the GIT. Additionally, Bifidobacteria contribute to digestive health by metabolizing complex carbohydrates, aiding nutrient absorption, and balancing the gut microbiome through competitive exclusion of pathogens (Arboleya et al., 2016; Fang et al., 2025; Fukuda et al., 2012).
A study on CNP‐Cu as a dietary supplement demonstrated a significant increase in Bifidobacterium populations in the jejunum and cecum, suggesting that CNP‐Cu supplementation promotes the growth of beneficial intestinal microbiota and supports gut morphology. These findings indicate that CNP‐Cu could serve as an effective alternative to chlortetracycline in the diets of weaned piglets (M.‐Q. Wang et al., 2012).
The incorporation of Canna edulis starch and starch nanoparticles (SNPs) into stabilized Pickering emulsions was also investigated by N. Wang et al. (2024). SNPs were derived via acid hydrolysis of native C. edulis starch and further modified with octenyl succinic anhydride (OSA) to produce OS‐starch and OS‐SNPs. These modified particles effectively stabilized curcumin‐loaded Pickering emulsions. In vitro fecal fermentation revealed that gut microbiota were affected by these particles, especially in their modulation of Bifidobacteria, underscoring their potential for applications in transport systems and as innovative prebiotic foods formulations. Inhibition of inflammatory factors such as NO and IL‐6 was a possible mechanism suggested by the authors through which OS‐starch and OS‐SNPs achieve the modulation of Bifidobacteria (N. Wang et al., 2024).
Additionally, a hydrogel microsphere composed of alginate and nano dietary fiber was developed to protect drugs from acidic and enzymatic degradation, ensuring targeted delivery to the colorectum and promoting the growth of Bifidobacteria. The NDF‐1 hydrogel microspheres facilitated the enhanced release of 5‐ASA specifically at irritable bowel syndrome (IBS)‐affected sites, leading to reduced gut inflammation and a reshaping of gut microbiota composition in chronic colitis models (L. Qiu et al., 2023).
4.1.3. PS‐NPs impact on Firmicutes
Modulating Firmicutes within the gut microbiota is essential for maintaining metabolic and immune health, as these bacteria play a crucial role in nutrient metabolism, energy balance, and pathogen defense. An optimal balance of Firmicutes supports metabolic processes, including enhanced insulin sensitivity and reduced inflammation, which are valuable in preventing or managing metabolic disorders such as obesity and diabetes (Umu et al., 2017). Firmicutes also produce beneficial compounds, including SCFAs, which help maintain gut integrity and promote immune resilience. Strategies for modulating Firmicutes, such as prebiotic fiber and probiotics, have been shown to promote beneficial bacteria while inhibiting pathogens, fostering a balanced gut ecosystem (Natividad et al., 2015; Xu et al., 2025). A prebiotic formulation made from phthalyl dextran nanoparticles (PDNs), created by conjugating phthalic anhydride with dextran, was developed to enhance antimicrobial activity. When Pediococcus acidilactici was internalized with these PDNs, it demonstrated the ability to reduce pathogen populations and increase beneficial bacterial species in mice, leading to shifts in the gut microbiome, particularly balancing the Firmicutes and Bacteroidetes communities, thus improving gut health (Xu et al., 2025).
In another PS‐NPs structure, β‐cyclodextrin complex NPs with olive oil Pickering emulsions were developed, using starch as a capping agent. Animal studies showed that this PS‐NPs complex influenced the gut microbiota in mice by enhancing microbial richness and diversity, resulting in a higher presence of health‐promoting bacteria. However, at high doses, the complex significantly reduced the Firmicutes/Bacteroidetes ratio (Q. Li et al., 2022). Low doses of sodium alginate‐coated nano ZnO, a novel zinc source, were developed as a potential alternative to traditional pharmacological levels of ZnO for weaned piglets. Functional analysis revealed that microbial changes induced by this formulation significantly influenced metabolic pathways through the modulation of Firmicutes. These findings suggest that this PS‐NPs formulation could serve as an effective substitute for ZnO, potentially reducing zinc emissions while supporting growth performance, antioxidant and immune functions, and restructuring gut microbiota in piglets (X. Xiao et al., 2023). Recently, NPs with embedded chitosan peptides were formulated as emulsions or lipid/peptide NPs.
The Pickering emulsion exhibited a significantly enhanced protective effect on the composition and function of intestinal microbiota by modulating the levels of Firmicutes, diminishing the secretion of interleukin‐1β, and suppressing the formation of the NLRP3 inflammasome. These results underscore the potential of chitosan‐based Pickering emulsions to regulate gut microbiota and confer anti‐inflammatory effects in gut‐related inflammatory diseases (M. Cao et al., 2023).
4.1.4. SCFAs influenced by PS‐NPs
SCFAs, primarily acetate, propionate, and butyrate, are essential metabolites produced by gut microbiota during the fermentation of non‐digestible carbohydrates. They play critical roles in maintaining gut health and modulating inflammation. SCFAs have been associated with enhanced gut barrier integrity, immune modulation, and the prevention of diseases such as IBD (Akhtar et al., 2022). These metabolites contribute to gut health by increasing mucosal layer thickness, promoting intestinal fluid secretion, stimulating colonic smooth muscle, accelerating intestinal transit, and improving contractility and motility, thus alleviating constipation.
PS capped on NPs can further promote SCFA production by serving as substrates for specific gut bacteria, thereby fostering a beneficial microbial environment. For instance, PS derived from sources like Cyclocarya paliurus and jackfruit have demonstrated the ability to enhance SCFA levels, improving metabolic functions and reducing inflammation (Wu et al., 2021; K. Zhu et al., 2021). This targeted modulation of gut microbiota via PS–NP systems presents promising therapeutic avenues, particularly in metabolic and inflammatory disorders, by boosting SCFA production and restoring gut homeostasis (Q. Huang et al., 2024).
In one study, treatment with nanocrystalline cellulose at a dose of 100 mg/kg per day resulted in a 25.02% increase in total SCFA levels, with butyric acid content showing a significant 65.45% rise, which was 20.41% higher than the control group levels. This enhancement in butyric acid matched or exceeded the effects of direct oral butyrate administration. Additionally, nanocrystalline cellulose treatment led to increases of over 24% in both acetic acid and valeric acid content (M. Wang et al., 2022). However, this study did not measure pentanoic acid, an SCFA integral to human health, as SCFAs like acetate support gut barrier integrity, modulate inflammatory responses, and provide energy to host cells (Pushpanathan et al., 2019).
In a separate study, sodium alginate‐coated nano zinc oxide (saZnO) affected the levels of specific SCFAs, while others showed no significant changes compared to the control group and dietary ZnO. Neither dietary ZnO nor saZnO supplementation significantly impacted the concentrations of acetic, propionic, butyric, isobutyric, or isovaleric acids, nor total SCFA levels in fecal samples (p > 0.05). However, saZnO significantly elevated valeric acid levels in fecal samples compared to the control group (p < 0.05), while dietary ZnO showed a nonsignificant increase in valeric acid (p > 0.05) (X. Xiao et al., 2023).
Testing PS‐NPs as modulators of gut microbiota without measuring SCFAs can lead to several criticisms and limitations, such as a lack of insight into metabolic pathways, overlooking key health implications, potential misinterpretation of microbial shifts, and difficulty in comparing findings with existing literature. This gap limits the study's relevance within the broader scientific context. Several in vivo studies have overlooked SCFA quantification, including those by M. Cao et al. (2023), Cheng et al. (2024), Khorasani and Shojaosadati (2017), W.‐S. Kim et al. (2019), Q. Li et al. (2022), Ma et al. (2024), and M.‐Q. Wang et al. (2012). Table 3 provides a summary of PS‐NPs structures, types of gut microbiota modulation, and enhanced SCFA production.
TABLE 3.
Polysaccharide‐based nanomaterials (PS‐NPs) structure, types of modulated gut‐microbiota community, and the enhanced short‐chain fatty acids (SCFAs).
| Type | In vivo/in vitro | Types of modulated gut–microbiota community | Modulated SCFAs | Reference |
|---|---|---|---|---|
| Copper‐loaded chitosan nanoparticles (CNP‐Cu) | In vivo |
↑ Levilactobacillus ↑ Bifidobacterium ↓ Escherichia coli |
— | M.‐Q. Wang et al. (2012) |
| Bacterial nanocellulose‐pectin bionanocomposites | In vitro |
↑ High survival rate of B. coagulans after microwave drying (99.43%) and sequential digestion under stimulated gastrointestinal fluids (94.76%) with optimum prebiotic score for B. coagulans (1.00) and for Escherichia coli (0.99) |
— | Khorasani and Shojaosadati (2016) |
| Pectin incorporated with nanofibers of chitin (NC), lignocellulose (NLC) | In vitro | ↑Exhibited the highest survival of the entrapped probiotic bacteria under simulated gastric (97.7%) and intestinal (95.8%) conditions | — | Khorasani and Shojaosadati (2017) |
| Phthalyl dextran nanoparticles | In vivo |
↓ Proteobacteria ↑Genera S24‐7 and Anaerostipes ↑Firmicutes and Bacteroidetes |
— | W.‐S. Kim et al. (2019) |
| Nanocrystalline cellulose | In vivo |
↑Firmicutes ↑Bacteroidetes ↑Lactobacillus ↑Anaerotruncus ↑Prevotellaceae ↑Anaerotruncus ↑Alloprevotella ↑Ruminiclostridium ↑Ruminococcaceae |
↑Acetic acid ↑Propionic acid ↑Butyric acid ↑Isobutyric acid ↑Valeric acid ↑Isovaleric acid ↑Total SCFAs |
M. Wang et al. (2022) |
| Tartary buckwheat flour capping nanovesicles | In vitro |
↑ Klebsiella, Escherichia, Lysinibacillus, and Salmonella ↓ Enterobacter, Citrobacter, Megasphaera, and Vibrio ‐ Lactobacillus and Escherichia ↑Leuconostoc |
↑Acetic acid ↑Propanoic acid ↑Butyric acid ↑Pentanoic acid |
Y. Liu et al. (2022) |
|
Starch capping β‐Cyclodextrin complex nanoparticles with olive oil Pickering emulsions |
In vivo |
↓ Ratio of phyla Firmicutes/Bacteroidetes ↓Proteobacteria and Epsilonbacteraeota ↑ Bacteroidetes and Deferribacteres ↓Proteobacteria ↑ Odoribacter |
— | Q. Li et al. (2022) |
| Alginate microsphere loaded nano dietary fiber carrier | In vivo |
↓Escherichia‐Shigella ↑Bifidobacterium ↓Dubosiella ↑Lactobacillus murinus ↑Lactobacillus johnsonii ↓Muribaculaceae |
↑Acetate ↑Propionate ↑Butyrate ↑Lacetate |
L. Qiu et al. (2023) |
| Sodium alginate‐coated nano‐zinc oxide | In vivo |
↑Firmicutes ↓Bacteroidota −Actinobacteriota −Spirochaetota −Proteobacteria −Cyanobacteria −Verrucomicrobiota |
↑Acetic acid −Propionic acid ↑Butyric acid ↓Isobutyric acid ↑Valeric acid ↓Isovaleric acid ↑Total SCFAs |
X. Xiao et al. (2023) |
| Chitosan peptide‐embedded nanoparticles emulsion/lipid/peptide nanoparticles emulsion | In vivo |
↑ Firmicutes ↓Bactericide, proteobacteria, deferribacteres ↓Bilophila, Deltaproteobacteria, Desulfovibrionales ↓Clostridium botulinum, Staphylococcus aureus ↑Lactobacillus |
— | M. Cao et al. (2023) |
|
Gum Arabic/lecithin capping nanoparticles from blueberry anthocyanins |
In vivo |
↑Clostridia and TM7–3 ↓Erysipelotrichi ↓Deltaproteobacteria ↓Desulfovibrionales ↑Coriobacteriales ↑CW040 |
— | Cheng et al. (2024) |
| Dextran capping nano‐aspirin particles | In vivo |
↑Lactobacillus ↑Akkermansia, ↓Bacteroides, Escherichia_coli, and Robinsoniella |
— | Ma et al. (2024) |
| Canna edulis starch and starch nanoparticles | In vitro |
↑Actinobacteriota ↓Firmicutes/Bacteroidota ratio ↓Proteobacteria ↑f Bifidobacteria ↑Prevotella ↓Escherichia‐Shigella ↓Comamonas ↑Subdoligranum, Lachnospira, and Streptococcus |
— | N. Wang et al. (2024) |
Note: ↓: decrease; ↑: increase.
Abbreviation: SCFAs, short‐chain fatty acids.
4.2. PS‐NPs applications in functional foods
Recent advancements in nanobiotechnology have significantly expanded the industrial applications of PS‐based NMs across diverse fields, including food, medical, cosmeceuticals, waste management, and pharmaceuticals. Notably, the development of functional foods with PS‐NPs has gained momentum due to the unique properties of PS hydrocolloids and their biocompatibility, which enhance both the intrinsic and extrinsic qualities of foods. PS have been widely employed in the development of functional foods, offering tailored viscosity, cohesivity, emulsification, extended shelf‐life, water and oil binding, sensory improvement, gelation, stability, and texture (Nikolić et al., 2024). These characteristics are highly valued by nanotechnologists and food technologists aiming to create novel functional foods that are safer, tastier, healthier, and nutritionally superior through efficient ingredient modification. PS‐NPs also serve as therapeutic agent carriers with minimized interactions with other food components.
The combined properties of NPs and bioactive PS make PS‐NPs highly effective in enhancing the functionality of functional foods (Lu et al., 2019). For instance, Bhopatkar et al. (2015) investigated SNPs as carriers for insoluble functional food ingredients through electrostatic interactions. Different concentrations of insoluble 1‐naphthol molecules were self‐assembled onto the SNPs. Molecular dynamic simulation, potentiometric titration, calorimetric analysis, and visual observation of the PS‐NPs revealed the residency of 1‐naphthol within the amylose helical structure, higher enthalpies of dissociation and reassociation, and uniform dispersion in aqueous media.
The increasing consumption of processed, fried, and energy‐dense foods, driven by population growth and modernization, has been linked to rising incidences of obesity, diabetes, and cardiovascular diseases (Ganesan et al., 2018). Moreover, many functional foods, such as nutraceuticals, are lipophilic molecules with poor bioavailability, solubility, chemical stability, and compatibility with other food ingredients (Plucinski et al., 2021). Incorporating PS‐NPs into processed foods has shown potential for reducing calorie intake, preventing the reabsorption of bile acids and cholesterol, and delivering SCFAs to improve gut health (Baek, 2021; Le et al., 2020; Lin et al., 2021). Andrade et al. (2015) explored nano‐fibrils complexed with cellulose extracted from peach palm residues at concentrations of 7%, 14%, and 21% in processed consumer diets using a mouse model. Their results demonstrated that supplementing diets with PS‐NPs improved the weight of mice by up to 10%. Similarly, in vitro digestion studies on nanocellulose‐coated lipid droplets showed restricted upper gut digestion and facilitated SCFA release in the colon (Mwangi et al., 2020).
In another in vitro study, the delivery of free fatty acids by corn oil‐in‐water Pickering emulsions decorated with plant‐based nanocellulose was 40% higher than in undecorated emulsions. The nanocellulose created a barrier that prevented bile salt and lipase absorption at the oil–water interface (Bai et al., 2021). Enzymatically synthesized Dextran‐NPs (DEXNPs) were used for delivering hydrophobic isoflavone Genistein nutraceuticals by Semyonov et al. (2014). The study reported improved bioavailability, solubility, and a larger surface area due to Genistein incorporation into spherical DEXNPs (85%, 105–400 nm). Additionally, chitosan‐NPs have been employed to transport bioactive polyphenols with health benefits, including anticancer, antimicrobial, antioxidant, and immune‐protective effects (Plucinski et al., 2021). Collectively, these findings validate the potential of PS‐NPs as functional ingredients for developing innovative functional foods. Although still a nascent research field, Table 4 summarizes previous applications of PS‐NPs in functional food development.
TABLE 4.
