Abstract
Background & Objective:
Phyllodes tumor (PT) is a rare fibroepithelial tumor of the breast exhibiting varied clinicopathologic behavior, ranging from benign to borderline to frankly malignant, based on the presence of infiltrative margins, stromal overgrowth, stromal atypia, cellularity, and mitotic activity. In this study, a detailed cytomorphological study of cases of PT with the clinical and histological correlation was performed.
Methods:
A cytomorphological study of 17 cases of histologically proven PT diagnosed between Jan 2014 and July 2021 was done retrospectively. Relevant data including age at the time of diagnosis, the duration of illness, presenting symptoms, personal and family history, tumor size, tumor localization, and surgical procedure were obtained. A detailed cytomorphological assessment of stromal and epithelial components was performed, and further histological correlation was obtained for each case.
Results:
Age of the patients ranged from 25 to 65 years old. The chief complaint was a palpable breast mass in all patients. The mean size of the lump was 11.86 cm. A complete cytohistological concordance was achieved among malignant cases. Stromal metaplasia was observed in only one case of benign phyllodes tumor, which was chondroid differentiation, and malignant heterologous component as fibrosarcomatous differentiation in one of the malignant PTs. Each of the benign and malignant phyllode tumors ductal carcinoma in situ (DCIS) of its epithelial component was seen in one case.
Conclusion:
Phyllodes should be considered in differential diagnosing of any rapidly growing breast lump. Breast imaging has limited role in diagnosis of phyllode tumors. FNAC or trucut biopsy is mandatory in preoperative diagnosis. An extended follow-up is needed in all cases.
Key Words: Cytohistologic correlation, fibroepithelial tumor, FNAC, Phyllodes
Introduction
Breast pathologies are among the most common lesions subjected to fine needle aspiration (FNA) for initial diagnosis, with carcinoma and fibroepithelial neoplasms comprising the majority. The latter includes fibroadenoma (FA) and phyllodes tumor (PT). Phyllodes tumors are rare fibroepithelial lesions, accounting for 0.3% to 1% of all breast tumors (1). While fibroadenomas are considered benign, the behavior of phyllodes tumors is highly unpredictable. Some cases behave as benign tumors and are amenable to simple excision, whereas others can metastasize or recur, requiring more aggressive treatment (2). Therefore, accurate preoperative diagnosis is essential to guide appropriate management. However, the cytologic features of fibroadenoma and benign phyllodes tumors show significant overlap (3).
We retrospectively analyzed 17 cases of phyllodes tumors for which both cytology smears and follow-up histopathology sections were available. The sensitivity and specificity of both methods in diagnosing phyllodes tumor were evaluated.
Material and Methods
The present retrospective study was conducted on a total of 17 cases of histologically proven phyllodes tumors diagnosed between Jan 2018 and July 2023 in which prior FNA was performed and smears were available for review. The cytology smears were reevaluated without the knowledge of the initial cytologic diagnosis, and an attempt was made to sub-classify the cases into benign, borderline, and malignant phyllodes. A detailed study of cases was undertaken for epithelial and stromal components, where the proportion, cellularity, and atypia of both the components were evaluated in additionto stromal-epithelial ratio, percentage of single scattered spindle cells in the background, and mitosis. For the number of fragments, a cut-off of less than and more than 05 fragments in the smear was used to indicate few fragments and many fragments, respectively for both the epithelial and stromal components as adopted by Krishnamurthy et al. (4). Stromal cellularity and atypia were assessed on the scale of 1+,2+, 3+ to represent mild, moderate and marked respectively. Mitosis was assessed as 1+ (<2/10hpf), 2+ (2-4/10 hpf), and 3+ (>4/10 hpf), similar to the criteria used by Bhattarai S et al. (5). Epithelial fragment architecture and stromal to epithelial ratio were also assessed. The stromal-to-epithelial ratio was evaluated as stromal predominant, epithelial predominant, or both components equally prevalent. Single scattered stromal cells were categorized as > 10%, 10-30%, and >30% as by Krishnamurthy et al. (4). To count the number of epithelial and stromal fragments, 10 medium power (10X) fields were observed, and an average was taken. Cytology slides available for review were Giemsa, hematoxylin, and eosin, and Papanicolaou stained, and histology slides were hematoxylin and eosin stained. The numbers of epithelial and stromal fragments were counted on 10 medium power fields, and an average of ten fields was taken as by Maritz et al. (6). The data was compiled in an Excel sheet and using SPSS software (SPSS Inc., Chicago, Ill., USA), the Fischer exact test was applied to calculate the P-value amongst various cytological parameters. A P-value of less than 0.05 was considered statistically significant.
