Abstract
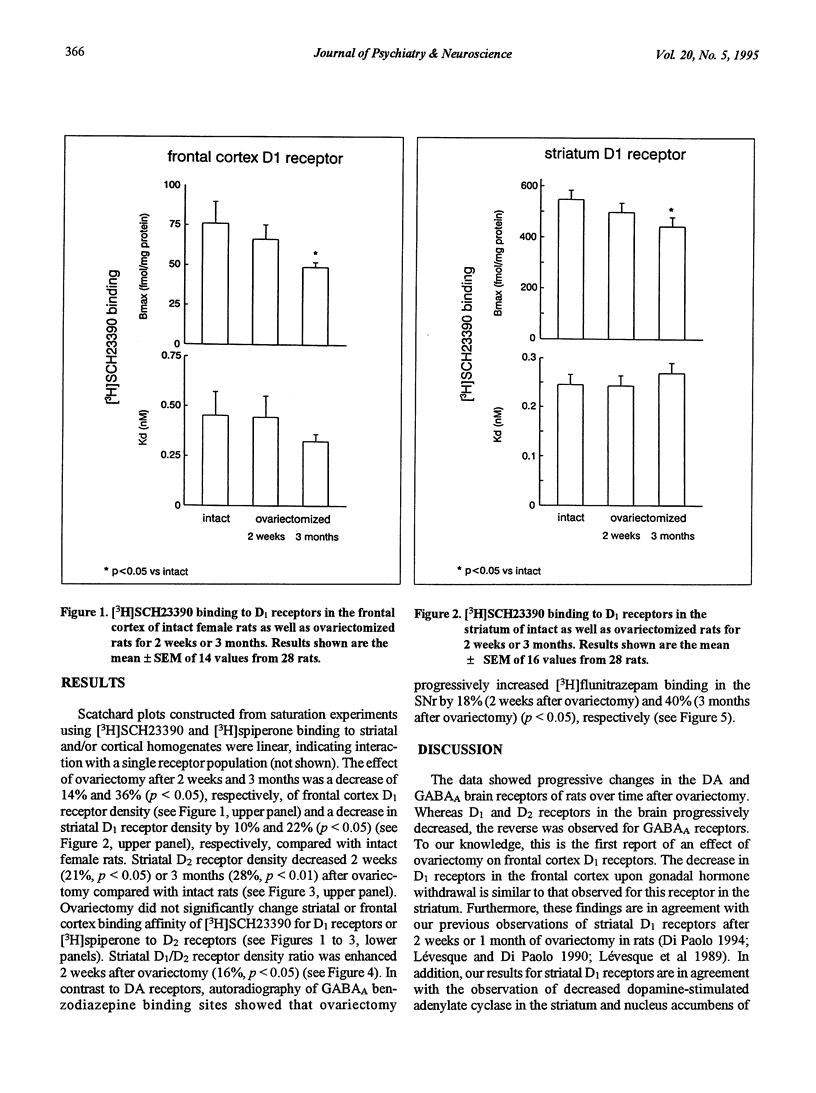
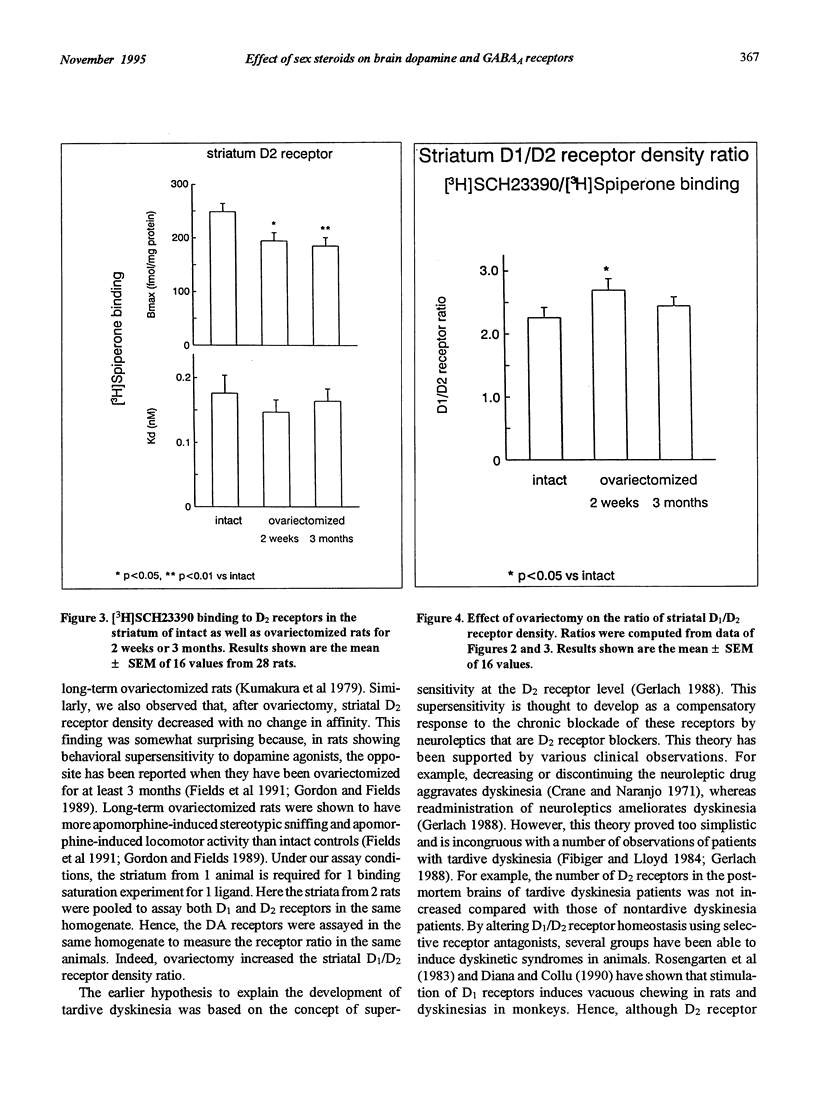
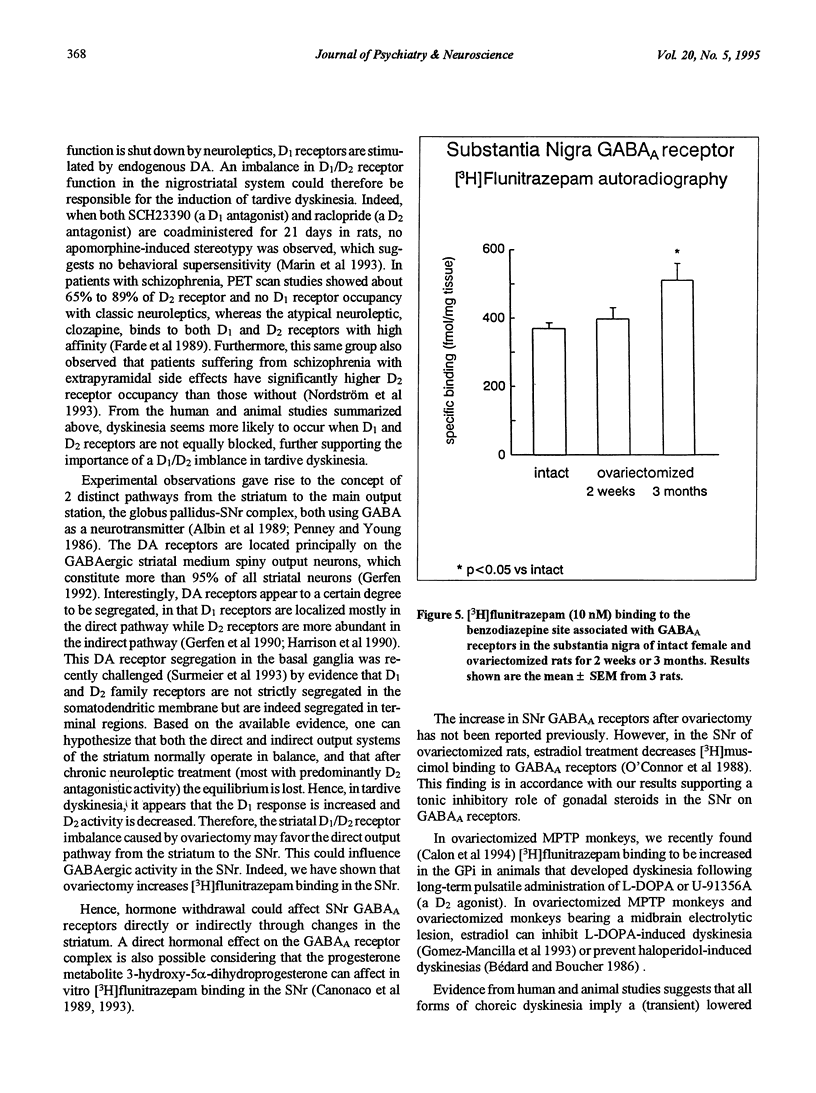
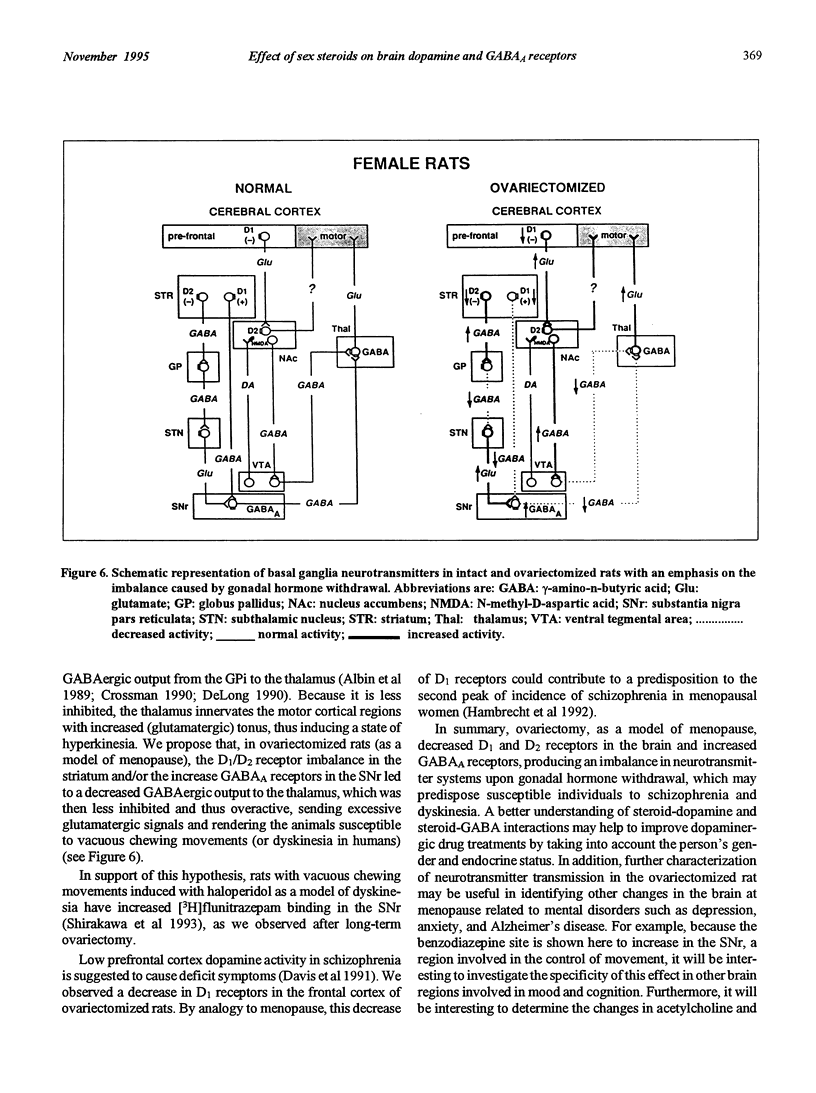
A peak of first episodes of schizophrenia can occur in postmenopausal women. Furthermore, tardive dyskinesia is more common in postmenopausal women than in men of comparable age. This study investigated the effect of ovariectomy (2 weeks or 3 months) in rats as a model of decreased gonadal function associated with menopause. After ovariectomy, frontal cortex D1 receptors progressively decreased in density with no change of affinity over time. Striatal D1 and D2 receptors also had decreased density after ovariectomy with no change of affinity. In the substantia nigra pars reticulata, a progressive increase in [3H]flunitrazepam-specific binding associated with GABAA receptors was observed as a function of time following ovariectomy. It is hypothesized that low prefrontal cortex dopamine activity has implications in negative symptoms of schizophrenia and, furthermore, that GABAergic overactivity in the internal globus pallidus-substantia nigra pars reticulata complex plays a role in tardive dyskinesia. The present results suggest that, by reducing brain dopamine receptors and increasing GABAA receptors, gonadal hormone withdrawal may predispose to schizophrenia and dyskinesia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Angermeyer M. C., Goldstein J. M., Kuehn L. Gender differences in schizophrenia: rehospitalization and community survival. Psychol Med. 1989 May;19(2):365–382. doi: 10.1017/s0033291700012411. [DOI] [PubMed] [Google Scholar]
- Angermeyer M. C., Kühn L. Gender differences in age at onset of schizophrenia. An overview. Eur Arch Psychiatry Neurol Sci. 1988 Sep;237(6):351–364. doi: 10.1007/BF00380979. [DOI] [PubMed] [Google Scholar]
- Angermeyer M. C. The association between family atmosphere and hospital career of schizophrenic patients. Br J Psychiatry. 1982 Jul;141:1–11. doi: 10.1192/bjp.141.1.1. [DOI] [PubMed] [Google Scholar]