Applications of polysaccharide‐based nanomaterials (PS‐NPs) in functional foods.
|
Food type |
Types of PS‐NPs | Functionalities | References |
|---|---|---|---|
| Ice cream | Nanocellulose |
Significant decrease in melting rate Increase in fiber content |
Contreras‐Ramírez et al. (2022) |
| Meat sausage | G. xylinus nanocellulose |
Decrease in fat content Improved rheological properties Improved textural properties Improved nutritional profile Improved structural profile |
Marchetti et al. (2017) |
| Muffins | Nanocellulose |
Development of gluten‐free muffins for celiac patients Increased batter consistency Increased fiber content |
Marchetti et al. (2020) |
|
Turkey deli meat Edible films |
Pullulan‐Ag NPs Pullulan‐ZnO NPs |
Enhanced antibacterial effect against L. monocytogenes and S. aureus | Khalaf et al. (2013) |
| Sausage |
Cellulose‐Ag NPs Collagen‐Ag NPs |
Antibacterial activities against E. coli and S. aureus | Fedotova et al. (2010) |
| Milk | Lactic acid‐Ag‐Cys NPs | Aggregation of Ag‐cysteine NPs and color change indicating spoilage | Lakade et al. (2017) |
| Set yoghurt |
Inulin‐ZnO NPs Inulin‐α−Fe2O3‐ZnO NPs Inulin‐CaHPO4 NPs |
Increased consistency and firmness Improved solubility |
Santillán‐Urquiza et al. (2017) |
| Mixed flour | Eugenol‐chitosan NPs |
Reduce eugenol loss during heating Enhanced antioxidant activities Improved thermal stability |
Woranuch and Yoksan (2013) |
|
Pineapple juice Melon juice |
Cellulose‐Cu NPs | Excellent antifungal activities against Saccharomyces cerevisiae | Llorens et al. (2012) |
| Melon | Cellulose‐Ag NPs |
Retardation of the melon cuts senescence Extended lag phase of yeast cells Reduced population of yeast Excellent antibacterial activities against E. coli |
Fernández et al. (2010) |
| Tuna fish | Gelatin‐ZnO NPs | Inhibition of lipid peroxidase | I. Kim et al. (2022) |
| Tomato juice | Soybean PS‐nisin NPs | Improved antibacterial activities against L. monocytogenes and B. subtilis | Luo et al. (2019) |
| Pork meat | PLA‐TiO2 NPs | Improved antimicrobial properties | S. Li et al. (2020) |
|
Carrot Pear |
Sodium alginate‐Ag NPs |
Enhanced antimicrobial activities against E. coli and S. aureus |
Mohammed Fayaz et al. (2009) |
| Pork frankfurters | Bacterial nanocellulose | Improved textural, sensorial, physicochemical, and microbiological properties | Yu and Lin (2014) |
Note: Ag‐Silver, NPs‐Nanoparticles, ZnO‐Zinc oxide, PLA‐Poly lactic acid, TiO2‐Titanium oxide, Cys‐Cysteine, α−Fe2O3‐Iron oxide, CaHPO4‐Di calcium phosphate.
5. FACTORS INFLUENCING THE EFFICACY OF POLYSACCHARIDE‐BASED NANOPARTICLES
Several techniques have been suggested for the preparation of NPs using natural PS. Choosing the right synthesis technique relies on the specific application and its needs. Before creating drug delivery nanocarriers, it is important to consider various factors like the active agent's thermal and chemical stability, reproducibility of release kinetics, particle size, stability of the final product, and any residual toxicity (Plucinski et al., 2021).
5.1. Impact of nanoparticle size, shape, and surface charge
The size of PS‐NPs influences their ability to modulate gut microbiota. Smaller NPs can enhance the delivery of bioactive compounds and improve interactions with gut bacteria, leading to changes in microbial composition and activity. Conversely, larger NPs may exhibit distinct distribution and interaction behaviors, potentially affecting their targeting efficiency within specific areas of the gut. Such adjustments support beneficial bacteria while inhibiting harmful ones, contributing to improved gut health. These NPs demonstrate rapid absorption and release properties, allowing for high diffusion capacity and volume adaptation. Additionally, their size and shape can be readily modified to minimize unwanted side effects (Malhaire et al., 2016).
The shape of PS‐NPs also plays a crucial role in influencing microbiota by altering interactions with gut bacteria. NPs of various shapes (e.g., spherical or rod‐like) impact gut microbiota composition and function differently. For instance, variations in surface area, hydrophobicity, and steric properties affect how microbial communities recognize and utilize these NPs. These differences may lead to changes in microbial metabolic activity and diversity, potentially promoting or suppressing beneficial bacteria associated with intestinal health. Understanding these effects is essential for developing targeted nutritional or therapeutic applications (Chaudhary et al., 2022; Ho Do et al., 2021). Therefore, it is vital to analyze PS‐NPs properties (such as size, surface charge, shape, and agglomeration) during their production, as they must navigate physiological barriers within the body, including cell uptake, tissue penetration, tumor targeting, and circulation within blood vessels (Figure 7) (Nidhi et al., 2016).
FIGURE 7.

Polysaccharides‐based nanoparticles overcome different barriers in human body.
NP characteristics, such as size and shape, encompass colloidal stability, specific surface area, optical properties, in vivo behavior, and cellular absorption. NMs are defined by having at least one dimension or surface structure within the nanometer range (∼1–100 nm). Governing bodies note that the upper nanoscale range may exceed 100 nm if the material exhibits certain physical or chemical characteristics, and its biological impact is often related to size, with benefits such as increased bioavailability, reduced toxicity, and lower required dosage (De Anda‐Flores et al., 2021; Patra et al., 2018).
Smaller particles have a higher surface area‐to‐volume ratio, facilitating quicker drug release. This size allows particles to penetrate cell membranes, organs, and tissues, supporting movement through the bloodstream, cellular uptake, intracellular movement, and uptake by cells. However, particles must not be too small, as studies indicate that the typical size threshold for renal excretion is 6–8 nm (Chiriac et al., 2019; Shah et al., 2020). NPs smaller than 6 nm can become coated with blood proteins, which enlarges their hydrodynamic diameter and prevents renal excretion. On the other hand, NPs larger than 100 nm are more likely to aggregate at the injection site or be captured by macrophages in the spleen, lungs, and liver (Shah et al., 2020; Zheng et al., 2020). The surface charge and clustering of PS‐NPs play a critical role in their interactions with gut microbiota, affecting their biological activity and modulation potential. PS‐NPs with specific surface charges interact with microbial cells accordingly. For instance, positively charged NPs can attract negatively charged bacterial cells, enhancing attachment and biofilm formation and potentially supporting beneficial microbial types.
Surface charge influences particle dispersion, aggregation, and flocculation. Neutral charges may lead to instability and precipitation of NPs, whereas a high zeta potential ensures particle stability, preventing aggregation. Conversely, a low zeta potential increases particle attraction, causing floc formation. Negatively charged NPs exhibit reduced phagocytic absorption, prolonging their presence in the bloodstream, as shown in Figure 7a. Meanwhile, positively charged particles enhance phagocytosis through interactions with the anionic cell membrane (Jafari & Esfanjani, 2017; Tiwari et al., 2019). Additionally, the charge can affect nutrient availability, altering how bacteria metabolize PS and ultimately affecting microbial composition. The aggregated NPs may be less accessible to gut bacteria, which decreases their capacity to influence the microbiota. Clumping could reduce the available surface area for interactions, potentially diminishing the benefits of the NPs, as shown in Figure 8a. Furthermore, larger clusters can modify the gut's physical properties, changing the way microbes interact with PS and each other, thus influencing the dynamics of the microbial community (C. Liu et al., 2023; Xie et al., 2022).
FIGURE 8.

(a) Effect of surface charge on the formation of nano‐particles aggregation and the bioavailability for microbial community and (b) overview of main nano‐polysaccharides barriers during the digestive system.
5.2. Role of chemical and enzymatic barriers
As illustrated in Figure 8b, during the digestive process, PS encounter harsh conditions as they travel through the stomach and small intestine before reaching the colon. The acidity of the stomach and the transit through the intestinal route are primary chemical barriers that can diminish the efficacy of administered molecules by degrading them (Huo et al., 2022). Electrostatic interactions can lead to the breakdown of PS‐NPs in the stomach, causing the polymer network to degrade and the NPs to disassemble. Therefore, a strategic approach involves developing pH‐responsive NP systems with durable matrices and enteric coatings to prevent premature drug release in the GIT (Huo et al., 2022; W. Yan et al., 2023). The interaction of PS‐NPs with microbial communities affects microbiota composition, activity, and behavior, thus modulating the microbiota through a chemical barrier effect. PS‐NPs derived from natural polymers such as chitosan, alginate, or cellulose are biocompatible and biodegradable, providing a gentler alternative for microbiota modulation compared to metallic or synthetic NPs (Homayun et al., 2019; Wahlgren et al., 2019). In studies simulating gastric conditions with simulated gastric fluid (SGF), 16% of the curcumin load was released from chitosan/guar gum‐based NPs after 3 h, while the NPs retained their structural integrity. This release system was governed by particle diffusion and swelling in the acidic environment, likely due to the protonation of the chitosan amide group (Tan et al., 2016).
Morphological analysis of modified nanogels made from soy protein and dextran revealed spherical core–layer structures for riboflavin encapsulation, with sizes ranging from 32 to 40 nm. These dimensions enabled an encapsulation efficiency of 65.9%. In vitro tests simulating gastrointestinal conditions showed that the nanogels remained stable in SGF, with a higher release observed in simulated intestinal fluid (Jin et al., 2016). Regarding enzymatic barriers, PS‐NPs face challenges from enzymatic breakdown in the GIT (Figure 7b). These PS are susceptible to degradation by digestive enzymes, with lipid and protein digestion starting in the stomach through gastric lipases and pepsins, followed by further breakdown in the small intestine via pancreatic enzymes such as lipase, trypsin, and elastase. This enzymatic action impacts the stability and effectiveness of NPs in delivering their intended bioactive compounds. PS that resist enzymatic breakdown in the stomach and intestine are eventually metabolized by colonic microbiota. This process converts PS into oligosaccharides, generating various metabolites and SCFAs that contribute to gut health and overall metabolism (Grewal & Salar, 2024; Homayun et al., 2019; T. Zhang, Yang, et al., 2018). For targeted colon delivery, citrus pectin‐based NPs coated with Eudragit S100 were developed for the release of 5‐fluorouracil to treat colorectal cancer. The study showed that pectinase degraded the NP complex, initiating drug release after 4 h in simulated intestinal fluid (pH 6.8). In vivo studies indicated minimal drug loss in the upper GIT, with increased concentrations of 5‐fluorouracil in the colon due to degradation by microbiota enzymes (Subudhi et al., 2015).
5.3. Surface modifications for enhanced functionality
Altering the surface of PS‐NPs can greatly affect gut microbiota by improving stability, targeting abilities, and interactions with microorganisms communities. By adjusting surface charge, hydrophilicity, and functional groups, modified NPs improve their resistance to digestive enzymes and are more likely to reach the colon, where they can be processed by microbiota. This approach can enhance the distribution of bioactive substances, support the growth of beneficial microbes, and regulate the overall balance of gut bacteria, potentially improving gut health and therapeutic outcomes (Ladaycia et al., 2021; Tamargo et al., 2022).
Surface modification techniques for PS‐NPs involve altering functional groups through reactions like esterification, etherification, or cross‐linking to improve properties. Additionally, stability and bioavailability can be enhanced by applying layers of other materials, such as polymers or lipids, for physical coating. Grafting methods also link synthetic or natural polymers to PS to adjust surface characteristics. Nanoprecipitation, which uses solvents to induce phase separation, alters surface morphology, while electrostatic assembly creates stable NPs complexes through charge interactions (Abdin et al., 2021; Aly & El‐Bisi, 2018).
The drying techniques used on PS‐capped bioactive compounds significantly impact their surface and internal structures. Freeze‐drying creates a porous surface, facilitating controlled release of grafted compounds by enhancing intestinal solvent penetration at different pH levels. The layered internal structure increases exposed surface area following solvent penetration. In contrast, vacuum drying produces a compact internal layer with fewer cracks, creating a smooth, uniform surface that effectively controls extract release under gastrointestinal conditions (Abdin et al., 2021; Hussain et al., 2018).
Incorporating nanofibers into pectin results in a gradual degradation of the pectin nanofiber bio‐composites, contrasting with their rapid swelling behavior. This effect is attributed to the homogeneous distribution of the interconnected nanofiber network within the pectin‐nanofiber bio‐composite, as well as intermolecular and intramolecular hydrogen bonding and ionic interactions between pectin and Ca2⁺ ions. The amorphous structure of the bio‐composites, compared to the crystalline structure of nanofibers, significantly improved gut microbiota by ensuring a high survival rate of encapsulated probiotic bacteria, achieving 97.7% survival under simulated gastric conditions and 95.8% under intestinal conditions (Khorasani & Shojaosadati, 2017).
Nanocrystalline cellulose was synthesized in rigid, rod‐like NPs with high crystallinity and purity. It was shown that nanocrystalline cellulose can effectively alleviate constipation by modulating gut microbiota metabolism, influencing populations such as Firmicutes, Bacteroidetes, Lactobacillus, Anaerotruncus, Prevotellaceae_UCG‐001, Alloprevotella, Ruminiclostridium_9, and Ruminococcaceae_UCG‐014. This approach required only a minimal dosage and showed no adverse effects on organs or intestinal health, presenting a viable alternative to conventional medications and dietary fibers for constipation management (M. Wang et al., 2022).
6. CHALLENGES AND FUTURE DIRECTIONS
6.1. Potential limitations of clinical applications
Accumulated evidence from this review highlights the significant nanomedical applications of PS‐NPs in gut modulation and functional food development, primarily due to their biocompatibility, bioavailability, and efficient cellular uptake. However, several limitations must be addressed to enable successful clinical translation of these findings. When therapeutic or pharmacological agents are developed for clinical applications, special attention is required to ensure their safety. Numerous studies have documented the biocompatibility, biodegradability, lower toxicity, and minimal immunological reactions of PS‐NPs compared to NPs based on synthetic polymers or inorganic agents.
Notably, R. Wang et al. (2021) fabricated a ternary nanocomposite (Zein/NaCas/Gum Arabic) through heat treatment and electrostatic interaction to form a protein–PS complex. An in vitro cytotoxicity assay of the synthesized, negatively charged nanocomposite (<150 nm particle size) was conducted using Caco‐2 carcinoma cells. The study confirmed that the survival rate of the cells exceeded 80% after 24 h of treatment with 200 µL of the nanocomposite, demonstrating its non‐toxic and biocompatible nature. Similarly, a systematic review of 70 studies published between 1998 and 2022 examined the cytotoxicity of chitosan‐based NPs. The review concluded that all experiments demonstrated low cytotoxicity, with survival rates exceeding 80%, irrespective of the assay method, particle composition, or cell lines used (Frigaard et al., 2022).
It is important to note, however, that variations in the behavior of PS‐NPs exist. These differences arise from factors such as the source, extraction methods, purification processes, and the temperature‐regulated physicochemical properties of the PS, as well as the NP precursors and synthesis methods (Barclay et al., 2019; Yuan et al., 2023). Additionally, PS can exhibit high molecular weight, instability, low mechanical strength, and excessive hydration under certain conditions, which can adversely impact the clinical efficacy of PS‐NPs (Q. Meng et al., 2022; Yuan et al., 2023). Therefore, considerable effort should be directed toward developing PS‐NPs tailored for clinical applications while preserving the natural properties of PS (Q. Meng et al., 2022). Furthermore, novel bioactive PS and their precursors intended for the synthesis of clinically relevant PS‐NPs should undergo comprehensive characterization and cytotoxicity testing prior to their adoption for clinical use, even though this may increase preparation costs and time. Despite these challenges, PS continue to hold immense potential for the development of clinically relevant PS‐NPs, offering a sustainable future for nanomedical and functional food applications.
6.2. Biological entry routes and toxicity considerations
NPs have attracted significant interest across various sectors, including energy, environmental science, the food industry, and medicine (Mir et al., 2013; Umar et al., 2016; Yaqoob et al., 2020). Specifically, polysaccharide‐based metallic nanoparticles (PMNPs) have seen substantial advancements in these fields. However, as with many emerging technologies, research has largely focused on their applications, leaving a gap in understanding their toxicity to cells, animals, and the environment. Several review articles offer valuable insights into the toxicological implications and recommended evaluation processes (Parveen et al., 2022; C. Wang et al., 2017). The risks associated with NPs exposure, potential entry pathways into the body, and metabolic processes remain incompletely understood. Nonetheless, various studies suggest that NPs accumulation in different organs can lead to toxic effects (Buzea et al., 2007; Yao et al., 2019). Although PS are appealing due to their low toxicity and minimal side effects, it is essential to know that not all PS are naturally non‐toxic and biologically compatible (Bushra et al., 2023; Goodarzi et al., 2013). Some sulphated PS, chitosan, and derivatives of certain bioactive PS have been reported to demonstrate cytotoxicity and immunogenicity at high doses or when administered using specific techniques. These characteristics vary depending on the source, influenced by the presence of impurities, degree of polymerization, charge density, and molecular weight (Bushra et al., 2023). Therefore, a comprehensive assessment of the biocompatibility and toxicity of natural PS, as well as the potential hazards associated with PS‐NPs, is crucial before their application.