Observation
The present study consisted of 17 cases of histologically proven phyllodes tumors with an age range of 22-63 years and a mean age being 44.06 years. The size of the lump ranged from 3.5 cm to 5.8 cm with the mean size being 4.2 cm. Table 1 shows the clinical characteristics of all the patients with phyllodes tumors.
Table 1.
Clinical characteristics of the patients with phyllodes tumor.
| Total No. of cases | 17 |
|---|---|
| Age range (years) | 22- 63 |
| Mean age | 44.06 |
| Gender | Females (n=17) |
| Laterality | Right= 06, Left=11 |
| Duration of a lump in months (mean) | 4.3 |
| Size range (cm) | 3.5- 5.8 |
| Surgical procedure | Wide excision (n=10) Simple mastectomy (n=01) MRM (n=06) |
| Outcome | Local recurrence (n=0), Metastasis (n=1) |
Table 2 shows the correlation between cytological and histological diagnoses of all cases.02 cases were reported as fibroadenoma on cytology and turned out to be benign phyllodes on histology. The correlation was not statistically significant (P=0.48). Considering histopathology as the gold standard, the sensitivity of cytology in diagnosing malignant phyllodes tumors was 100%. The sensitivity and specificity of cytology in diagnosing benign and borderline PT was 87.5% and 100%, respectively.
Table 2.
Cytohistological correlation.
| Cytopathology (n= 17) | Histopathology (n= 17) | ||
|---|---|---|---|
| Benign PT (08) | Borderline PT (03) | Malignant PT (06) | |
| Benign PT = (07) | 06 | 01 | - |
| Fibroadenoma = (02) | 02 | - | - |
| Borderline PT = (02) | - | 02 | - |
| Malignant PT= (04) | - | - | 04 |
| Malignant PT/ Metaplastic carcinoma = (02) | - | - | 02 |
Discussion
Phyllodes tumor was first described by Johannes Muller in 1838 in Germany (7). Fibroadenoma (FA) and benign phyllodes tumor (BPT) share similar morphologic features, as both exhibit dimorphic patterns comprising stromal and epithelial components. Consequently, distinguishing between the two in cytology can be challenging (8). FNA is a commonly used first-line preoperative test in the investigation of palpable breast masses. For diagnosis of breast carcinoma, its sensitivity is reported to be 100% when combined with the clinical and mammography results (9). But its sensitivity ranges from 32% to 77% for diagnosis of phyllodes (10). Malignant phyllodes can be diagnosed on FNAC with the prominent stromal component, marked atypia, pleomorphism, and high mitotic count. The main problem arises in diagnosis of benign and borderline phyllodes cases (11). Phyllodes were considered to be benign, until in 1943, Cooper and Ackerman reported its malignant counterpart (12). In 1981, World Health Organization (WHO) classified these tumors into benign, borderline, and malignant as given in Table 4.
Table 4.