- Bardenstein K. K., McGlashan T. H. Gender differences in affective, schizoaffective, and schizophrenic disorders. A review. Schizophr Res. 1990 May-Jun;3(3):159–172. doi: 10.1016/0920-9964(90)90034-5. [DOI] [PubMed] [Google Scholar]
- Bédard P. J., Boucher R. Estradiol can suppress haloperidol-induced supersensitivity in dyskinetic monkeys. Neurosci Lett. 1986 Feb 28;64(2):206–210. doi: 10.1016/0304-3940(86)90101-1. [DOI] [PubMed] [Google Scholar]
- Canonaco M., Carelli A., Maggi A. Steroid hormones and receptors of the GABAA supramolecular complex. I. Benzodiazepine receptor level changes in some extrahypothalamic brain areas of the female rat following sex steroid treatment. Neuroendocrinology. 1993 May;57(5):965–973. doi: 10.1159/000126461. [DOI] [PubMed] [Google Scholar]
- Canonaco M., Valenti A., Tavolaro R., Bettini E., Maggi A. Differential modulation of [3H]flunitrazepam binding in female rat brain by sex steroid hormones. Eur J Pharmacol. 1989 Oct 24;170(1-2):95–99. doi: 10.1016/0014-2999(89)90140-4. [DOI] [PubMed] [Google Scholar]
- Childers S. E., Harding C. M. Gender, premorbid social functioning, and long-term outcome in DSM-III schizophrenia. Schizophr Bull. 1990;16(2):309–318. doi: 10.1093/schbul/16.2.309. [DOI] [PubMed] [Google Scholar]
- Crane G. E., Naranjo E. R. Motor disorders induced by neuroleptics: a proposed new classification. Arch Gen Psychiatry. 1971 Feb;24(2):179–184. doi: 10.1001/archpsyc.1971.01750080083014. [DOI] [PubMed] [Google Scholar]
- Crossman A. R. A hypothesis on the pathophysiological mechanisms that underlie levodopa- or dopamine agonist-induced dyskinesia in Parkinson's disease: implications for future strategies in treatment. Mov Disord. 1990;5(2):100–108. doi: 10.1002/mds.870050203. [DOI] [PubMed] [Google Scholar]