The nanotoxicity of PS‐NPs is not absolute and depends on several factors, including health status, pre‐existing conditions, genetics, and PS‐NP properties such as electromagnetic state, size, agglutination, and shape. The nanoscale size of PS‐NPs, which is significantly smaller than most cellular organelles and cells, is a key factor in understanding their nanotoxicity (Buzea et al., 2007; Yao et al., 2019). Primary routes of entry for PS‐NPs include the respiratory system, oral intake, and skin absorption (C. Wang et al., 2017). Once inside the body, NPs can circulate through the bloodstream to various organs and systems, including the lymphatic, nervous, circulatory systems, and brain, potentially disrupting normal physiological functions (Buzea et al., 2007). Reported nanotoxicity conditions include disrupted cellular mechanisms, imbalances in cellular redox reactions, abnormal cellular functionalities, and tissue inflammation.
For example, colloidal gold NPs (30 nm) have been detected in rat platelets following tracheal administration, while Tc‐labeled carbon particles (99 nm) entered the bloodstream within 1 min (Simões, 2021). In vivo studies on macrophage clearance mechanisms have shown that NPs are removed less efficiently from the lungs compared to larger macroparticles, leading to various pulmonary diseases (Buzea et al., 2007). Although the field of nanotoxicity is relatively new, researchers suggest that understanding creation‐exposure pathways of nanotoxins and reducing fossil fuel combustion may help minimize human and animal exposure to nanotoxicity (Buzea et al., 2007; Yao et al., 2019).
6.3. Hemolysis, cytotoxicity, and inflammatory responses
Hemolysis, the rupture of red blood cells, can be induced by NPs through oxidative stress, leading to cell destruction and death. Previous studies have assessed the harmful effects of PMNPs by measuring hemolysis rates. Notably, Gum karaya‐based AuPNs (15.0–20.0 nm) and xanthan‐based gold nanoparticles AuPNs (20.0–25.0 nm) demonstrated lower hemolysis rates, even at doses exceeding 200 µg/mL (C. Wang et al., 2017). AgNPs, synthesized using PS from the edible fungus Lentinus squarrosulus (Mont.) Singer, as a reducing and stabilizing agent, was found to be compatible with human red blood cells at concentrations below 5 µg/mL (C. Wang et al., 2017). In contrast, significant hemolysis and a concentration‐dependent increase in hemagglutination were observed with starch‐based Ag NPs (5.8 nm) compared to gold and platinum NPs (Asharani et al., 2010). Consequently, a hemolysis risk assessment of PMNPs is essential before their application.
While hemolysis specifically affects red blood cells, cytotoxicity refers to the degree of cellular damage an agent can cause. Cytotoxicity evaluations are crucial to identify additional adverse effects associated with PS‐NPs. In a study by Rocha Amorim et al. (2016) on Fucan‐coated AgNPs (210.4 nm), minimal harmful effects were observed on mouse embryonic fibroblasts (NIH3T3), human renal (HEK 293), and murine fibroblasts (3T3) cells. Similarly, AuNPs decorated with porphyran (14.0 nm) and pectin (7.0–13.0 nm) did not significantly affect the viability of normal monkey kidney cells, even at doses up to 100 µM (Reena et al., 2016). Moreover, chitosan‐coated CuNPs (260.0 nm) significantly improved the survival rate of human alveolar epithelial (A549) cells compared to uncoated CuNPs. However, administering PS‐NPs via the lungs may increase the risk of inflammation, underscoring the need for comprehensive investigations before use (Worthington et al., 2013).
Macrophages, responsible for removing NPs that breach the mucociliary barrier, can trigger the release of pro‐inflammatory substances, leading to both acute and chronic inflammation (Braydich‐Stolle et al., 2014). In vitro studies on PMNP‐induced inflammation showed that chitosan‐coated Ag/ZnO NPs (100.0–150.0 nm) did not elicit significant inflammatory responses in murine macrophages (RAW264.7 cells) at concentrations of 50 µg/mL, nor did they alter cell morphology (Iram et al., 2014). Furthermore, evidence suggests that PMNPs exhibit lower cytotoxicity than their corresponding metals. Fluid exposure techniques were also found to significantly impact macrophage viability (Hwang et al., 2017). Additionally, several studies confirmed that PMNPs are neither neurotoxic nor phytotoxic (Iram et al., 2014).
6.4. Long‐term toxicity and recovery efficiency
There have been significant advancements in nanoscience, particularly in medicine, pharmaceuticals, and the food industries. However, considerable limitations persist in their commercialization, largely due to consumer concerns regarding safety and toxicity. There remains a paucity of information on the ethical approval processes and the safety‐to‐risk ratio of these products. As a burgeoning field, nanomedicine and nanofood require a comprehensive understanding of their pharmacognosy, immunoreactions, toxicity, and recovery mechanisms.
Researchers have highlighted that variations in surface area, size, and surface charge of PS‐NPs pose significant challenges to their comprehensive characterization and behavior in biosystems (Ahmad et al., 2022). Furthermore, their high biocompatibility allows them to translocate more rapidly and cross biological barriers such as the placenta, blood–brain barrier, and cell membranes, potentially facilitating uncontrolled activities, accumulation, and toxicity (Sharma et al., 2021).
Despite these limitations, there are continuous studies on bridging the narrow gap between toxicity and safety of PS‐NPs. In a study, sub‐acute oral toxicity evaluations revealed that PS‐NPs, administered to rats at a dose of 1500 ppm over 28 days, did not affect hematological or biochemical parameters. Similar results were observed in in vivo toxicity studies conducted on zebrafish (Venkatpurwar et al., 2012). Additionally, skin penetration studies showed the presence of PS‐NPs in the receptor compartment of intact skin (Dhar & Patil, 2012). A recent study indicated that NPs smaller than 22 nm could penetrate the deeper layers of the stratum corneum, although true skin penetration was not observed (Raju et al., 2018). Iswarya et al. (2017) conducted an evaluation of the toxicity associated with glucan‐ZnO NPs on HepG2 liver cancer cells as well as various bacterial pathogens. The researchers confirmed that treatment with 75 µg/mL PS‐NPs resulted in increased membrane permeability and the generation of ROS in S. aureus and Prunella vulgaris, consequently leading to cellular mortality. At the same concentration, significant inhibition of viability was observed in human liver carcinoma (HepG2) cells.
Advances in toxicity analysis have emphasized the safety and risks associated with PS‐NPs, but the risks related to PS‐NPs exposure still warrant consideration. Concerningly, in vivo studies fail to provide complete physiological data, toxicity evaluation, and management necessary for human trials. Sharma et al. (2021) noted that administering PS‐NPs in a monoclonal antibodies study led to serious illness in humans, contrary to the favorable outcomes observed in animal trials. Consequently, further in vivo studies are necessary to address these concerns and ensure comprehensive safety assessments.
Despite notable advancements, PS‐NPs recovery efficiency remains relatively low. There is an urgent need for physical techniques to enhance recovery efficiency, such as the application of magnetic fields to improve the retrieval of magnetic PS‐NPs. Additionally, various synthesis strategies employing the host–guest approach in PS‐NPs production have shown promise and are frequently recommended.
6.5. Synthesis challenges and potential applications
The synthesis process of PS‐NPs can pose challenges to their development and practical application. Therefore, there is a need to develop cost‐effective and environmentally friendly methods. Unlike natural polymers, synthetic polymers can be used in this process, yielding homogeneous PS‐NPs with a controllable and modifiable synthesis process. PS‐NPs have applications across various fields, including biosensing, targeted drug delivery, wound healing, and as antiviral, anticancer, and antibacterial agents (Bhardwaj et al., 2015; C. Wang et al., 2017). Many PS‐NPs applications are closely related to the characteristics of metallic ions. Consequently, incorporating guest metallic ions with beneficial properties during PS‐NPs synthesis is particularly valuable for applications in optics, imaging, diagnostics, and nanomedicine. However, recent in vitro and in vivo studies have yielded conflicting toxicological evaluations of PS‐NPs (Karuppaiah et al., 2023). Further research is necessary to assess the potential risks associated with PS‐NPs, including comprehensive long‐term toxicity studies that examine both acute and sub‐acute toxicity.
6.6. Future perspectives
There will be an increasing demand for advanced PS‐NPs in the future. Innovative protocols, targeted strategies, medical applications, and collaborative research methods will drive their development. Sustainable synthesis approaches will not only reduce production costs and enhance NPs production efficiency but will also incorporate green chemistry practices, promoting environmentally friendly production processes. The primary objective in developing PS‐NPs is to improve efficiency and precision in targeted drug delivery. These NPs have the potential to enhance therapeutic effects, transforming the treatment of complex diseases (Curcio et al., 2022; De Anda‐Flores et al., 2021). Modifying NP treatments can increase the precision and efficacy of medications. This approach involves aligning NPs with the microbiota profile of patients to optimize therapeutic outcomes. If researchers succeed in exploring new applications for gene delivery, food technology, and immunotherapy, they could significantly expand the role of PS‐NPs, particularly in enhancing functional foods and developing innovative nutraceuticals. Translating research findings into practical applications will rely heavily on contributions from academic institutions and the fields of biomedical science, clinical research, and materials science. Through these efforts, PS‐NPs are poised to become integral components of future therapies and food technologies.
7. CONCLUSION
PS have emerged as key components in functional foods due to their remarkable biological activities and prebiotic benefits. These natural macromolecular polymers, abundantly available in various food sources, play a significant role in enhancing the colonic environment, regulating gut microbiota, preventing metabolic disorders, maintaining mucosal immune balance, and managing intestinal permeability. Their biological effects are closely linked to their structure, conformation, molecular weight, shape, and monosaccharide composition. Recent studies indicate that dietary PS resist digestion and absorption in the upper GIT, undergoing microbial fermentation primarily in the colon. This process generates beneficial metabolites, such as SCFAs, which are crucial for nutrient metabolism, energy regulation, and immune function. The interaction of PS with gut bacteria, including Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes, underscores their critical role in maintaining gut health.
NPs have gained considerable attention for their ability to modulate colonic microflora and act as innovative drug delivery systems, enhancing solubility, targeted localization, and drug protection. However, their oral administration faces challenges, such as instability in the gastrointestinal environment. Structural modifications using PS have been explored to overcome these limitations, improving stability, extending residence time, and enhancing delivery to the colon. PS‐NPs, including nanohydrogels, NPs, and nanofibers, have demonstrated significant potential in nanomedicine and functional food applications due to their low cost, bioavailability, biodegradability, biocompatibility, and non‐toxicity. They have been incorporated into various foods such as milk, ice cream, yogurt, meatballs, sausages, and muffins to enhance both intrinsic and extrinsic properties, functionality, and health benefits.
Despite these advancements, there remain critical gaps in the understanding and application of PS‐NPs. Challenges such as aggregation, burst release of substances, and the need for precise control over particle properties require further research. Additionally, while promising results have been achieved in preclinical in vitro and in vivo studies, translating these findings to human applications remains a significant hurdle. A more comprehensive understanding of the mechanisms of action, evaluation methods, and long‐term safety of PS‐NPs is essential.
In summary, PS‐NPs represent a transformative approach in gut microbiota modulation and functional food development. They hold immense potential as biological nanocarriers, emulsifiers, and material‐reinforcing additives. However, future research must focus on optimizing their fabrication methods, addressing safety concerns, and ensuring sustainable applications in the medical and food industries. This review underscores the necessity for interdisciplinary collaboration to bridge the current knowledge gaps and advance PS‐NPs toward clinical and commercial success.
AUTHOR CONTRIBUTIONS
Gafar Babatunde Bamigbade: Writing—original draft; writing—review and editing. Mohamed Abdin: Writing—original draft; investigation; writing—review and editing; visualization. Athira Subhash: Writing—original draft; writing—review and editing. Maduni Paththuwe Arachchi: Writing—original draft; writing—review and editing. Naeem Ullah: Writing—original draft; writing—review and editing. Ren‐You Gan: Writing—review and editing; supervision. Abdelmoneim Ali: Writing—review and editing. Afaf Kamal‐Eldin: Writing—review and editing. Mutamed Ayyash: Conceptualization; writing—original draft; funding acquisition; writing—review and editing; project administration; resources; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the United Arab Emirates University for the financial support of this review paper.