Criteria for phyllodes classification according to WHO (12)
| Criteria | Benign | Borderline | Malignant |
|---|---|---|---|
| Stromal cellularity and atypia | Minimal | Moderate | Marked |
| Stromal overgrowth | Minimal | Moderate | Marked |
| Mitosis/ 10 high power field | 0-4 | 5-9 | ≥10 |
| Tumor margins | Well-circumscribed with pushing tumor margins | Zone of microscopic invasion around tumor margins | Infiltrative tumor margins |
On histopathology, features mentioned in WHO criteria can be evaluated but on FNAC, features like tumor margins cannot be assessed. Also, there might be focal atypia and focal mitotic focus that may be missed on FNAC due to limited sampling. In our study, 88.2% of phyllodes cases showed stromal fragments >5, differentiating fibroadenoma from phyllodes. However, this was not a significant criterion to distinguish between benign, borderline, and malignant cases (p=0.47). Similar results were obtained in studies by Scolyer et al., El Hag et al., Maritz et al., Veneti et al., Jayaram et al., and Bandyopadhyay et al. (6,11,13,14,15,16). Low stromal cellularity was seen in benign PT and all malignant PTs showed high stromal cellularity in our study. In numerous previously reported s studies, presence of hypercellular stroma was one of the most distinguishing features between fibroadenoma and phyllodes. In a study by Krishnamurthy et al. and Maritz et al. approximately 30% of cases of fibroadenomas showed hypercellularity and so they concluded that this feature cannot be used unequivocally for diagnosing phyllodes (4,6).
In the present study, marked stromal atypia was seen in borderline and malignant PTs, while no atypia was seen in the benign PTs category. No significant association was seen between the degree of atypia and categories of phyllodes tumors. In a study by Maritz et al., there was a significant association between stromal atypia in fibroadenomas and phyllodes (6). Stromal atypia was found to be an important feature in studies by Bhattarai et al., Krishnamurthy et al., El Hag et al., and Rao et al. (4,5,11,18).
Shimizu K emphasized the patterns of the epithelial component in differentiating between BPT and fibroadenoma where tubular, monolayered, and blunt branching small to medium-sized fragments favored fibroadenoma and folded and wavy large-sized (> 1mm in diameter) epithelial fragments were seen in BPT (19). In our study, phyllodes tumors mainly showed a wavy pattern with 02 cases of BPT showing monolayered sheets and hence were misdiagnosed as fibroadenoma on FNAC. The behavior of BPT is also unpredictable as some cases may show a recurrence which is largely attributable to incomplete surgical excision (20,21). Rarely it undergoes malignant transformation and hence correct preoperative diagnosis is essential for clinical management (20). In a study by Ashfaq et al., they found that stromal cells in PTs are larger and wavy while in fibroadenomas they are small and round to oval (22). However, they found no significant association between these features and PTs. Another study by Deen et al. also didn’t find any significant difference between in type of stromal fragments and FAs, variants of FAs, and BPTs (23).
In our study, phyllodes tumors showed a lesser number of epithelial fragments, and the stromal-to-epithelial ratio was higher in malignant PTs than in BPTs. However, this difference was not significant (P=0.21). In a study by Bandyopadhyay et al., the S:E ratio was found to be an important factor in distinguishing FAs from PTs, while in a study by Bhattarai et al., it was considered an important feature in distinguishing three grades of PTs (5,16).
In our study, the presence of singly scattered stromal cells showed a significant association in differentiating three grades of phyllodes. The presence of >30% single scattered cells was seen in 84% of malignant PT cases while 02 cases of BPT showed <10% scattered spindle cells. According to Krishnamurthy et al., <10% of spindle cells favor the diagnosis of FA, and >30 % favor PTs, while 10-30% indicate the intermediate zone (4). However, El Hag differed in this cut-off and concluded in their study that, a background of 10% spindled cells rather than 30% would increase sensitivity in diagnosing phyllodes tumors and will reduce the possibility of misdiagnosing PT as FA (11). Our study and study by Maritz et al. (6) favor that cut-off given by Krishnamurthy et al. are better discriminator of these tumors.
Two cases in our study were wrongly diagnosed as fibroadenoma instead of BPT on cytology. This may be because of sampling error as the diagnostic area was focally present in the tumor and may be missed on FNAC. Therefore, a thorough sampling of all breast lesions should be done to improve diagnostic accuracy. Clinical and radiological features alone cannot reliably distinguish fibroadenoma and phyllodes tumor and FNA is frequently used as a first-line investigation for breast lumps (6,24).
It can be difficult to distinguish between a PT and a fibroadenoma using FNA due to overlapping features. Lack of familiarity with cytological features, disease rarity, morphological heterogeneity, and inadequate sampling can lead to poor FNAC outcomes. While individual cytological parameters may not be decisive, when considered together, they can effectively differentiate between the two groups (25).