- Davis K. L., Kahn R. S., Ko G., Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991 Nov;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- DeLong M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990 Jul;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deister A., Marneros A. Geschlechtsabhängige Unterschiede bei endogenen Psychosen. Ein Vergleich zwischen schizophrenen, schizoaffektiven und affektiven Psychosen. Fortschr Neurol Psychiatr. 1992 Nov;60(11):407–419. doi: 10.1055/s-2007-1000664. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994 Jan-Mar;5(1):27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Di Paolo T., Poyet P., Labrie F. Effect of prolactin and estradiol on rat striatal dopamine receptors. Life Sci. 1982 Dec 20;31(25):2921–2929. doi: 10.1016/0024-3205(82)90684-1. [DOI] [PubMed] [Google Scholar]
- Diana M., Collu M. D1 receptors mediated vacuous chewing in the rat: a model of tardive dyskinesia. Pharmacol Res. 1990 Sep-Oct;22 (Suppl 3):45–45. doi: 10.1016/s1043-6618(09)80019-4. [DOI] [PubMed] [Google Scholar]
- Dworkin R. H. Patterns of sex differences in negative symptoms and social functioning consistent with separate dimensions of schizophrenic psychopathology. Am J Psychiatry. 1990 Mar;147(3):347–349. doi: 10.1176/ajp.147.3.347. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Schizophrenia: sex differences. Can J Psychiatry. 1985 Aug;30(5):319–322. doi: 10.1177/070674378503000504. [DOI] [PubMed] [Google Scholar]
- Gender and schizophrenia. Schizophr Bull. 1990;16(2):179–344. [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr, Sibley D. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990 Dec 7;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerlach J. Tardive dyskinesia. Pathophysiological mechanisms and clinical trials. Encephale. 1988 Sep;14(Spec No):227–232. [PubMed] [Google Scholar]