Bamigbade, G. B. , Abdin, M. , Subhash, A. , Arachchi, M. P. , Ullah, N. , Gan, R.‐Y. , Ali, A. , Kamal‐Eldin, A. , & Ayyash, M. (2025). Plant polysaccharide‐capped nanoparticles: A sustainable approach to modulate gut microbiota and advance functional food applications. Comprehensive Reviews in Food Science and Food Safety, 24, e70156. 10.1111/1541-4337.70156
REFERENCES
- Abdin, M. , Salama, M. A. , Riaz, A. , Saleem Akhtar, H. M. , & Elsanat, S. Y. (2021). Enhanced the entrapment and controlled release of Syzygium cumini seeds polyphenols by modifying the surface and internal organization of Alginate‐based microcapsules. Journal of Food Processing and Preservation, 45(1), e15100. 10.1111/jfpp.15100 [DOI] [Google Scholar]
- Abedini, F. , Ebrahimi, M. , Hemmati Roozbehani, A. , Domb, A. J. , & Hosseinkhani, H. (2018). Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polymers for Advanced Technologies, 29(10), 2564–2573. 10.1002/pat.4375 [DOI] [Google Scholar]
- Ahmad, A. , Gulraiz, Y. , Ilyas, S. , & Bashir, S. (2022). Polysaccharide based nano materials: Health implications. Food Hydrocolloids for Health, 2, 100075. 10.1016/j.fhfh.2022.100075 [DOI] [Google Scholar]
- Akhtar, M. , Chen, Y. , Ma, Z. , Zhang, X. , Shi, D. , Khan, J. A. , & Liu, H. (2022). Gut microbiota‐derived short chain fatty acids are potential mediators in gut inflammation. Animal Nutrition, 8, 350–360. 10.1016/j.aninu.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, A. , Mitchell, A. L. , Boland, M. , Forster, S. C. , Gloor, G. B. , Tarkowska, A. , Lawley, T. D. , & Finn, R. D. (2019). A new genomic blueprint of the human gut microbiota. Nature, 568(7753), 499–504. 10.1038/s41586-019-0965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly, A. A. , & El‐Bisi, M. K. (2018). Grafting of polysaccharides: Recent advances. Biopolymer grafting (pp. 469–519). Elsevier. 10.1016/B978-0-323-48104-5.00011-1 [DOI] [Google Scholar]
- Andrade, D. R. M. , Mendonça, M. H. , Helm, C. V. , Magalhães, W. L. E. , de Muniz, G. I. B. , & Kestur, S. G. (2015). Assessment of nano cellulose from peach palm residue as potential food additive: Part II: Preliminary studies. Journal of Food Science and Technology, 52(9), 5641–5650. 10.1007/s13197-014-1684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbain, R. , Othman, M. , & Palaniandy, S. (2011). Preparation of iron oxide nanoparticles by mechanical milling. Minerals Engineering, 24(1), 1–9. 10.1016/j.mineng.2010.08.025 [DOI] [Google Scholar]
- Arboleya, S. , Watkins, C. , Stanton, C. , & Ross, R. P. (2016). Gut bifidobacteria populations in human health and aging. Frontiers in Microbiology, 7, 1204. 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfat, Y. A. , Ahmed, J. , Hiremath, N. , Auras, R. , & Joseph, A. (2017). Thermo‐mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver‐copper nanoparticles. Food Hydrocolloids, 62, 191–202. 10.1016/j.foodhyd.2016.08.009 [DOI] [Google Scholar]
- Arulmozhi, K. T. , & Mythili, N. (2013). Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. AIP Advances, 3(12), 122122. 10.1063/1.4858419 [DOI] [Google Scholar]
- Asharani, P. V. , Sethu, S. , Vadukumpully, S. , Zhong, S. , Teck Lim, C. , Prakash Hande, M. , & Valiyaveettil, S. (2010). Investigations on the structural damage in human erythrocytes exposed to silver, gold, and platinum nanoparticles. Advanced Functional Materials, 20(8), 1233–1242. 10.1002/adfm.200901846 [DOI] [Google Scholar]
- Ashraf, H. , Cossu, D. , Ruberto, S. , Noli, M. , Jasemi, S. , Simula, E. R. , & Sechi, L. A. (2023). Latent potential of multifunctional selenium nanoparticles in neurological diseases and altered gut microbiota. Materials, 16(2), 699. 10.3390/ma16020699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azehaf, H. , Benzine, Y. , Tagzirt, M. , Skiba, M. , & Karrout, Y. (2023). Microbiota‐sensitive drug delivery systems based on natural polysaccharides for colon targeting. Drug Discovery Today, 28(7), 103606. 10.1016/j.drudis.2023.103606 [DOI] [PubMed] [Google Scholar]
- Baek, J. (2021). “Modified Cellulose Nanocrystals (CNC) for Functional Foods.” https://hdl.handle.net/10012/17083
- Bai, L. , Huan, S. , Zhu, Y. , Chu, G. , Julian McClements, D. , & Rojas, O. J. (2021). Recent advances in food emulsions and engineering foodstuffs using plant‐based nanocelluloses. Annual Review of Food Science and Technology, 12, 383–406. 10.1146/annurev-food-061920-123242 [DOI] [PubMed] [Google Scholar]
- Bamigbade, G. B. , Subhash, A. J. , Al‐Ramadi, B. , Kamal‐Eldin, A. , Gan, R. Y. , Liu, S. Q. , & Ayyash, M. (2024). Gut microbiota modulation, prebiotic and bioactive characteristics of date pomace polysaccharides extracted by microwave‐assisted deep eutectic solvent. International Journal of Biological Macromolecules, 262(Pt 2), 130167. 10.1016/j.ijbiomac.2024.130167 [DOI] [PubMed] [Google Scholar]
- Bamigbade, G. B. , Subhash, A. J. , Tarique, M. , al‐Ramadi, B. , Abu‐Jdayil, B. , Kamal‐Eldin, A. , Nyström, L. , & Ayyash, M. (2024). Date pomace polysaccharide: Ultrasonic‐assisted deep eutectic solvent extraction, physicochemical properties, biological activities, gut microbiota modulation, and rheological properties. Chemical and Biological Technologies in Agriculture, 11(1), 79. 10.1186/s40538-024-00601-0 [DOI] [Google Scholar]
- Barclay, T. G. , Day, C. M. , Petrovsky, N. , & Garg, S. (2019). Review of polysaccharide particle‐based functional drug delivery. Carbohydrate Polymers, 221, 94–112. 10.1016/j.carbpol.2019.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoum, A. , García‐Betancourt, M. L. , Jeevanandam, J. , Hussien, E. A. , Mekkawy, S. A. , Mostafa, M. , Omran, M. M. , Abdalla, M. S. , & Bechelany, M. (2022). Review on natural, incidental, bioinspired, and engineered nanomaterials: history, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials, 12(2), 177. 10.3390/nano12020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr, M. , & Ganesan, K. (2022). Improving polysaccharide‐based chitin/chitosan‐aerogel materials by learning from genetics and molecular biology. Materials, 15(3), 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathi, D. , Ranjithkumar, R. , Vasantharaj, S. , Chandarshekar, B. , & Bhuvaneshwari, V. (2019). Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. International Journal of Biological Macromolecules, 132, 880–887. 10.1016/j.ijbiomac.2019.03.233 [DOI] [PubMed] [Google Scholar]
- Bhardwaj, V. , Srinivasan, S. , & McGoron, A. J. (2015). Efficient intracellular delivery and improved biocompatibility of colloidal silver nanoparticles towards intracellular SERS immuno‐sensing. Analyst, 140(12), 3929–3934. 10.1039/C5AN00435G [DOI] [PubMed] [Google Scholar]
- Bhattacharya, S. , & Srivastava, A. (2003). Synthesis and characterization of novel cationic lipid and cholesterol‐coated gold nanoparticles and their interactions with dipalmitoylphosphatidylcholine membranes. Langmuir, 19(10), 4439–4447. 10.1021/la0269513 [DOI] [Google Scholar]
- Bhopatkar, D. , Feng, T. , Chen, F. , Zhang, G. , Carignano, M. , Park, S. H. , Zhuang, H. , Campanella, O. H. , & Hamaker, B. R. (2015). Self‐assembled nanoparticle of common food constituents that carries a sparingly soluble small molecule. Journal of Agricultural and Food Chemistry, 63(17), 4312–4319. 10.1021/acs.jafc.5b00037 [DOI] [PubMed] [Google Scholar]
- Biscari, G. , Malkoch, M. , Fiorica, C. , Fan, Y. , Palumbo, F. S. , Indelicato, S. , Bongiorno, D. , & Pitarresi, G. (2024). Gellan gum‐dopamine mediated in situ synthesis of silver nanoparticles and development of nano/micro‐composite injectable hydrogel with antimicrobial activity. International Journal of Biological Macromolecules, 258(Pt 1), 128766. 10.1016/j.ijbiomac.2023.128766 [DOI] [PubMed] [Google Scholar]
- Bokov, D. , Jalil, A. T. , Chupradit, S. , Suksatan, W. , Ansari, M. J. , Shewael, I. H. , Valiev, G. H. , & Kianfar, E. (2021). Nanomaterial by sol‐gel method: Synthesis and application. Advances in Materials Science and Engineering, 2021(1), 5102014. 10.1155/2021/5102014 [DOI] [Google Scholar]
- Bouwmeester, H. , van der Zande, M. , & Jepson, M. A. (2018). Effects of food‐borne nanomaterials on gastrointestinal tissues and microbiota. WIREs Nanomedicine and Nanobiotechnology, 10(1), e1481. 10.1002/wnan.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouziane, G. , Henni, A. , ’hamed Bouricha, M. , Boual, Z. , Belkhalfa, H. , Bachari, K. , & El Hadj, M. D. O. (2024). A new galactomannan extracted from the seeds of Astragalus gombiformis Pomel (Fabaceae) and its utilization in the biosynthesis of silver nanoparticles. Process Biochemistry, 136, 48–59. 10.1016/j.procbio.2023.11.009 [DOI] [Google Scholar]
- Braydich‐Stolle, L. K. , Breitner, E. K. , Comfort, K. K. , Schlager, J. J. , & Hussain, S. M. (2014). Dynamic characteristics of silver nanoparticles in physiological fluids: Toxicological implications. Langmuir, 30(50), 15309–15316. 10.1021/la5036079 [DOI] [PubMed] [Google Scholar]
- Bushra, R. , Ahmad, M. , Seidi, F. , Song, Q. J. , Jin, Y. , & Xiao, H. (2023). Polysaccharide‐based nanoassemblies: From synthesis methodologies and industrial applications to future prospects. Advances in Colloid and Interface Science, 318, 102953. 10.1016/j.cis.2023.102953 [DOI] [PubMed] [Google Scholar]
- Buzea, C. , Pacheco, I. I. , & Robbie, K. (2007). Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases, 2(4), Mr17–Mr71. 10.1116/1.2815690 [DOI] [PubMed] [Google Scholar]
- Calderón‐Jiménez, B. , Johnson, M. E. , Bustos, A. R. M. , Murphy, K. E. , Winchester, M. R. , & Baudrit, J. R. V. (2017). Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Frontiers in Chemistry, 5, 6. 10.3389/fchem.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi, S. , Schiavoni, M. , Chan‐Thaw, C. , & Villa, A. (2016). Untangling the role of the capping agent in nanocatalysis: Recent advances and perspectives. Catalysts, 6(12), 185. 10.3390/catal6120185 [DOI] [Google Scholar]
- Cao, B. , Zhang, Q. , Guo, J. , Guo, R. , Fan, X. , & Bi, Y. (2021). Synthesis and evaluation of Grateloupia Livida polysaccharides‐functionalized selenium nanoparticles. International Journal of Biological Macromolecules, 191, 832–839. 10.1016/j.ijbiomac.2021.09.087 [DOI] [PubMed] [Google Scholar]
- Cao, M. , Cao, A. , Xing, J. , Zhang, J. , Zhu, W. , Wang, Q. , & Cai, L. (2023). Pickering emulsion stabilized by parasin I and chitosan nanoparticles enhances protection against intestinal microbiota homeostasis by reducing inflammation in peritonitis mice. International Journal of Biological Macromolecules, 242, 125016. 10.1016/j.ijbiomac.2023.125016 [DOI] [PubMed] [Google Scholar]
- Chandrarathna, H. P. S. U. , Liyanage, T. D. , Edirisinghe, S. L. , Dananjaya, S. H. S. , Thulshan, E. H. T. , Nikapitiya, C. , Oh, C. , Kang, D.‐H. , & De Zoysa, M. (2020). Marine microalgae, spirulina maxima‐derived modified pectin and modified pectin nanoparticles modulate the gut microbiota and trigger immune responses in mice. Marine Drugs, 18(3), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, S. , Jain, V. P. , & Jaiswar, G. (2022). The composition of polysaccharides: Monosaccharides and binding, group decorating, polysaccharides chains. Innovation in nano‐polysaccharides for eco‐sustainability (pp. 83–118). Elsevier. [Google Scholar]
- Chelakkot, C. , Ghim, J. , & Ryu, S. H. (2018). Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine, 50(8), 1–9. 10.1038/s12276-018-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Zhao, X. , Gao, Y. , Yin, J. , Bai, M. , & Wang, F. (2018). Green synthesis of gold nanoparticles using carrageenan oligosaccharide and their in vitro antitumor activity. Marine Drugs, 16(8), 277. 10.3390/md16080277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Wei, W. , Chen, Y. , Xu, A. , Wang, Y. , & Li, B. (2024). Construction of nanoparticles from blueberry anthocyanins‐lecithin/gum Arabic improves lipid droplet accumulation and gut microbiota disturbance in HFD‐induced obese mice. International Journal of Biological Macromolecules, 264, 130595. 10.1016/j.ijbiomac.2024.130595 [DOI] [PubMed] [Google Scholar]
- Chiesa, E. , Dorati, R. , Pisani, S. , Conti, B. , Bergamini, G. , Modena, T. , & Genta, I. (2018). The microfluidic technique and the manufacturing of polysaccharide nanoparticles. Pharmaceutics, 10(4), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriac, A. P. , Ghilan, A. , Neamtu, I. , Nita, L. E. , Rusu, A. G. , & Chiriac, V. M. (2019). Advancement in the biomedical applications of the (nano) gel structures based on particular polysaccharides. Macromolecular Bioscience, 19(9), 1900187. 10.1002/mabi.201900187 [DOI] [PubMed] [Google Scholar]
- Cinteza, L. O. , Scomoroscenco, C. , Voicu, S. N. , Nistor, C. L. , Nitu, S. G. , Trica, B. , Jecu, M. L. , & Petcu, C. (2018). Chitosan‐stabilized ag nanoparticles with superior biocompatibility and their synergistic antibacterial effect in mixtures with essential oils. Nanomaterials, 8(10), 826. 10.3390/nano8100826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras‐Ramírez, J. I. , Patel, A. R. , Gallegos‐Infante, J. A. , Toro‐Vázquez, J. F. , Pérez‐Martínez, J. D. , Rosas‐Flores, W. , & González‐Laredo, R. F. (2022). Organogel‐based emulsified systems, food applications, microstructural and rheological features—A review. Biointerface Research in Applied Chemistry, 12, 1601–1627. 10.33263/BRIAC122.16011627 [DOI] [Google Scholar]
- Corrie, L. , Gulati, M. , Awasthi, A. , Vishwas, S. , Kaur, J. , Khursheed, R. , Kumar, R. , Kumar, A. , Imran, M. , Chellappan, D. K. , Gupta, G. , Pinto, T. d. J. A. , Morris, A. , Choonara, Y. E. , Adams, J. , Dua, K. , & Singh, S. K. (2022). Polysaccharide, fecal microbiota, and curcumin‐based novel oral colon‐targeted solid self‐nanoemulsifying delivery system: Formulation, characterization, and in‐vitro anticancer evaluation. Materials Today Chemistry, 26, 101165. 10.1016/j.mtchem.2022.101165 [DOI] [Google Scholar]
- Cui, M. , Zhang, M. , & Liu, K. (2021). Colon‐targeted drug delivery of polysaccharide‐based nanocarriers for synergistic treatment of inflammatory bowel disease: A review. Carbohydrate Polymers, 272, 118530. 10.1016/j.carbpol.2021.118530 [DOI] [PubMed] [Google Scholar]
- Curcio, M. , Brindisi, M. , Cirillo, G. , Frattaruolo, L. , Leggio, A. , Rago, V. , Nicoletta, F. P. , Cappello, A. R. , & Iemma, F. (2022). Smart lipid‐polysaccharide nanoparticles for targeted delivery of doxorubicin to breast cancer cells. International Journal of Molecular Sciences, 23(4), 2386. 10.3390/ijms23042386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya, D. K. , Renuka, & Puniya, A. K. (2018). Impact of nanosilver on gut microbiota: A vulnerable link. Future Microbiology, 13(4), 483–492. 10.2217/fmb-2017-0103 [DOI] [PubMed] [Google Scholar]
- Danchin, A. , & Braham, S. (2017). Coenzyme B12 synthesis as a baseline to study metabolite contribution of animal microbiota. Microbial Biotechnology, 10(4), 688–701. 10.1111/1751-7915.12722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, L. P. , Manchineella, S. , & Govindaraju, T. (2020). Biomolecules‐derived biomaterials. Biomaterials, 230, 119633. 10.1016/j.biomaterials.2019.119633 [DOI] [PubMed] [Google Scholar]