Kumar PVet al in their study reported that the presence of stromal hypercellularity, bonsai-like epithelial clusters, amorphous pinkish material at the border of stromal fragments, intranuclear inclusions, and popcorn-like nuclei in stromal cells may aid in diagnosing phyllodes tumors (26).
In FNA smears features favoring a malignant PT include high stromal cellularity, prominent stromal nuclear atypia, presence of mitotic figures, scattered single atypical cells, multinucleated tumor giant cells, and heterologous differentiation of sarcomatous stroma with features of osteosarcoma, liposarcoma, chondrosarcoma, or rhabdomyosarcoma (27).
Kuppusamy DA reported a case of a 58-year-old woman who presented with a large breast mass and on FNAC showed features consistent with malignant PT, with prominent areas of heterologous liposarcomatous differentiation. The cytological findings were also confirmed by histology. Authors concluded that malignant PT and its different tissue components can be accurately diagnosed through FNA cytology when performed optimally. This can be crucial for preoperatively assessing patients suspected of having malignancies to plan surgical procedures accordingly (28). Li JJ et al. presented a case of 54-year-old woman with recurrent malignant phyllodes tumor that metastasized to left pleura leading to massive unilateral malignant pleural effusion (29). PTs are rare and difficult to diagnose preoperatively. Grading them pathologically is important for predicting recurrence and survival rates. Benign and borderline PTs have a less aggressive course than malignant PTs. Excision with negative margins is the recommended treatment. The role of adjuvant radiation therapy in borderline and malignant PTs needs further investigation (30).
The main limitation of our study is the low number of cases. This is because of the rarity of PTs, as they constitute only 1 % of breast tumors.1 Another limitation would be the lack of definite criteria to describe atypia, counting stromal and epithelial fragments and counting single scattered stromal cells amongst various studies done so far. Due to the limited amount of material in core biopsies and FNACs, there are difficulties in obtaining a correct diagnosis. To reduce diagnostic inaccuracy, vacuum-assisted breast biopsy (VABB) was introduced in 1995. This helps in more accurate diagnosis and complete removal of the lesions with the help of real-time USG (31).
Table 3 shows cytomorphological characteristics observed in the present study (Figure 1-7). There was no significant association between the number of stromal fragments, atypia, or number of epithelial fragments (P=0.47, P=0.08, P= 0.21 respectively) with benign, borderline, and malignant phyllodes category. Only a statistically significant association was found between singly scattered stromal cells and three categories of phyllodes tumors (P=0.012). On histopathology, stromal metaplasia was observed in only one case of benign phyllodes tumor, which was chondroid differentiation (Figure 8) and malignant heterologous component as fibrosarcomatous differentiation (Figure 9) in one of the malignant PTs. In one case each of the benign and malignant phyllodes tumors ductal carcinoma in situ (DCIS) of its epithelial component was seen.
Table 3.
Details of cytomorphological analysis with the case-wise distribution.