- Goldstein J. M., Santangelo S. L., Simpson J. C., Tsuang M. T. The role of gender in identifying subtypes of schizophrenia: a latent class analytic approach. Schizophr Bull. 1990;16(2):263–275. doi: 10.1093/schbul/16.2.263. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B., Boucher R., Gagnon C., Di Paolo T., Markstein R., Bédard P. J. Effect of adding the D1 agonist CY 208-243 to chronic bromocriptine treatment. I: Evaluation of motor parameters in relation to striatal catecholamine content and dopamine receptors. Mov Disord. 1993 Apr;8(2):144–150. doi: 10.1002/mds.870080205. [DOI] [PubMed] [Google Scholar]
- Gordon J. H., Fields J. Z. A permanent dopamine receptor up-regulation in the ovariectomized rat. Pharmacol Biochem Behav. 1989 May;33(1):123–125. doi: 10.1016/0091-3057(89)90440-1. [DOI] [PubMed] [Google Scholar]
- Gureje O. Gender and schizophrenia: age at onset and sociodemographic attributes. Acta Psychiatr Scand. 1991 May;83(5):402–405. doi: 10.1111/j.1600-0447.1991.tb05564.x. [DOI] [PubMed] [Google Scholar]
- Hambrecht M., Maurer K., Häfner H. Evidence for a gender bias in epidemiological studies of schizophrenia. Schizophr Res. 1993 Jan;8(3):223–231. doi: 10.1016/0920-9964(93)90020-j. [DOI] [PubMed] [Google Scholar]
- Harrison M. B., Wiley R. G., Wooten G. F. Selective localization of striatal D1 receptors to striatonigral neurons. Brain Res. 1990 Oct 1;528(2):317–322. doi: 10.1016/0006-8993(90)91674-6. [DOI] [PubMed] [Google Scholar]
- Häfner H., Behrens S., De Vry J., Gattaz W. F. Oestradiol enhances the vulnerability threshold for schizophrenia in women by an early effect on dopaminergic neurotransmission. Evidence from an epidemiological study and from animal experiments. Eur Arch Psychiatry Clin Neurosci. 1991;241(1):65–68. doi: 10.1007/BF02193758. [DOI] [PubMed] [Google Scholar]
- Häfner H., Riecher A., Maurer K., Löffler W., Munk-Jørgensen P., Strömgren E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol Med. 1989 Nov;19(4):903–918. doi: 10.1017/s0033291700005626. [DOI] [PubMed] [Google Scholar]