- De Anda‐Flores, Y. , Carvajal‐Millan, E. , Campa‐Mada, A. , Lizardi‐Mendoza, J. , Rascon‐Chu, A. , Tanori‐Cordova, J. , & Martínez‐López, A. L. (2021). Polysaccharide‐based nanoparticles for colon‐targeted drug delivery systems. Polysaccharides, 2(3), 626–647. 10.3390/polysaccharides2030038 [DOI] [Google Scholar]
- Desai, M. P. , Labhasetwar, V. , Walter, E. , Levy, R. J. , & Amidon, G. L. (1997). The mechanism of uptake of biodegradable microparticles in Caco‐2 cells is size dependent. Pharmaceutical Research, 14(11), 1568–1573. 10.1023/A:1012126301290 [DOI] [PubMed] [Google Scholar]
- Devatha, C. P. , & Thalla, A. K. (2018). Green synthesis of nanomaterials. In Bhagyaraj S. M., Oluwafemi O. S., Kalarikkal N., & Thomas S. (Eds.), Synthesis of inorganic nanomaterials (pp. 169–184). Woodhead Publishing. [Google Scholar]
- Dhar, J. , & Patil, S. (2012). Self‐assembly and catalytic activity of metal nanoparticles immobilized in polymer membrane prepared via layer‐by‐layer approach. ACS Applied Materials & Interfaces, 4(3), 1803–1812. 10.1021/am300068e [DOI] [PubMed] [Google Scholar]
- Ding, W. , & Wu, Y. (2020). Sustainable dialdehyde polysaccharides as versatile building blocks for fabricating functional materials: An overview. Carbohydrate Polymers, 248, 116801. 10.1016/j.carbpol.2020.116801 [DOI] [PubMed] [Google Scholar]
- dos Santos, A. M. , Meneguin, A. B. , Akhter, D. T. , Fletcher, N. , Houston, Z. H. , Bell, C. , Thurecht, K. J. , & Gremião, M. P. D. (2021). Understanding the role of colon‐specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. European Journal of Pharmaceutics and Biopharmaceutics, 158, 371–378. 10.1016/j.ejpb.2020.12.004 [DOI] [PubMed] [Google Scholar]
- dos Santos Corrêa, A. , Contreras, L. A. , Keijok, W. J. , Barcelos, D. H. F. , Pereira, A. C. H. , Kitagawa, R. R. , Scherer, R. , de Oliveira Gomes, D. C. , da Silva, A. R. , Endringer, D. C. , de Oliveira, J. P. , & Guimarães, M. C. C. (2018). Virola oleifera‐capped gold nanoparticles showing radical‐scavenging activity and low cytotoxicity. Materials Science and Engineering: C, 91, 853–858. 10.1016/j.msec.2018.06.027 [DOI] [PubMed] [Google Scholar]
- Dubey, R. S. , Rajesh, Y. B. R. D. , & More, M. A. (2015). Synthesis and characterization of SiO2 nanoparticles via sol‐gel method for industrial applications. Materials Today: Proceedings, 2(4), 3575–3579. 10.1016/j.matpr.2015.07.098 [DOI] [Google Scholar]
- El Aidy, S. , Hooiveld, G. , Tremaroli, V. , Bäckhed, F. , & Kleerebezem, M. (2013). The gut microbiota and mucosal homeostasis. Gut Microbes, 4(2), 118–124. 10.4161/gmic.23362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsupikhe, R. F. , Shameli, K. , Ahmad, M. B. , Ibrahim, N. A. , & Zainudin, N. (2015). Green sonochemical synthesis of silver nanoparticles at varying concentrations of kappa‐carrageenan. Nanoscale Research Letters, 10(1), 916. 10.1186/s11671-015-0916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. , He, Y.‐X. , Wang, H.‐Q. , Zhang, Y.‐L. , Zhong, Y.‐d. , Hu, X.‐T. , Nie, S.‐P. , Xie, M.‐Y. , & Hu, J.‐L. (2025). Impact of eight extruded starchy whole grains on glycemic regulation and fecal microbiota modulation. Food Hydrocolloids, 160, 110756. 10.1016/j.foodhyd.2024.110756 [DOI] [Google Scholar]
- Fedotova, A. V. , Snezhko, A. G. , Sdobnikova, O. A. , Samoilova, L. G. , Smurova, T. A. , Revina, A. A. , & Khailova, E. B. (2010). Packaging materials manufactured from natural polymers modified with silver nanoparticles. International Polymer Science and Technology, 37(10), 59–64. 10.1177/0307174x1003701010 [DOI] [Google Scholar]
- Feng, Z. , Peng, S. , Wu, Z. , Jiao, L. , Xu, S. , Wu, Y. , Liu, Z. , Hu, Y. , Liu, J. , Wu, Y. , & Wang, D. (2021). Ramulus mori polysaccharide‐loaded PLGA nanoparticles and their anti‐inflammatory effects in vivo . International Journal of Biological Macromolecules, 182, 2024–2036. 10.1016/j.ijbiomac.2021.05.200 [DOI] [PubMed] [Google Scholar]
- Fernández, A. , Picouet, P. , & Lloret, E. (2010). Cellulose‐silver nanoparticle hybrid materials to control spoilage‐related microflora in absorbent pads located in trays of fresh‐cut melon. International Journal of Food Microbiology, 142(1), 222–228. 10.1016/j.ijfoodmicro.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Franconetti, A. , Carnerero, J. M. , Prado‐Gotor, R. , Cabrera‐Escribano, F. , & Jaime, C. (2019). Chitosan as a capping agent: Insights on the stabilization of gold nanoparticles. Carbohydrate Polymers, 207, 806–814. 10.1016/j.carbpol.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Frigaard, J. , Liaaen Jensen, J. , Galtung, H. K. , & Hiorth, M. (2022). The potential of chitosan in nanomedicine: An overview of the cytotoxicity of chitosan based nanoparticles. Frontiers in Pharmacology, 13, 880377. 10.3389/fphar.2022.880377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, S. , Toh, H. , Taylor, T. D. , Ohno, H. , & Hattori, M. (2012). Acetate‐producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes, 3(5), 449–454. 10.4161/gmic.21214 [DOI] [PubMed] [Google Scholar]
- Ganesan, K. , Sukalingam, K. , & Xu, B. (2018). Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases—A critical review. Trends in Food Science & Technology, 71, 132–154. 10.1016/j.tifs.2017.11.003 [DOI] [Google Scholar]
- Gangadoo, S. , Dinev, I. , Chapman, J. , Hughes, R. J. , Van, T. T. H. , Moore, R. J. , & Stanley, D. (2018). Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii . Applied Microbiology and Biotechnology, 102(3), 1455–1466. 10.1007/s00253-017-8688-4 [DOI] [PubMed] [Google Scholar]
- Garmasheva, I. , Kovalenko, N. , Voychuk, S. , Ostapchuk, A. , Livins'ka, O. , & Oleschenko, L. (2016). Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro . Bioimpacts, 6(4), 219–223. 10.15171/bi.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatoo, M. A. , Naseem, S. , Arfat, M. Y. , Dar, A. M. , Qasim, K. , & Zubair, S. (2014). Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Research International, 2014(1), 498420. 10.1155/2014/498420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard, C. , & Vidal, H. (2019). Impact of gut microbiota on host glycemic control. Frontiers in Endocrinology, 10, 29. 10.3389/fendo.2019.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarian, R. , Pérez‐Herrero, E. , Oh, H. , Raghavan, S. R. , & Muro, S. (2016). Chitosan–alginate microcapsules provide gastric protection and intestinal release of ICAM‐1‐targeting nanocarriers, enabling gi targeting in vivo . Advanced Functional Materials, 26(20), 3382–3393. 10.1002/adfm.201600084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini, C. , Ladisa, M. , Altamura, D. , Siliqi, D. , Sibillano, T. , & De Caro, L. (2016). X‐ray diffraction: A powerful technique for the multiple‐length‐scale structural analysis of nanomaterials. Crystals, 6(8), 87. 10.3390/cryst6080087 [DOI] [Google Scholar]
- Goodarzi, N. , Varshochian, R. , Kamalinia, G. , Atyabi, F. , & Dinarvand, R. (2013). A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydrate Polymers, 92(2), 1280–1293. 10.1016/j.carbpol.2012.10.036 [DOI] [PubMed] [Google Scholar]
- Gordon, H. A. , Wostmann, B. S. , & Bruckner‐Kardoss, E. (1963). Effects of microbial flora on cardiac output and other elements of blood circulation. Proceedings of the Society for Experimental Biology and Medicine, 114(2), 301–304. 10.3181/00379727-114-28658 [DOI] [PubMed] [Google Scholar]
- Grewal, A. K. , & Salar, R. K. (2024). Chitosan nanoparticle delivery systems: An effective approach to enhancing efficacy and safety of anticancer drugs. Nano TransMed, 3, 100040. 10.1016/j.ntm.2024.100040 [DOI] [Google Scholar]
- Guan, Z. , Jia, J. , Zhang, C. , Sun, T. , Zhang, W. , Yuan, W. , Leng, H. , & Song, C. (2020). Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clinical Science, 134(23), 3159–3174. 10.1042/cs20201224 [DOI] [PubMed] [Google Scholar]
- Han, J. , Chen, F. , Gao, C. , Zhang, Y. , & Tang, X. (2020). Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. International Journal of Biological Macromolecules, 157, 202–211. 10.1016/j.ijbiomac.2020.04.177 [DOI] [PubMed] [Google Scholar]
- Harish, B. S. , Uppuluri, K. B. , & Anbazhagan, V. (2015). Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohydrate Polymers, 132, 104–110. 10.1016/j.carbpol.2015.06.069 [DOI] [PubMed] [Google Scholar]
- Hashemi Tabatabaei, R. , Jafari, S. M. , Mirzaei, H. , Nafchi, A. M. , & Dehnad, D. (2018). Preparation and characterization of nano‐SiO2 reinforced gelatin‐k‐carrageenan biocomposites. International Journal of Biological Macromolecules, 111, 1091–1099. 10.1016/j.ijbiomac.2018.01.116 [DOI] [PubMed] [Google Scholar]
- Hasnidawani, J. N. , Azlina, H. N. , Norita, H. , Bonnia, N. N. , Ratim, S. , & Ali, E. S. (2016). Synthesis of ZnO nanostructures using sol‐gel method. Procedia Chemistry, 19, 211–216. 10.1016/j.proche.2016.03.095 [DOI] [Google Scholar]
- Hochella, M. F. , Mogk, D. W. , Ranville, J. , Allen, I. C. , Luther, G. W. , Marr, L. C. , Peter McGrail, B. , Murayama, M. , Qafoku, N. P. , Rosso, K. M. , Sahai, N. , Schroeder, P. A. , Vikesland, P. , Westerhoff, P. , & Yang, Y. (2019). Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science, 363(6434), eaau8299. 10.1126/science.aau8299 [DOI] [PubMed] [Google Scholar]
- Ho Do, M. , Seo, Y. S. , & Park, H.‐Y. (2021). Polysaccharides: Bowel health and gut microbiota. Critical Reviews in Food Science and Nutrition, 61(7), 1212–1224. 10.1080/10408398.2020.1755949 [DOI] [PubMed] [Google Scholar]
- Homayun, B. , Lin, X. , & Choi, H.‐J. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S.‐J. , Das, P. R. , & Eun, J.‐B. (2021). Effects of superfine grinding using ball‐milling on the physical properties, chemical composition, and antioxidant properties of Quercus salicina (Blume) leaf powders. Journal of the Science of Food and Agriculture, 101(8), 3123–3131. 10.1002/jsfa.10941 [DOI] [PubMed] [Google Scholar]
- Hosseinpour‐Mashkani, S. M. , & Ramezani, M. (2014). Silver and silver oxide nanoparticles: Synthesis and characterization by thermal decomposition. Materials Letters, 130, 259–262. 10.1016/j.matlet.2014.05.133 [DOI] [Google Scholar]
- Hu, Q. , & Luo, Y. (2018). Recent advances of polysaccharide‐based nanoparticles for oral insulin delivery. International Journal of Biological Macromolecules, 120, 775–782. 10.1016/j.ijbiomac.2018.08.152 [DOI] [PubMed] [Google Scholar]
- Hu, S. , & Hsieh, Y. L. (2016). Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. International Journal of Biological Macromolecules, 82, 856–862. 10.1016/j.ijbiomac.2015.09.066 [DOI] [PubMed] [Google Scholar]
- Hua, Y. , Wei, Z. , & Xue, C. (2023). Chitosan and its composites‐based delivery systems: Advances and applications in food science and nutrition sector. Critical Reviews in Food Science and Nutrition, 63(20), 4579–4598. 10.1080/10408398.2021.2004992 [DOI] [PubMed] [Google Scholar]
- Huang, Q. , Zhang, Y. , Chu, Q. , & Song, H. (2024). The influence of polysaccharides on lipid metabolism: Insights from gut microbiota. Molecular Nutrition & Food Research, 68(1), 2300522. 10.1002/mnfr.202300522 [DOI] [PubMed] [Google Scholar]
- Huang, W. , Zhang, Y. , Li, Z. , Li, M. , Li, F. , Mortimer, M. , & Guo, L.‐H. (2022). Silver and hyaluronic acid‐coated gold nanoparticles modulate the metabolism of a model human gut bacterium Lactobacillus casei . Nanomaterials, 12(19), 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, J. , Wu, Z. , Sun, W. , Wang, Z. , Wu, J. , Huang, M. , Wang, B. , & Sun, B. (2022). Protective effects of natural polysaccharides on intestinal barrier injury: A review. Journal of Agricultural and Food Chemistry, 70(3), 711–735. 10.1021/acs.jafc.1c05966 [DOI] [PubMed] [Google Scholar]
- Hussain, S. A. , Hameed, A. , Nazir, Y. , Naz, T. , Wu, Y. , Suleria, H. A. R. , & Song, Y. (2018). Microencapsulation and the characterization of polyherbal formulation (PHF) rich in natural polyphenolic compounds. Nutrients, 10(7), 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, P. A. , Lin, X. Z. , Kuo, K. L. , & Hsu, F. Y. (2017). Fabrication and cytotoxicity of fucoidan‐cisplatin nanoparticles for macrophage and tumor cells. Materials, 10(3), 291. 10.3390/ma10030291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz, I. , Gilani, E. , Nazir, A. , & Bukhari, A. (2020). Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chemistry Letters and Reviews, 13(3), 223–245. 10.1080/17518253.2020.1802517 [DOI] [Google Scholar]
- Iram, F. , Iqbal, M. S. , Athar, M. M. , Saeed, M. Z. , Yasmeen, A. , & Ahmad, R. (2014). Glucoxylan‐mediated green synthesis of gold and silver nanoparticles and their phyto‐toxicity study. Carbohydrate Polymers, 104, 29–33. 10.1016/j.carbpol.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Iswarya, A. , Vaseeharan, B. , Anjugam, M. , Ashokkumar, B. , Govindarajan, M. , Alharbi, N. S. , Kadaikunnan, S. , Khaled, J. M. , & Benelli, G. (2017). Multipurpose efficacy of ZnO nanoparticles coated by the crustacean immune molecule β‐1, 3‐glucan binding protein: Toxicity on HepG2 liver cancer cells and bacterial pathogens. Colloids and Surfaces B: Biointerfaces, 158, 257–269. 10.1016/j.colsurfb.2017.06.035 [DOI] [PubMed] [Google Scholar]
- Jafari, S. M. , & Esfanjani, A. F. (2017). Instrumental analysis and characterization of nanocapsules. Nanoencapsulation technologies for the food and nutraceutical industries (pp. 524–544). Elsevier. [Google Scholar]
- Javed, R. , Zia, M. , Naz, S. , Aisida, S. O. , Ain, N. U. , & Ao, Q. (2020). Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. Journal of Nanobiotechnology, 18(1), 172. 10.1186/s12951-020-00704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar, R. , Menon, D. , Manzoor, K. , Nair, S. V. , & Tamura, H. (2010). Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydrate Polymers, 82(2), 227–232. 10.1016/j.carbpol.2010.04.074 [DOI] [Google Scholar]
- Jayasree Subhash, A. , Babatunde Bamigbade, G. , al‐Ramadi, B. , Kamal‐Eldin, A. , Gan, R.‐Y. , Ranadheera, C. S. , & Ayyash, M. (2024). Characterizing date seed polysaccharides: A comprehensive study on extraction, biological activities, prebiotic potential, gut microbiota modulation, and rheology using microwave‐assisted deep eutectic solvent. Food Chemistry, 444, 138618. 10.1016/j.foodchem.2024.138618 [DOI] [PubMed] [Google Scholar]
- Jha, A. K. , Prasad, K. , & Kulkarni, A. R. (2009). Synthesis of TiO2 nanoparticles using microorganisms. Colloids and Surfaces B: Biointerfaces, 71(2), 226–229. 10.1016/j.colsurfb.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Jin, B. , Zhou, X. , Li, X. , Lin, W. , Chen, G. , & Qiu, R. (2016). Self‐assembled modified soy protein/dextran nanogel induced by ultrasonication as a delivery vehicle for riboflavin. Molecules, 21(3), 282. 10.3390/molecules21030282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JyothiKumar, A. , Kailasnath, M. , Simon, J. , & Mathew, S. (2019). BSA stabilized gold nanoparticles: Synthesis and characterization. Materials Today: Proceedings, 9, 111–115. 10.1016/j.matpr.2019.02.044 [DOI] [Google Scholar]
- Kandpal, N. D. , Sah, N. , Loshali, R. , Joshi, R. , & Prasad, J. (2014). Co‐precipitation method of synthesis and characterization of iron oxide nanoparticles. Journal of Scientific and Industrial Research, 73, 87–90. https://nopr.niscpr.res.in/bitstream/123456789/26444/1/JSIR%2073(2)%2087‐90.pdf [Google Scholar]