| Histopathology (n= 17) | Cytopathology (n= 17) | Cytologic features | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromal Fragments | ||||||||||||||||||||||||
| Number | Stromal cellularity | Atypia | ||||||||||||||||||||||
| <5 (Few) | >5 (Many) | + | ++ | +++ | - | + | ++ | +++ | ||||||||||||||||
| Benign PT (08) | Benign PT = (06) | - | 06 | 01 | 05 | - | 01 | 05 | - | - | ||||||||||||||
| Fibroadenoma = (02) | - | 02 | 01 | 01 | - | 01 | - | - | - | |||||||||||||||
| Borderline PT (03) | Benign PT = (01) | - | 01 | - | 01 | - | - | 01 | - | - | ||||||||||||||
| Borderline PT = (01) | - | 01 | - | 01 | - | - | - | 01 | - | |||||||||||||||
| Borderline PT/ Malignant PT = (01) | 01 | - | - | - | 01 | - | - | 01 | - | |||||||||||||||
| Malignant PT (06) | Malignant PT (06) | 01 | 05 | - | 02 | 04 | - | - | 02 | 04 | ||||||||||||||
| Histopathology (n= 17) | Cytopathology (n= 17) | Cytologic features | ||||||||||||||||||||||
| Epithelial Fragments | Stromal: Epithelial ratio |
Single scattered stromal cells | Mitosis | |||||||||||||||||||||
| Pattern of epithelial fragments |
Number | SP | S=E | EP | <10% | 10-30% | >30% | 0 | + | ++ | +++ | |||||||||||||
| <5 (Few) |
>5 (Many) |
|||||||||||||||||||||||
| Benign PT (08) | Benign PT = (06) | Folded | 06 | - | 01 | 02 | 03 | - | 06 | - | 06 | - | - | - | ||||||||||
| Fibroadenoma = (02) | Mono-layered | - | 02 | - | - | 02 | 02 | - | - | 02 | - | - | - | |||||||||||
| Borderline PT (03) | Benign PT = (01) | Wavy | - | 01 | - | 01 | - | 01 | - | - | 1 | - | - | - | ||||||||||
| Borderline PT = (01) | Folded | 01 | - | 1 | - | - | - | 01 | - | - | 1 | - | - | |||||||||||
| Borderline PT/ Malignant PT = (01) | Wavy | 01 | - | 1 | - | - | - | - | 01 | - | - | 01 | - | |||||||||||
| Malignant PT (06) | Malignant PT (06) | Wavy | 04 | 02 | 06 | - | - | - | 01 | 05 | - | 01 | 03 | 02 | ||||||||||
PT= Phyllodes Tumor, SP= Stromal Predominant, S=E = Stromal= Epithelial, EP= Epithelial Predominant
Fig. 1.

FNA smears of benign phyllodes tumour showing prominent epithelial and fewer stromal fragments. (H/E 40X).
Fig 7.
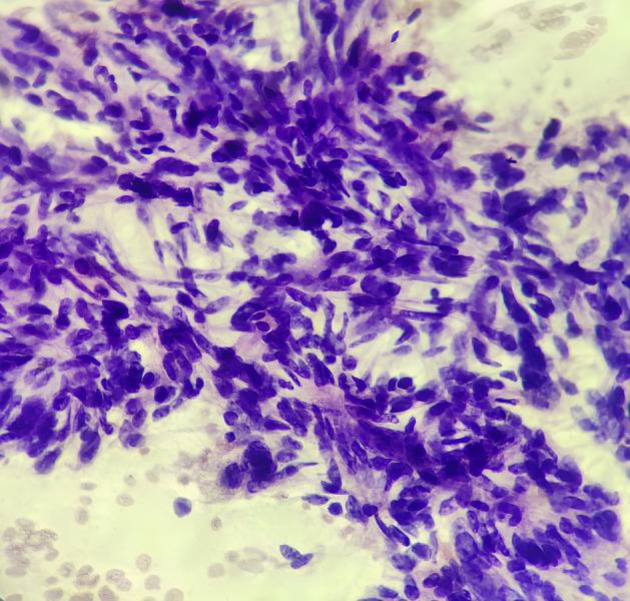
FNA smear of malignant Phyllodes tumor showing highly cellular cluster of malignant stromal cells with nuclear hyperchromasia and pleomorphism. (PAP400X).
Fig 8.

Section from benign Phyllodes tumor showing foci of chondroid differentiation. (H/E 100X)
Fig 9.

Section from Malignant Phyllodes tumor with fibrosarcomatous area. (H/E 100X)
Fig 2.

FNA smears of benign phyllodes tumour showing more than five epithelial fragments and low stromal to epithelial ratio (MGG 40X).
Fig 3.

FNA smears of benign phyllodes tumour showing marked myxoid degeneration in stromal fragments. (MGG 40X).
Fig 4.

FNA smear with moderate stromal cellularity and pleomorphic stromal cells. (H/E200X)
Fig 5.

FNA smear of malignant Phyllodes tumor showing high cellularity with predominantly stromal clusters. (MGG100X).
Fig 6.