- Iacono W. G., Beiser M. Where are the women in first-episode studies of schizophrenia? Schizophr Bull. 1992;18(3):471–480. doi: 10.1093/schbul/18.3.471. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Hoffman M., Cocchi D., Trabucchi M., Spano P. F., Müller E. E. Long-term effect of ovariectomy on dopamine-stimulated adenylate cyclase in rat striatum and nucleus accumbens. Psychopharmacology (Berl) 1979 Mar 14;61(1):13–16. doi: 10.1007/BF00426803. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewine R. Schizophrenia: an amotivational syndrome in men. Can J Psychiatry. 1985 Aug;30(5):316–318. doi: 10.1177/070674378503000503. [DOI] [PubMed] [Google Scholar]
- Loranger A. W. Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry. 1984 Feb;41(2):157–161. doi: 10.1001/archpsyc.1984.01790130053007. [DOI] [PubMed] [Google Scholar]
- Lévesque D., Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990 Feb;24(2):281–284. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- Lévesque D., Gagnon S., Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989 Apr 10;98(3):345–350. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- Marin C., Parashos S. A., Kapitzoglou-Logothetis V., Peppe A., Chase T. N. D1 and D2 dopamine receptor-mediated mechanisms and behavioral supersensitivity. Pharmacol Biochem Behav. 1993 May;45(1):195–200. doi: 10.1016/0091-3057(93)90104-2. [DOI] [PubMed] [Google Scholar]
- McCabe M. S. Demographic differences in functional psychoses. Br J Psychiatry. 1975 Oct;127:320–323. doi: 10.1192/bjp.127.4.320. [DOI] [PubMed] [Google Scholar]
- Nicole L., Lesage A., Lalonde P. Lower incidence and increased male:female ratio in schizophrenia. Br J Psychiatry. 1992 Oct;161:556–557. doi: 10.1192/bjp.161.4.556. [DOI] [PubMed] [Google Scholar]
- Nordström A. L., Farde L., Wiesel F. A., Forslund K., Pauli S., Halldin C., Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993 Feb 15;33(4):227–235. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- O'Connor L. H., Nock B., McEwen B. S. Regional specificity of gamma-aminobutyric acid receptor regulation by estradiol. Neuroendocrinology. 1988 Jun;47(6):473–481. doi: 10.1159/000124958. [DOI] [PubMed] [Google Scholar]