- Karimzadeh, M. , Eslampanah‐Seyyedi, E. , & Behniafar, H. (2018). Poly(tetramethylene oxide)‐coated silica nanoparticles incorporated into poly(4,4′‐oxydiphenylene‐pyromellitimide) matrix. Materials and Manufacturing Processes, 33(10), 1093–1099. 10.1080/10426914.2017.1364856 [DOI] [Google Scholar]
- Karuppaiah, A. , Selvaraj, D. , Sellappan, M. , Nagarajan, A. , Babu, D. , Rahman, H. , Madheswaran, T. , Bose, B. , & Natrajan, T. (2023). A perspective on therapeutic applications and strategies to mitigate toxicity of metallic nanoparticles. Current Pharmaceutical Design, 29(4), 239–245. 10.2174/1381612829666230109111635 [DOI] [PubMed] [Google Scholar]
- Kato, Y. , & Suzuki, M. (2020). Synthesis of metal nanoparticles by microorganisms. Crystals, 10(7), 589. 10.3390/cryst10070589 [DOI] [Google Scholar]
- Khalaf, H. H. , Sharoba, A. M. , El‐Tanahi, H. H. , & Morsy, M. K. (2013). Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. Journal of Food and Dairy Sciences, 4(11), 557–573. 10.21608/jfds.2013.72104 [DOI] [Google Scholar]
- Khorasani, A. C. , & Shojaosadati, S. A. (2016). Bacterial nanocellulose‐pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. International Journal of Biological Macromolecules, 83, 9–18. 10.1016/j.ijbiomac.2015.11.041 [DOI] [PubMed] [Google Scholar]
- Khorasani, A. C. , & Shojaosadati, & S. A. (2017). Pectin‐non‐starch nanofibers biocomposites as novel gastrointestinal‐resistant prebiotics. International Journal of Biological Macromolecules, 94, 131–144. 10.1016/j.ijbiomac.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Kim, I. , Viswanathan, K. , Kasi, G. , Thanakkasaranee, S. , Sadeghi, K. , & Seo, J. (2022). ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Reviews International, 38(4), 537–565. 10.1080/87559129.2020.1737709 [DOI] [Google Scholar]
- Kim, M. , Osone, S. , Kim, T. , Higashi, H. , & Seto, T. (2017). Synthesis of nanoparticles by laser ablation: A review. KONA Powder and Particle Journal, 34, 80–90. 10.14356/kona.2017009 [DOI] [Google Scholar]
- Kim, W.‐S. , Han, G. G. , Hong, L. , Kang, S.‐K. , Shokouhimehr, M. , Choi, Y.‐J. , & Cho, C.‐S. (2019). Novel production of natural bacteriocin via internalization of dextran nanoparticles into probiotics. Biomaterials, 218, 119360. 10.1016/j.biomaterials.2019.119360 [DOI] [PubMed] [Google Scholar]
- Kong, H. , Yang, J. , Zhang, Y. , Fang, Y. , Nishinari, K. , & Phillips, G. O. (2014). Synthesis and antioxidant properties of gum arabic‐stabilized selenium nanoparticles. International Journal of Biological Macromolecules, 65, 155–162. 10.1016/j.ijbiomac.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Ladaycia, A. , Passirani, C. , & Lepeltier, E. (2021). Microbiota and nanoparticles: Description and interactions. European Journal of Pharmaceutics and Biopharmaceutics, 169, 220–240. 10.1016/j.ejpb.2021.10.015 [DOI] [PubMed] [Google Scholar]
- Lakade, A. J. , Sundar, K. , & Shetty, P. H. (2017). Nanomaterial‐based sensor for the detection of milk spoilage. LWT, 75, 702–709. 10.1016/j.lwt.2016.10.031 [DOI] [Google Scholar]
- Lamas, E. S. , & Osti, A. V. G. (2020). Patent foramen ovale closure for splenic infarction: An unusual presentation and an unusual indication. Case Reports in Cardiology, 2020(1), 9802908. 10.1155/2020/9802908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurienzo, P. (2010). Marine polysaccharides in pharmaceutical applications: An overview. Marine Drugs, 8(9), 2435–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, H. D. , Loveday, S. M. , Singh, H. , & Sarkar, A. (2020). Gastrointestinal digestion of Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Release of short‐chain fatty acids. Food Chemistry, 320, 126650. 10.1016/j.foodchem.2020.126650 [DOI] [PubMed] [Google Scholar]
- Lesnichaya, M. , Perfileva, A. , Nozhkina, O. , Gazizova, A. , & Graskova, I. (2022). Synthesis, toxicity evaluation and determination of possible mechanisms of antimicrobial effect of arabinogalactane‐capped selenium nanoparticles. Journal of Trace Elements in Medicine and Biology, 69, 126904. 10.1016/j.jtemb.2021.126904 [DOI] [PubMed] [Google Scholar]
- Li, H. , Cheng, Y. , Cui, L. , Yang, Z. , Wang, J. , Zhang, Z. , Chen, K. , Zhao, C. , He, N. , & Li, S. (2024). Combining gut microbiota modulation and enzymatic‐triggered colonic delivery by prebiotic nanoparticles improves mouse colitis therapy. Biomaterials Research, 28, 0062. 10.34133/bmr.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Liu, D. , Li, S. , & Xue, C. (2019). Synthesis and cytotoxicity of selenium nanoparticles stabilized by α‐D‐glucan from Castanea mollissima Blume. International Journal of Biological Macromolecules, 129, 818–826. 10.1016/j.ijbiomac.2019.02.085 [DOI] [PubMed] [Google Scholar]
- Li, H. F. , Pan, Z. C. , Chen, J. M. , Zeng, L. X. , Xie, H. J. , Liang, Z. Q. , Wang, Y. , & Zeng, N. K. (2024b). Green synthesis of silver nanoparticles using Phlebopus portentosus polysaccharide and their antioxidant, antidiabetic, anticancer, and antimicrobial activities. International Journal of Biological Macromolecules, 254(Pt 1), 127579. 10.1016/j.ijbiomac.2023.127579 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Huang, Y. , Du, Y. , Chen, Y. , Wu, Y. , Zhong, K. , Huang, Y. , & Gao, H. (2022). Food‐grade olive oil Pickering emulsions stabilized by starch/β‐cyclodextrin complex nanoparticles: Improved storage stability and regulatory effects on gut microbiota. LWT, 155, 112950. 10.1016/j.lwt.2021.112950 [DOI] [Google Scholar]
- Li, Q. , & Liu, S. (2021). Applications of nanocellulose in the food industry. In Yang C., Wajid Ullah M., & Shi Z. (Eds.), Nanocellulose: Synthesis, structure, properties and applications (pp. 257–285). World Scientific Publishing Co. Ltd. 10.1142/9781786349477_0008 [DOI] [Google Scholar]
- Li, S. , Chen, G. , Qiang, S. , Yin, Z. , Zhang, Z. , & Chen, Y. (2020). Synthesis and evaluation of highly dispersible and efficient photocatalytic TiO2/poly lactic acid nanocomposite films via sol‐gel and casting processes. International Journal of Food Microbiology, 331, 108763. 10.1016/j.ijfoodmicro.2020.108763 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Kröger, M. , & Kam Liu, W. (2015). Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale, 7(40), 16631–16646. 10.1039/C5NR02970H [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zheng, Y. , Zhang, Y. , Yang, Y. , Wang, P. , Imre, B. , Wong, A. C. Y. , Hsieh, Y. S. Y. , & Wang, D. (2021). Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Marine Drugs, 19(11), 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, A. L. , Gratieri, T. , Cunha‐Filho, M. , & Gelfuso, G. M. (2022). Polymeric nanocapsules: A review on design and production methods for pharmaceutical purpose. Methods, 199, 54–66. 10.1016/j.ymeth.2021.07.009 [DOI] [PubMed] [Google Scholar]
- Lin, Y.‐J. , Qin, Z. , Paton, C. M. , Fox, D. M. , & Kong, F. (2021). Influence of cellulose nanocrystals (CNC) on permeation through intestinal monolayer and mucus model in vitro . Carbohydrate Polymers, 263, 117984. 10.1016/j.carbpol.2021.117984 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Chen, S. , Sun, C. , Zuo, W. , Wu, P. , Wang, S. , Dai, J. , Xing, Y. , Hou, Y. , & Ju, Y. (2023). Protonated charge reversal nanodrugs for active targeting clearance of Helicobacter pylori accompanied by gut microbiota protection. Advanced Functional Materials, 33(35), 2300682. 10.1002/adfm.202300682 [DOI] [Google Scholar]
- Liu, G. , Yang, X. , Zhang, J. , Liang, L. , Miao, F. , Ji, T. , Ye, Z. , Chu, M. , Ren, J. , & Xu, X. (2021). Synthesis, stability and anti‐fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. International Journal of Biological Macromolecules, 179, 418–428. 10.1016/j.ijbiomac.2021.03.018 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Huang, W. , Han, W. , Li, C. , Zhang, Z. , Hu, B. , Chen, S. , Cui, P. , Luo, S. , Tang, Z. , Wu, W. , & Luo, Q. (2021). Structure characterization of Oudemansiella radicata polysaccharide and preparation of selenium nanoparticles to enhance the antioxidant activities. LWT, 146, 111469. 10.1016/j.lwt.2021.111469 [DOI] [Google Scholar]
- Liu, Y. , Tan, M.‐L. , Zhu, W.‐J. , Cao, Y.‐N. , Peng, L.‐X. , Yan, Z.‐Y. , & Zhao, G. (2022). In vitro effects of Tartary buckwheat‐derived nanovesicles on gut microbiota. Journal of Agricultural and Food Chemistry, 70(8), 2616–2629. 10.1021/acs.jafc.1c07658 [DOI] [PubMed] [Google Scholar]
- Llorens, A. , Lloret, E. , Picouet, P. , & Fernandez, A. (2012). Study of the antifungal potential of novel cellulose/copper composites as absorbent materials for fruit juices. International Journal of Food Microbiology, 158(2), 113–119. 10.1016/j.ijfoodmicro.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Lu, X. , Chen, J. , Guo, Z. , Zheng, Y. , Rea, M. C. , Su, H. , Zheng, X. , Zheng, B. , & Miao, S. (2019). Using polysaccharides for the enhancement of functionality of foods: A review. Trends in Food Science & Technology, 86, 311–327. 10.1016/j.tifs.2019.02.024 [DOI] [Google Scholar]
- Luo, L. , Wu, Y. , Liu, C. , Huang, L. , Zou, Y. , Shen, Y. , & Lin, Q. (2019). Designing soluble soybean polysaccharides‐based nanoparticles to improve sustained antimicrobial activity of nisin. Carbohydrate Polymers, 225, 115251. 10.1016/j.carbpol.2019.115251 [DOI] [PubMed] [Google Scholar]
- Ma, S. , Yao, H. , Si, X. , Huang, Z. , Wang, R. , Wan, R. , Tang, Z. , Wang, G. , & Song, W. (2024). Orally available dextran‐aspirin nanomedicine modulates gut inflammation and microbiota homeostasis for primary colorectal cancer therapy. Journal of Controlled Release, 370, 528–542. 10.1016/j.jconrel.2024.05.002 [DOI] [PubMed] [Google Scholar]
- Mackevica, A. , Emil Olsson, M. , & Hansen, S. F. (2017). The release of silver nanoparticles from commercial toothbrushes. Journal of Hazardous Materials, 322, 270–275. 10.1016/j.jhazmat.2016.03.067 [DOI] [PubMed] [Google Scholar]
- Malhaire, H. , Gimel, J. C. , Roger, E. , BenoiT, J. P. , & Lagarce, F. (2016). How to design the surface of peptide‐loaded nanoparticles for efficient oral bioavailability? Advanced Drug Delivery Reviews, 106, 320–336. 10.1016/j.addr.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Manivasagan, P. , & Oh, J. (2016). Marine polysaccharide‐based nanomaterials as a novel source of nanobiotechnological applications. International Journal of Biological Macromolecules, 82, 315–327. 10.1016/j.ijbiomac.2015.10.081 [DOI] [PubMed] [Google Scholar]
- Marchetti, L. , Andrés, S. C. , Cerruti, P. , & Califano, A. N. (2020). Effect of bacterial nanocellulose addition on the rheological properties of gluten‐free muffin batters. Food Hydrocolloids, 98, 105315. 10.1016/j.foodhyd.2019.105315 [DOI] [Google Scholar]
- Marchetti, L. , Muzzio, B. , Cerrutti, P. , Andrés, S. C. , & Califano, A. N. (2017). Impact of bacterial nanocellulose on the rheological and textural characteristics of low‐lipid meat emulsions. In Oprea A. E. & Grumezescu A. M. (Eds.), Nanotechnology applications in food (pp. 345–361). Academic Press. [Google Scholar]
- Mekuye, B. , & Abera, B. (2023). Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select, 4(8), 486–501. 10.1002/nano.202300038 [DOI] [Google Scholar]
- Meng, Q. , Zhong, S. , Xu, L. , Wang, J. , Zhang, Z. , Gao, Y. , & Cui, X. (2022). Review on design strategies and considerations of polysaccharide‐based smart drug delivery systems for cancer therapy. Carbohydrate Polymers, 279, 119013. 10.1016/j.carbpol.2021.119013 [DOI] [PubMed] [Google Scholar]
- Meng, Y. , Zhang, Y. , Jia, N. , Qiao, H. , Zhu, M. , Meng, Q. , Lu, Q. , & Zu, Y. (2018). Synthesis and evaluation of a novel water‐soluble high Se‐enriched Astragalus polysaccharide nanoparticles. International Journal of Biological Macromolecules, 118, 1438–1448. 10.1016/j.ijbiomac.2018.06.153 [DOI] [PubMed] [Google Scholar]
- Mensah, M. B. , Awudza, J. A. M. , & O'Brien, P. (2018). Castor oil: A suitable green source of capping agent for nanoparticle syntheses and facile surface functionalization. Royal Society Open Science, 5(8), 180824. 10.1098/rsos.180824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, N. , Khan, A. , Umar, K. , & Muneer, M. (2013). Photocatalytic study of a xanthene dye derivative, phloxine B in aqueous suspension of TiO2: Adsorption isotherm and decolourization kinetics. Energy and Environment Focus, 2, 208–216. 10.1166/eef.2013.1052 [DOI] [Google Scholar]
- Mittal, A. K. , Chisti, Y. , & Banerjee, U. C. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnology Advances, 31(2), 346–356. 10.1016/j.biotechadv.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Mohammadi, H. , Nekobahr, E. , Akhtari, J. , Saeedi, M. , Akbari, J. , & Fathi, F. (2021). Synthesis and characterization of magnetite nanoparticles by co‐precipitation method coated with biocompatible compounds and evaluation of in‐vitro cytotoxicity. Toxicology Reports, 8, 331–336. 10.1016/j.toxrep.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Fayaz, A. , Balaji, K. , Girilal, M. , Kalaichelvan, P. T. , & Venkatesan, R. (2009). Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. Journal of Agricultural and Food Chemistry, 57(14), 6246–6252. 10.1021/jf900337h [DOI] [PubMed] [Google Scholar]
- Montes, C. , Villaseñor, M. J. , & Ríos, Á. (2019). Analytical control of nanodelivery lipid‐based systems for encapsulation of nutraceuticals: Achievements and challenges. Trends in Food Science & Technology, 90, 47–62. 10.1016/j.tifs.2019.06.001 [DOI] [Google Scholar]
- Moonwiriyakit, A. , Pathomthongtaweechai, N. , Steinhagen, P. R. , Chantawichitwong, P. , Satianrapapong, W. , & Pongkorpsakol, P. (2023). Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers, 11(2), 2077620. 10.1080/21688370.2022.2077620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi, W. W. , Lim, H. P. , Ee Low, L. , Tey, B. T. , & Chan, E. S. (2020). Food‐grade Pickering emulsions for encapsulation and delivery of bioactives. Trends in Food Science & Technology, 100, 320–332. 10.1016/j.tifs.2020.04.020 [DOI] [Google Scholar]
- Nagaraja, K. , Hemalatha, D. , Ansar, S. , Rao, K. , & Hwan, O. T. (2023). Novel, biosynthesis of palladium nanoparticles using strychnos potatorum polysaccharide as a green sustainable approach; and their effective catalytic hydrogenation of 4‐nitrophenol. International Journal of Biological Macromolecules, 253(Pt 4), 126983. 10.1016/j.ijbiomac.2023.126983 [DOI] [PubMed] [Google Scholar]
- Natividad, J. M. , Pinto‐Sanchez, M. I. , Galipeau, H. J. , Jury, J. , Jordana, M. , Reinisch, W. , Collins, S. M. , Bercik, P. , Surette, M. G. , & Allen‐Vercoe, E. (2015). Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflammatory Bowel Diseases, 21(8), 1883–1893. 10.1097/mib.0000000000000422 [DOI] [PubMed] [Google Scholar]
- Nidhi, A. D. , Hallan, S. S. , Sharma, S. , & Mishra, N. (2016). Development of enteric‐coated microspheres of embelin for their beneficial pharmacological potential in ulcerative colitis. Artificial Cells, 45, 1092–1100. 10.1080/21691401.2016.1202258 [DOI] [PubMed] [Google Scholar]
- Nikolić, I. , Šoronja‐Simović, D. , Zahorec, J. , Dokić, L. , Lončarević, I. , Stožinić, M. , & Petrović, J. (2024). Polysaccharide‐based fat replacers in the functional food products. Processes, 12(12), 2701. 10.3390/pr12122701 [DOI] [Google Scholar]