FNA smear of malignant Phyllodes tumor showing numerous singly scattered stromal cells. (MGG 200X)
Conclusion
Cytological features of benign phyllodes tumor and fibroadenoma show significant overlap, whereas those of malignant phyllodes tumors are quite characteristic; however, it is challenging to diagnose and subclassify phyllodes tumors correctly on cytology. Moreover, rarity of phyllodes tumors makes the scenario even more difficult. In the present study, only a limited number of cases were studied, which found only the proportion of singly scattered stromal cells to be a statistically significant feature helpful in diagnosing and discriminating between different types of phyllodes tumors. However, there is a conspicuous lack of well-defined cytologic criteria to correctly differentiate the two fibroepithelial tumors that exhibit drastically different clinical behavior. Compared to core needle biopsy, fine needle aspiration (FNA) is a simpler, more affordable, and less invasive procedure. Therefore, further studies with larger sample sizes are needed to enhance the diagnostic accuracy of FNA in identifying phyllodes tumors.
Acknowledgments
None
Ethical Approval
This study had been approved by the ethical committees of UPUMS, Saifai under the following ethical code number IEC-149/2018-19.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Authors' Contributions
Noun
Conflict of Interest
The authors declared no conflict of interest.
References
- 1.Limaiem F, Kashyap S. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing ; 2023 . Phyllodes Tumor of the Breast. [PubMed] [Google Scholar]
- 2.Tummidi S, Kothari K, Agnihotri M, Naik L, Sood P. Fibroadenoma versus phyllodes tumor: a vexing problem revisited! BMC Cancer. 2020;20(1):648 . doi: 10.1186/s12885-020-07129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast. 2007;16:27–37. doi: 10.1016/j.breast.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy S, Ashfaq R, Shin HJ, Sneige N. Distinction of phyllodes tumor from fibroadenoma: a reappraisal of an old problem. Cancer. 2000;90:342–9. [PubMed] [Google Scholar]
- 5.Bhattarai S, Kapila K, Verma K. Phyllodes tumor of the breast: a cytohistologic study of 80 cases. Acta Cytol. 2000;44:790–6. doi: 10.1159/000328563. [DOI] [PubMed] [Google Scholar]
- 6.Maritz RM, Michelow PM. Cytological criteria to distinguish phyllodes tumour of the breast from fibroadenoma. Acta Cytol. 2017;61:418–24. doi: 10.1159/000477573. [DOI] [PubMed] [Google Scholar]
- 7.Fiks A. Cystosarcoma phyllodes of the mammary gland - Müller's tumor For the 180th birthday of Johannes Müller. Virchows Arch A Pathol Anat Histol. 1981;392:1–6. doi: 10.1007/BF00430543. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Korematsu M. Phyllodes Tumor of the Breast A cytomorphologic approach based on evaluation of epithelial cluster architecture. Acta Cytol. 2002;46:332–6. doi: 10.1159/000326730. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman Z, Shiptz B, Shapiro M, Rona R, Lew S, Dinbar A. Triple approach in the diagnosis of dominant breast mass: combined physical examination, mammography, and fine needle aspiration. J Surg Oncol. 1994;56:254–7. doi: 10.1002/jso.2930560413. [DOI] [PubMed] [Google Scholar]
- 10.Jackin RK, Fridgway PF, Ziprin P, Healy V, Hadjiminas A, Darzi A. Optimizing preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol. 2006;59:454–9. doi: 10.1136/jcp.2005.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Hag IA, Aodah A, Kollur SM, Attallah A, Mohamed AAE, Hussaini HA. Cytological clues in the distinction between phyllodes tumor and fibroadenoma. Cancer Cytopathol. 2010;118:33–40. doi: 10.1002/cncy.20057. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Histologic typing of breast tumors. 2nd ed. Geneva: WHO; 1981. [Google Scholar]