- Opjordsmoen S. Long-term clinical outcome of schizophrenia with special reference to gender differences. Acta Psychiatr Scand. 1991 Apr;83(4):307–313. doi: 10.1111/j.1600-0447.1991.tb05545.x. [DOI] [PubMed] [Google Scholar]
- Ring N., Tantam D., Montague L., Newby D., Black D., Morris J. Gender differences in the incidence of definite schizophrenia and atypical psychosis--focus on negative symptoms of schizophrenia. Acta Psychiatr Scand. 1991 Dec;84(6):489–496. doi: 10.1111/j.1600-0447.1991.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Rosengarten H., Schweitzer J. W., Friedhoff A. J. Induction of oral dyskinesias in naive rats by D1 stimulation. Life Sci. 1983 Dec 19;33(25):2479–2482. doi: 10.1016/0024-3205(83)90155-8. [DOI] [PubMed] [Google Scholar]
- Seeman M. V. Gender differences in schizophrenia. Can J Psychiatry. 1982 Mar;27(2):107–112. doi: 10.1177/070674378202700204. [DOI] [PubMed] [Google Scholar]
- Seeman M. V. Sex and schizophrenia. Can J Psychiatry. 1985 Aug;30(5):313–315. doi: 10.1177/070674378503000502. [DOI] [PubMed] [Google Scholar]
- Shirakawa O., Maeda K., Sakai K. Dysregulation of striato-nigral GABAergic pathway by chronic haloperidol treatment: the role of dopamine D1 receptor in the substantia nigra pars reticulata on the development of tardive dyskinesia. Jpn J Psychiatry Neurol. 1993 Jun;47(2):429–430. doi: 10.1111/j.1440-1819.1993.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Reiner A., Levine M. S., Ariano M. A. Are neostriatal dopamine receptors co-localized? Trends Neurosci. 1993 Aug;16(8):299–305. doi: 10.1016/0166-2236(93)90103-s. [DOI] [PubMed] [Google Scholar]
- Yassa R., Jeste D. V. Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull. 1992;18(4):701–715. doi: 10.1093/schbul/18.4.701. [DOI] [PubMed] [Google Scholar]