- Niu, Z. , & Li, Y. (2014). Removal and utilization of capping agents in nanocatalysis. Chemistry of Materials, 26(1), 72–83. 10.1021/cm4022479 [DOI] [Google Scholar]
- Novickij, V. , Stanevičienė, R. , Staigvila, G. , Gruškienė, R. , Sereikaitė, J. , Girkontaitė, I. , Novickij, J. , & Servienė, E. (2020). Effects of pulsed electric fields and mild thermal treatment on antimicrobial efficacy of nisin‐loaded pectin nanoparticles for food preservation. LWT, 120, 108915. 10.1016/j.lwt.2019.108915 [DOI] [Google Scholar]
- Nyabadza, A. , McCarthy, É. , Makhesana, M. , Heidarinassab, S. , Plouze, A. , Vazquez, M. , & Brabazon, D. (2023). A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Advances in Colloid and Interface Science, 321, 103010. 10.1016/j.cis.2023.103010 [DOI] [PubMed] [Google Scholar]
- Parveen, S. , Sharma, G. , Khajuria, A. K. , & Kandwal, A. (2022). A review synthesis and biological evaluation of plant‐based metallic gold nanoparticles. Journal of Pharmaceutical Research International, 34(7B), 68–88. [Google Scholar]
- Patil, N. , Bhaskar, R. , Vyavhare, V. , Dhadge, R. , Khaire, V. , & Patil, Y. (2021). Overview on methods of synthesis of nanoparticles. International Journal of Current Pharmaceutical Research, 13(2), 11–16. 10.22159/ijcpr.2021v13i2.41556 [DOI] [Google Scholar]
- Patra, J. K. , Das, G. , Fraceto, L. F. , Campos, E. V. R. , Rodriguez‐Torres, M. d. P. , Acosta‐Torres, L. S. , Diaz‐Torres, L. A. , Grillo, R. , Swamy, M. K. , & Sharma, S. (2018). Nano based drug delivery systems: Recent developments and future prospects. Journal of Nanobiotechnology, 16, 1–33. 10.1186/s12951-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso‐Santana, S. , & Fleitas‐Salazar, N. (2023). The use of capping agents in the stabilization and functionalization of metallic nanoparticles for biomedical applications. Particle & Particle Systems Characterization, 40(2), 2200146. 10.1002/ppsc.202200146 [DOI] [Google Scholar]
- Perumal, A. B. , Nambiar, R. B. , Sellamuthu, P. S. , & Sadiku, E. R. (2019). Application of biosynthesized nanoparticles in food, food packaging and dairy industries. Biological synthesis of nanoparticles and their applications (pp. 145–158). CRC Press. [Google Scholar]
- Phan, C. M. , & Nguyen, H. M. (2017). Role of capping agent in wet synthesis of nanoparticles. Journal of Physical Chemistry A, 121(17), 3213–3219. 10.1021/acs.jpca.7b02186 [DOI] [PubMed] [Google Scholar]
- Pilmis, B. , Monnier, A. L. , & Zahar, J.‐R. (2020). Gut microbiota, antibiotic therapy and antimicrobial resistance: A narrative review. Microorganisms, 8(2), 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirsaheb, M. , Gholami, T. , Seifi, H. , Dawi, E. A. , Said, E. A. , Hamoody, A.‐H. M. , Altimari, U. S. , & Salavati‐Niasari, M. (2024). Green synthesis of nanomaterials by using plant extracts as reducing and capping agents. Environmental Science and Pollution Research, 31(17), 24768–24787. 10.1007/s11356-024-32983-x [DOI] [PubMed] [Google Scholar]
- Plucinski, A. , Lyu, Z. , & Schmidt, B. V. K. J. (2021). Polysaccharide nanoparticles: From fabrication to applications. Journal of Materials Chemistry B, 9(35), 7030–7062. 10.1039/D1TB00628B [DOI] [PubMed] [Google Scholar]
- Ponce, S. , Murillo, H. A. , Alexis, F. , Alvarez‐Barreto, J. , & Mora, J. R. (2023). Green synthesis of nanoparticles mediated by deep eutectic solvents and their applications in water treatment. Sustainability, 15(12), 9703. 10.3390/su15129703 [DOI] [Google Scholar]
- Priya, N. , Kaur, K. , & Sidhu, A. K. (2021). Green synthesis: An eco‐friendly route for the synthesis of iron oxide nanoparticles. Frontiers in Nanotechnology, 3, 655062. 10.3389/fnano.2021.655062 [DOI] [Google Scholar]
- Pushpanathan, P. , Mathew, G. S. , Selvarajan, S. , Seshadri, K. G. , & Srikanth, P. (2019). Gut microbiota and its mysteries. Indian Journal of Medical Microbiology, 37(2), 268–277. 10.4103/ijmm.IJMM_19_373 [DOI] [PubMed] [Google Scholar]
- Qi, S. , Luo, R. , Han, X. , Nie, W. , Ye, N. , Fu, C. , & Gao, F. (2022). pH/ROS dual‐sensitive natural polysaccharide nanoparticles enhance ”one stone four birds“ effect of rhein on ulcerative colitis. ACS Applied Materials & Interfaces, 14(45), 50692–50709. 10.1021/acsami.2c17827 [DOI] [PubMed] [Google Scholar]
- Qiu, L. , Shen, R. , Wei, L. , Xu, S. , Xia, W. , Hou, Y. , Cui, J. , Qu, R. , Luo, J. , & Cao, J. (2023). Designing a microbial fermentation‐functionalized alginate microsphere for targeted release of 5‐ASA using nano dietary fiber carrier for inflammatory bowel disease treatment. Journal of Nanobiotechnology, 21(1), 344. 10.1186/s12951-023-02097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, W. Y. , Wang, Y. Y. , Wang, M. , & Yan, J. K. (2018). Construction, stability, and enhanced antioxidant activity of pectin‐decorated selenium nanoparticles. Colloids and Surfaces B: Biointerfaces, 170, 692–700. 10.1016/j.colsurfb.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Rabadan‐Chavez, G. , & Lugo‐Cervantes, E. (2018). Phenolic compounds in cocoa and chocolate. Phenolic compounds in food (pp. 375–394). CRC Press. [Google Scholar]
- Rahal, H. T. , Awad, R. , Abdel‐Gaber, A. M. , & El‐Said Bakeer, D. (2017). Synthesis, characterization, and magnetic properties of pure and EDTA‐capped NiO nanosized particles. Journal of Nanomaterials, 2017(1), 7460323. 10.1155/2017/7460323 [DOI] [Google Scholar]
- Raju, G. , Katiyar, N. , Vadukumpully, S. , & Shankarappa, S. A. (2018). Penetration of gold nanoparticles across the stratum corneum layer of thick‐skin. Journal of Dermatological Science, 89(2), 146–154. 10.1016/j.jdermsci.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Ran, M. , Wu, T. , Jiao, Y. , Wu, J. , & Li, J. (2024). Selenium bio‐nanocomposite based on extracellular polymeric substances (EPS): Synthesis, characterization and application in alleviating cadmium toxicity in rice (Oryza sativa L.). International Journal of Biological Macromolecules, 258(Pt 2), 129089. 10.1016/j.ijbiomac.2023.129089 [DOI] [PubMed] [Google Scholar]
- Raveendran, S. , Yoshida, Y. , Maekawa, T. , & Kumar, D. S. (2013). Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomedicine: Nanotechnology, Biology and Medicine, 9(5), 605–626. 10.1016/j.nano.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Reena, K. , Balashanmugam, P. , Gajendiran, M. , & Antony, S. A. (2016). Synthesis of Leucas Aspera extract loaded gold‐PLA‐PEG‐PLA amphiphilic copolymer nanoconjugates: In vitro cytotoxicity and anti‐inflammatory activity studies. Journal of Nanoscience and Nanotechnology, 16(5), 4762–4770. 10.1166/jnn.2016.12404 [DOI] [PubMed] [Google Scholar]
- Restrepo, C. V. , & Villa, C. C. (2021). Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environmental Nanotechnology, Monitoring & Management, 15, 100428. 10.1016/j.enmm.2021.100428 [DOI] [Google Scholar]
- Rocha Amorim, M. O. , Gomes, D. L. , Dantas, L. A. , Silva Viana, R. L. , Chiquetti, S. C. , Almeida‐Lima, J. , Costa, L. S. , & Oliveira Rocha, H. A. (2016). Fucan‐coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. International Journal of Biological Macromolecules, 93(Pt A), 57–65. 10.1016/j.ijbiomac.2016.08.043 [DOI] [PubMed] [Google Scholar]
- Salassi, S. , Caselli, L. , Cardellini, J. , Lavagna, E. , Montis, C. , Berti, D. , & Rossi, G. (2021). A martini coarse grained model of citrate‐capped gold nanoparticles interacting with lipid bilayers. Journal of Chemical Theory and Computation, 17(10), 6597–6609. 10.1021/acs.jctc.1c00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavati‐Niasari, M. , & Davar, F. (2009). Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Materials Letters, 63(3), 441–443. 10.1016/j.matlet.2008.11.023 [DOI] [Google Scholar]
- Saleh, T. A. (2020). Nanomaterials: Classification, properties, and environmental toxicities. Environmental Technology & Innovation, 20, 101067. 10.1016/j.eti.2020.101067 [DOI] [Google Scholar]
- Santillán‐Urquiza, E. , Ángel Méndez‐Rojas, M. , & Vélez‐Ruiz, J. F. (2017). Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT, 80, 462–469. 10.1016/j.lwt.2017.03.025 [DOI] [Google Scholar]
- Saptarshi, S. R. , Duschl, A. , & Lopata, A. L. (2013). Interaction of nanoparticles with proteins: Relation to bio‐reactivity of the nanoparticle. Journal of Nanobiotechnology, 11(1), 26. 10.1186/1477-3155-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyonov, D. , Ramon, O. , Shoham, Y. , & Shimoni, E. (2014). Enzymatically synthesized dextran nanoparticles and their use as carriers for nutraceuticals. Food & Function, 5(10), 2463–2474. 10.1039/c4fo00103f [DOI] [PubMed] [Google Scholar]
- Senthil Kumar, C. , Thangam, R. , Mary, S. A. , Kannan, P. R. , Arun, G. , & Madhan, B. (2020). Targeted delivery and apoptosis induction of trans‐resveratrol‐ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydrate Polymers, 231, 115682. 10.1016/j.carbpol.2019.115682 [DOI] [PubMed] [Google Scholar]
- Shah, B. M. , Srivatsa Palakurthi, S. , Khare, T. , Khare, S. , & Palakurthi, S. (2020). Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. International Journal of Biological Macromolecules, 165, 722–737. 10.1016/j.ijbiomac.2020.09.214 [DOI] [PubMed] [Google Scholar]
- Shang, Q. , Jiang, H. , Cai, C. , Hao, J. , Li, G. , & Yu, G. (2018). Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydrate Polymers, 179, 173–185. 10.1016/j.carbpol.2017.09.059 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , Parveen, R. , & Chatterji, B. P. (2021). Toxicology of nanoparticles in drug delivery. Current Pathobiology Reports, 9(4), 133–144. 10.1007/s40139-021-00227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Lu, Z. , Wang, J. , Zhang, T. , Yang, J. , Li, Y. , Liu, G. , & Zhang, X. (2020). Advances of nanoparticles for leukemia treatment. ACS Biomaterials Science & Engineering, 6(12), 6478–6489. 10.1021/acsbiomaterials.0c01040 [DOI] [PubMed] [Google Scholar]
- Shevchenko, V. Y. , & Madison, A. E. (2002). Structure of nanoparticles: II. Magic numbers of zirconia nanoparticles. Glass Physics and Chemistry, 28(1), 44–49. 10.1023/A:1014253514099 [DOI] [Google Scholar]
- Sidhu, A. K. , Verma, N. , & Kaushal, P. (2022). Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Frontiers in Nanotechnology, 3, 801620. 10.3389/fnano.2021.801620 [DOI] [Google Scholar]
- Simões, J. C. S. (2021). Radiolabelled porphyrinic metalla‐assemblies linked to cellulose nanocrystals as PDT and imaging agents. Université de Limoges; Université de Neuchâtel. Faculté Des sciences. https://theses.hal.science/tel‐03638616v1/file/2021LIMO0115.pdf [Google Scholar]
- Singh, A. , & Dutta, P. K. (2020). Green synthesis, characterization and biological evaluation of chitin glucan based zinc oxide nanoparticles and its curcumin conjugation. International Journal of Biological Macromolecules, 156, 514–521. 10.1016/j.ijbiomac.2020.04.081 [DOI] [PubMed] [Google Scholar]
- Skiba, M. I. , Vorobyova, V. I. , Pivovarov, A. , & Makarshenko, N. P. (2020). Green synthesis of silver nanoparticles in the presence of polysaccharide: Optimization and characterization. Journal of Nanomaterials, 2020(1), 3051308. 10.1155/2020/3051308 [DOI] [Google Scholar]
- Sood, A. , Arora, V. , Shah, J. , Kotnala, R. K. , & Jain, T. K. (2016). Ascorbic acid‐mediated synthesis and characterisation of iron oxide/gold core–shell nanoparticles. Journal of Experimental Nanoscience, 11(5), 370–382. 10.1080/17458080.2015.1066514 [DOI] [Google Scholar]
- Subhash, A. , Bamigbade, G. , Abdin, M. , Jarusheh, H. , Abu‐Jdayil, B. , Liu, S.‐Q. , Palmisano, G. , Ali, A. , Kamal‐Eldin, A. , & Ayyash, M. (2025). Date seeds polysaccharides as novel capping agents for selenium nanoparticles: Synthesis, characterization, stability, biological activities, and gut microbiota modulation. Food Chemistry, 470, 142746. 10.1016/j.foodchem.2024.142746 [DOI] [PubMed] [Google Scholar]
- Subhash, A. J. , Bamigbade, G. B. , Tarique, M. , Al‐Ramadi, B. , Abu‐Jdayil, B. , Kamal‐Eldin, A. , Nyström, L. , & Ayyash, M. (2024). Bioactive properties and gut microbiota modulation by date seed polysaccharides extracted using ultrasound‐assisted deep eutectic solvent. Food Chemistry: X, 22, 101354. 10.1016/j.fochx.2024.101354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi, M. B. , Jain, A. , Jain, A. , Hurkat, P. , Shilpi, S. , Gulbake, A. , & Jain, S. K. (2015). Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5‐fluorouracil. Materials, 8(3), 832–849. 10.1016/j.intimp.2024.112661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo, N. (2006). Stress and gut microbiota: Does postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response? International Congress Series, 1287, 350–354. 10.1016/j.ics.2005.12.019 [DOI] [Google Scholar]
- Sudo, N. , Chida, Y. , Aiba, Y. , Sonoda, J. , Oyama, N. , Yu, X.‐N. , Kubo, C. , & Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. The Journal of Physiology, 558(1), 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo, A. , Molinero, N. , Reinosa, J. J. , Alcolea‐Rodriguez, V. , Portela, R. , Bañares, M. A. , Fernández, J. F. , & Victoria Moreno‐Arribas, M. (2022). PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Scientific Reports, 12(1), 528. 10.1038/s41598-021-04489-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, C. , Xie, J. , Zhang, X. , Cai, J. , & Xia, S. (2016). Polysaccharide‐based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocolloids, 57, 236–245. 10.1016/j.foodhyd.2016.01.021 [DOI] [Google Scholar]
- Tang, L. , Luo, X. , Wang, M. , Wang, Z. , Guo, J. , Kong, F. , & Bi, Y. (2021). Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis . International Journal of Biological Macromolecules, 193(Pt A), 923–932. 10.1016/j.ijbiomac.2021.10.189 [DOI] [PubMed] [Google Scholar]
- Tarique, M. , Ali, A. H. , Kizhakkayil, J. , Gan, R.‐Y. , Liu, S.‐Q. , Kamal‐Eldin, A. , & Ayyash, M. (2023). Investigating the biological activities and prebiotic potential of exopolysaccharides produced by Lactobacillus delbrueckii and Lacticaseibacillus rhamnosus: Implications for gut microbiota modulation and rheological properties in fermented milk. Food Hydrocolloids for Health, 4, 100162. 10.1016/j.fhfh.2023.100162 [DOI] [Google Scholar]
- Tarique, M. , Ali, A. H. , Kizhakkayil, J. , Liu, S.‐Q. , Oz, F. , Dertli, E. , Kamal‐Eldin, A. , & Ayyash, M. (2024). Exopolysaccharides from Enterococcus faecium and Streptococcus thermophilus: Bioactivities, gut microbiome effects, and fermented milk rheology. Food Chemistry: X, 21, 101073. 10.1016/j.fochx.2023.101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, A. , Verma, A. , Kumar Panda, P. , Saraf, S. , Jain, A. , & Jain, S. K. (2019). Stimuli‐responsive polysaccharides for colon‐targeted drug delivery. Stimuli responsive polymeric nanocarriers for drug delivery applications (pp. 547–566). Elsevier. [Google Scholar]