- 13.Scolyer RA, McKenzie PR, Achmed D, Lee CS. Can phyllodes tumours of the breast be distinguished from fibroadenomas using fine needle aspiration cytology? Pathology. 2001;33:437–43. doi: 10.1080/00313020120083151. [DOI] [PubMed] [Google Scholar]
- 14.Veneti S, Manek S. Benign phyllodes tumour vs fibroadenoma: FNA cytological differentiation. Cytopathology. 2001;12:321–8. doi: 10.1046/j.1365-2303.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 15.Jayaram G, Sthaneshwar P. Fine-needle aspiration cytology of phyllodes tumors. Diagn Cytopathol. 2002;26:222–7. doi: 10.1002/dc.10085. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay R, Nag D, Mondal SK, Mukhopadhyay S, Roy S, Sinha SK. Distinction of phyllodes tumor from fibroadenoma: cytologists' perspective. J Cytol. 2010;27:59–62. doi: 10.4103/0970-9371.70739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simi U, Moretti D, Iacconi P, Arganini M, Roncella M, Miccoli P, et al. Fine needle aspiration cytopathology of phyllodes tumor: differential diagnosis with fibroadenoma. Acta Cytol. 1988;32:63–6. [PubMed] [Google Scholar]
- 18.Rao CR, Narasimhamurthy NK, Jaganathan K, Mukherjee G, Hazarika D. Cystosarcoma phyllodes: diagnosis by fine needle aspiration cytology. Acta Cytol. 1992;36:203–7. [PubMed] [Google Scholar]
- 19.Shimizu K, Masawa N, Yamada T, Okamoto K, Kanda K. Cytologic evaluation of phyllodes tumours as compared to fibroadenomas of the breast. Acta Cytol. 1994;38:891–7. [PubMed] [Google Scholar]
- 20.Hadju SI, Espinosa MH, Robbins GF. Recurrent cystosarcoma phyllodes: a clinicopathologic study of 32 cases. Cancer. 1976;38:1402–6. doi: 10.1002/1097-0142(197609)38:3<1402::aid-cncr2820380346>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–6. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Ashfaq R, Sneige N, Staerkel G. Cytologic features distinguishing phyllodes tumours from fibroadenomas in fine needle aspirates of the breast: a review of 48 cases. Mod Pathol. 1993;7:27A. [Google Scholar]
- 23.Deen SA, McKee GT, Kissin MW. Differential cytological features of fibroepithelial lesions of the breast. Diagn Cytopathol. 1999;20:53–6. doi: 10.1002/(sici)1097-0339(199902)20:2<53::aid-dc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol. 2002;20:64–71. doi: 10.1046/j.1469-0705.2002.00736.x. [DOI] [PubMed] [Google Scholar]
- 25.Chatura KR, Deeparani T, Patil SB. A revisit of cytological features in phyllodes tumors. J Med Radiol Pathol Surg. 2018;5(6):3–8. [Google Scholar]
- 26.Kumar PV, Mokhtari M. Cytological findings in benign phyllodes tumors. Acta Cytol. 2019;63(1):23–7. doi: 10.1159/000493677. [DOI] [PubMed] [Google Scholar]
- 27.Lissidini G, Mulè A, Santoro A, Papa G, Nicosia L, Cassano E, et al. Malignant phyllodes tumor of the breast: a systematic review. Pathologica. 2022;114(2):111 . doi: 10.32074/1591-951X-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuppusamy DA, Jinkala S, Thambiraj P, Stephen N, Gochhait D, Siddaraju N, et al. Malignant phyllodes tumor with liposarcomatous differentiation: diagnosed on cytology. Diagn Cytopathol. 2021;49(6):E226–30. doi: 10.1002/dc.24681. [DOI] [PubMed] [Google Scholar]
- 29.Li JJ, Chan WC, Chau HH, Wu C, Tse GM. Cytologic diagnosis of metastatic malignant phyllodes tumor of the breast in pleural effusion. Diagn Cytopathol. 2019;47(6):599–602. doi: 10.1002/dc.24151. [DOI] [PubMed] [Google Scholar]
- 30.Rayzah M. Phyllodes tumors of the breast: a literature review. Cureus. 2020;12(9):e10211. doi: 10.7759/cureus.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett IC, Saboo A. The evolving role of vacuum-assisted biopsy of the breast: a progression from fine-needle aspiration biopsy. World J Surg. 2019;43(4):1054–61. doi: 10.1007/s00268-018-04892-x. [DOI] [PubMed] [Google Scholar]