- Tsamesidis, I. , Lymperaki, E. , Egwu, C. O. , Pouroutzidou, G. K. , Kazeli, K. , Reybier, K. , Bourgeade‐Delmas, S. , Valentin, A. , & Kontonasaki, E. (2021). Effect of silica based nanoparticles against Plasmodium falciparum and Leishmania infantum parasites. Journal of Xenobiotics, 11(4), 155–162. 10.3390/jox11040011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulve, N. S. , Stefaniak, A. B. , Vance, M. E. , Rogers, K. , Mwilu, S. , LeBouf, R. F. , Schwegler‐Berry, D. , Willis, R. , Thomas, T. A. , & Marr, L. C. (2015). Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children's potential exposures. International Journal of Hygiene and Environmental Health, 218(3), 345–357. 10.1016/j.ijheh.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar, K. , Aris, A. , Ahmad, H. , Parveen, T. , Jaafar, J. , Abdul Majid, Z. , Vijaya Bhaskar Reddy, A. , & Talib, J. (2016). Synthesis of visible light active doped TiO2 for the degradation of organic pollutants—Methylene blue and glyphosate. Journal of Analytical Science and Technology, 7(1), 29. 10.1186/s40543-016-0109-2 [DOI] [Google Scholar]
- Umu, Ö. Z. C. O. , Rudi, K. , & Diep, D. B. (2017). Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microbial Ecology in Health and Disease, 28(1), 1348886. 10.1080/16512235.2017.1348886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatpurwar, V. , Mali, V. , Bodhankar, S. , & Pokharkar, V. (2012). In vitro cytotoxicity and in vivo sub‐acute oral toxicity assessment of porphyran reduced gold nanoparticles. Toxicological & Environmental Chemistry, 94(7), 1357–1367. 10.1080/02772248.2012.697731 [DOI] [Google Scholar]
- Verma, M. , Kumar, V. , & Katoch, A. (2018). Sputtering based synthesis of CuO nanoparticles and their structural, thermal and optical studies. Materials Science in Semiconductor Processing, 76, 55–60. 10.1016/j.mssp.2017.12.018 [DOI] [Google Scholar]
- Vijayakumar, S. , Chen, J. , Kalaiselvi, V. , Tungare, K. , Bhori, M. , Gonzalez‐Sanchez, Z. I. , & Duran‐Lara, E. F. (2022). Marine polysaccharide laminarin embedded ZnO nanoparticles and their based chitosan capped ZnO nanocomposites: Synthesis, characterization and in vitro and in vivo toxicity assessment. Environmental Research, 213, 113655. 10.1016/j.envres.2022.113655 [DOI] [PubMed] [Google Scholar]
- Vishakha, V. , Abdel‐Mohsen, A. M. , Michalicka, J. , White, P. B. , Lepcio, P. , Navarro, L. K. T. , & Jančář, J. (2023). Carboxymethyl starch as a reducing and capping agent in the hydrothermal synthesis of selenium nanostructures for use with three‐dimensional‐printed hydrogel carriers. Royal Society Open Science, 10(10), 230829. 10.1098/rsos.230829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren, M. , Axenstrand, M. , Håkansson, Å. s. , Marefati, A. , & Pedersen, B. L. (2019). In vitro methods to study colon release: State of the art and an outlook on new strategies for better in‐vitro biorelevant release media. Pharmaceutics, 11(2), 95. 10.3390/pharmaceutics11020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. W. , & Lawley, T. D. (2013). Therapeutic modulation of intestinal dysbiosis. Pharmacological Research, 69(1), 75–86. 10.1016/j.phrs.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Gao, X. , Chen, Z. , Chen, Y. , & Chen, H. (2017). Preparation, characterization and application of polysaccharide‐based metallic nanoparticles: A review. Polymers (Basel), 9(12), 689. 10.3390/polym9120689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Wang, M. Q. , Ye, S. S. , Tao, W. J. , & Du, Y. J. (2011). Effects of copper‐loaded chitosan nanoparticles on growth and immunity in broilers. Poultry Science, 90(10), 2223–2228. 10.3382/ps.2011-01511 [DOI] [PubMed] [Google Scholar]
- Wang, M. , Cha, R. , Hao, W. , Du, R. , Zhang, P. , Hu, Y. , & Jiang, X. (2022). Nanocrystalline cellulose cures constipation via gut microbiota metabolism. ACS Nano, 16(10), 16481–16496. 10.1021/acsnano.2c05809 [DOI] [PubMed] [Google Scholar]
- Wang, M.‐Q. , Du, Y.‐J. , Wang, C. , Tao, W.‐J. , He, Y.‐D. , & Li, H. (2012). Effects of copper‐loaded chitosan nanoparticles on intestinal microflora and morphology in weaned piglets. Biological Trace Element Research, 149(2), 184–189. 10.1007/s12011-012-9410-0 [DOI] [PubMed] [Google Scholar]
- Wang, N. , Zhang, C. , Li, H. , Zhang, D. , Wu, J. , Li, Y. , Yang, L. , Zhang, N. , & Wang, X. (2024). Addition of Canna edulis starch and starch nanoparticles to stabilized Pickering emulsions: In vitro digestion and fecal fermentation. International Journal of Biological Macromolecules, 258, 128993. 10.1016/j.ijbiomac.2023.128993 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Wang, Y. , Guo, W. , & Zeng, M. (2021). Stability and bioactivity of carotenoids from Synechococcus sp. PCC 7002 in Zein/NaCas/Gum Arabic composite nanoparticles fabricated by pH adjustment and heat treatment antisolvent precipitation. Food Hydrocolloids, 117, 106663. 10.1016/j.foodhyd.2021.106663 [DOI] [Google Scholar]
- Woranuch, S. , & Yoksan, R. (2013). Eugenol‐loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydrate Polymers, 96(2), 578–585. 10.1016/j.carbpol.2012.08.117 [DOI] [PubMed] [Google Scholar]
- Worthington, K. L. , Adamcakova‐Dodd, A. , Wongrakpanich, A. , Mudunkotuwa, I. A. , Mapuskar, K. A. , Joshi, V. B. , Allan Guymon, C. , Spitz, D. R. , Grassian, V. H. , Thorne, P. S. , & Salem, A. K. (2013). Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology, 24(39), 395101. 10.1088/0957-4484/24/39/395101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T. , Shen, M. , Yu, Q. , Chen, Y. , Chen, X. , Yang, J. , Huang, L. , Guo, X. , & Xie, J. (2021). Cyclocarya paliurus polysaccharide improves metabolic function of gut microbiota by regulating short‐chain fatty acids and gut microbiota composition. Food Research International, 141, 110119. 10.1016/j.foodres.2021.110119 [DOI] [PubMed] [Google Scholar]
- Xiao, X. , Guo, K. , Liu, J. , Liu, Y. , Yang, C. , Xu, Y. , & Deng, B. (2023). The effect of sodium alginate‐coated nano‐zinc oxide on the growth performance, serum indexes and fecal microbial structure of weaned piglets. Animals, 14(1), 146. 10.3390/ani14010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Huang, Q. , Zheng, Z. , Guan, H. , & Liu, S. (2017). Construction of a Cordyceps sinensis exopolysaccharide‐conjugated selenium nanoparticles and enhancement of their antioxidant activities. International Journal of Biological Macromolecules, 99, 483–491. 10.1016/j.ijbiomac.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Xie, J. , Zhao, M. , Wang, C. , Yong, Y. , & Gu, Z. (2022). Recent advances in understanding the effects of nanomaterials on gut microbiota. Chemical Engineering Journal, 435, 134976. 10.1016/j.cej.2022.134976 [DOI] [Google Scholar]
- Xu, B. , Sun, P. , Lu, J. , Wang, Y. , Lin, X. , Chen, C. , Zhu, J. , Jia, H. , Wang, X. , & Shen, J. (2025). Studies of peach gum polysaccharide on gut microbiota in vitro fermentation by human feces. Journal of Future Foods, 5(1), 79–87. 10.1016/j.jfutfo.2024.01.007 [DOI] [Google Scholar]
- Yan, L. , Wei, L. , Zheng, W. , Liu, S. , Yin, L. , Liu, C. , & Zheng, L. (2021). Oral delivery of chitosan derivative‐based nanoparticles encapsulating quercetin to attenuate intestinal injury and regulate gut microbiota dysbiosis in mucositis treatment. ACS Food Science & Technology, 1(3), 399–409. 10.1021/acsfoodscitech.0c00121 [DOI] [Google Scholar]
- Yan, W. , Luo, J. , Yu, Z. , & Xu, B. (2023). Critical review on intestinal mucosal barrier protection effects of dietary polysaccharides. Food & Function, 15, 481–492. 10.1039/d3fo03412g [DOI] [PubMed] [Google Scholar]
- Yang, L. , Cui, Y. , Liang, H. , Li, Z. , Wang, N. , Wang, Y. , & Zheng, G. (2022). Multifunctional selenium nanoparticles with different surface modifications ameliorate neuroinflammation through the gut microbiota‐NLRP3 inflammasome‐brain axis in APP/PS1 mice. ACS Applied Materials & Interfaces, 14(27), 30557–30570. 10.1021/acsami.2c06283 [DOI] [PubMed] [Google Scholar]
- Yang, L. , Wang, Y. , Li, Z. , Wu, X. , Mei, J. , & Zheng, G. (2023). Brain targeted peptide‐functionalized chitosan nanoparticles for resveratrol delivery: Impact on insulin resistance and gut microbiota in obesity‐related Alzheimer's disease. Carbohydrate Polymers, 310, 120714. 10.1016/j.carbpol.2023.120714 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Hu, Y. , Yue, P. , Li, H. , Wu, Y. , Hao, X. , & Peng, F. (2023). Structure, stability, antioxidant activity, and controlled‐release of selenium nanoparticles decorated with lichenan from Usnea longissima . Carbohydrate Polymers, 299, 120219. 10.1016/j.carbpol.2022.120219 [DOI] [PubMed] [Google Scholar]
- Yao, Y. , Zang, Y. , Qu, J. , Tang, M. , & Zhang, T. (2019). The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. International Journal of Nanomedicine, 14, 8787–8804. 10.2147/ijn.S212907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob, A. A. , Ahmad, H. , Parveen, T. , Ahmad, A. , Oves, M. , Ismail, I. M. I. , Qari, H. A. , Umar, K. , & Mohamad Ibrahim, M. N. (2020). Recent advances in metal decorated nanomaterials and their various biological applications: A review. Frontiers in Chemistry, 8, 341. 10.3389/fchem.2020.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yausheva, Е. , Miroshnikov, S. , & Sizova, Е. (2018). Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environmental Science and Pollution Research, 25(18), 18109–18120. 10.1007/s11356-018-1991-5 [DOI] [PubMed] [Google Scholar]
- Ye, R. , Guo, Q. , Huang, J. , Wang, Z. , Chen, Y. , & Dong, Y. (2023). Eucommia ulmoides polysaccharide modified nano‐selenium effectively alleviated DSS‐induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. Journal of Nanobiotechnology, 21(1), 222. 10.1186/s12951-023-01965-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosri, N. , Khalifa, S. A. M. , Guo, Z. , Xu, B. , Zou, X. , & El‐Seedi, H. R. (2021). Marine organisms: Pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. International Journal of Biological Macromolecules, 193(Pt B), 1767–1798. 10.1016/j.ijbiomac.2021.10.229 [DOI] [PubMed] [Google Scholar]
- Yu, S. Y. , & Lin, K. W. (2014). Influence of bacterial cellulose (nata) on the physicochemical and sensory properties of frankfurter. Journal of Food Science, 79(6), C1117–C1122. 10.1111/1750-3841.12494 [DOI] [PubMed] [Google Scholar]
- Yuan, H. , Guo, C. , Liu, L. , Zhao, L. , Zhang, Y. , Yin, T. , He, H. , Gou, J. , Pan, B. , & Tang, X. (2023). Progress and prospects of polysaccharide‐based nanocarriers for oral delivery of proteins/peptides. Carbohydrate Polymers, 312, 120838. 10.1016/j.carbpol.2023.120838 [DOI] [PubMed] [Google Scholar]
- Zarrintaj, P. , Ghorbani, S. , Barani, M. , Pal Singh Chauhan, N. , Yazdi, M. K. , Saeb, M. R. , Ramsey, J. D. , Hamblin, M. R. , Mozafari, M. , & Mostafavi, E. (2022). Polylysine for skin regeneration: A review of recent advances and future perspectives. Bioengineering & Translational Medicine, 7(1), e10261. 10.1002/btm2.10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Zhai, X. , Zhao, G. , Ren, F. , & Leng, X. (2015). Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydrate Polymers, 134, 158–166. 10.1016/j.carbpol.2015.07.065 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Liu, J. , Cheng, H. , Wang, H. , Tan, Y. , Feng, W. , & Peng, C. (2022). Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review. Food Research International, 160, 111653. 10.1016/j.foodres.2022.111653 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Yang, X. , Ji, T. , Wen, C. , Ye, Z. , Liu, X. , Liang, L. , Liu, G. , & Xu, X. (2022). Digestion and absorption properties of Lycium barbarum polysaccharides stabilized selenium nanoparticles. Food Chemistry, 373(Pt B), 131637. 10.1016/j.foodchem.2021.131637 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Wang, Y. , Wang, M. , Jiang, L. , Ma, X. , Huang, Y. , Liu, T. , Zheng, L. , & Li, Y. (2024). Construction and anti‐pancreatic cancer activity of selenium nanoparticles stabilized by Prunella vulgaris polysaccharide. International Journal of Biological Macromolecules, 278, 134924. 10.1016/j.ijbiomac.2024.134924 [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Yang, Y. , Liang, Y. , Jiao, X. , & Zhao, C. (2018). Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients, 10(8), 1055. 10.3390/nu10081055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Zhang, J. , Ding, D. , Zhang, L. , Muehlmann, L. A. , Deng, S. E. , Wang, X. , Li, W. , & Zhang, W. (2018). Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artificial Cells, Nanomedicine, and Biotechnology, 46(7), 1463–1470. 10.1080/21691401.2017.1373657 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Mortimer, M. , & Guo, L.‐H. (2020). Interplay between engineered nanomaterials and microbiota. Environmental Science: Nano, 7(9), 2454–2485. 10.1039/D0EN00557F [DOI] [Google Scholar]
- Zhao, L. , Du, X. , Tian, J. , Kang, X. , Li, Y. , Dai, W. , Li, D. , Zhang, S. , & Li, C. (2021). Berberine‐loaded carboxylmethyl chitosan nanoparticles ameliorate DSS‐induced colitis and remodel gut microbiota in mice. Frontiers in Pharmacology, 12, 644387. 10.3389/fphar.2021.644387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Gao, Y. , Tan, J. , Zhu, Y. , Ying, X. , Zhang, M. , Yu, X. , & You, B. (2019). Facile synthesis and antibacterial applications of cuprous oxide/bovine serum albumin hierarchical nanocomposite particles. SN Applied Sciences, 1(8), 917. 10.1007/s42452-019-0963-9 [DOI] [Google Scholar]
- Zhao, X. , Li, Q. , Li, X. , Xia, Y. , Wang, B. , & Zhao, Z. (2017). Antibacterial activity and in vitro cytotoxicity evaluation of alginate‐AgNP fibers. Textile Research Journal, 87(11), 1377–1386. 10.1177/0040517516652350 [DOI] [Google Scholar]
- Zhao, X. , Xia, Y. , Li, Q. , Ma, X. , Quan, F. , Geng, C. , & Han, Z. (2014). Microwave‐assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 444, 180–188. 10.1016/j.colsurfa.2013.12.008 [DOI] [Google Scholar]
- Zhao, Y. , Chen, H. , Li, W. , He, Q. , Liang, J. , Yan, X. , Yuan, Y. , & Yue, T. (2022). Selenium‐containing tea polysaccharides ameliorate DSS‐induced ulcerative colitis via enhancing the intestinal barrier and regulating the gut microbiota. International Journal of Biological Macromolecules, 209, 356–366. 10.1016/j.ijbiomac.2022.04.028 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. , Xie, Q. , Wang, H. , Hu, Y. , Ren, B. , & Li, X. (2020). Recent advances in plant polysaccharide‐mediated nano drug delivery systems. International Journal of Biological Macromolecules, 165, 2668–2683. 10.1016/j.ijbiomac.2020.10.173 [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Wang, L. , An, S. , Wang, C. , Jiang, Q. , & Li, X. (2022). Fabrication of quercetin‐loaded nanoparticles based on Hohenbuehelia serotina polysaccharides and their modulatory effects on intestinal function and gut microbiota in vivo . Innovative Food Science & Emerging Technologies, 78, 102993. 10.1016/j.ifset.2022.102993 [DOI] [Google Scholar]
- Zhu, C. , Zhang, S. , Song, C. , Zhang, Y. , Ling, Q. , Hoffmann, P. R. , Li, J. , Chen, T. , Zheng, W. , & Huang, Z. (2017). Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF‐κB mediated hyper inflammation. Journal of Nanobiotechnology, 15(1), 20. 10.1186/s12951-017-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, K. , Fan, H. , Zeng, S. , Nie, S. , Zhang, Y. , Tan, L. , Li, C. , Xu, F. , Liu, Q. , & Wu, G. (2021). Polysaccharide from Artocarpus heterophyllus Lam. (jackfruit) pulp modulates gut microbiota composition and improves short‐chain fatty acids production. Food Chemistry, 364, 130434. 10.1016/j.foodchem.2021.130434 [DOI] [PubMed] [Google Scholar]


