Abstract
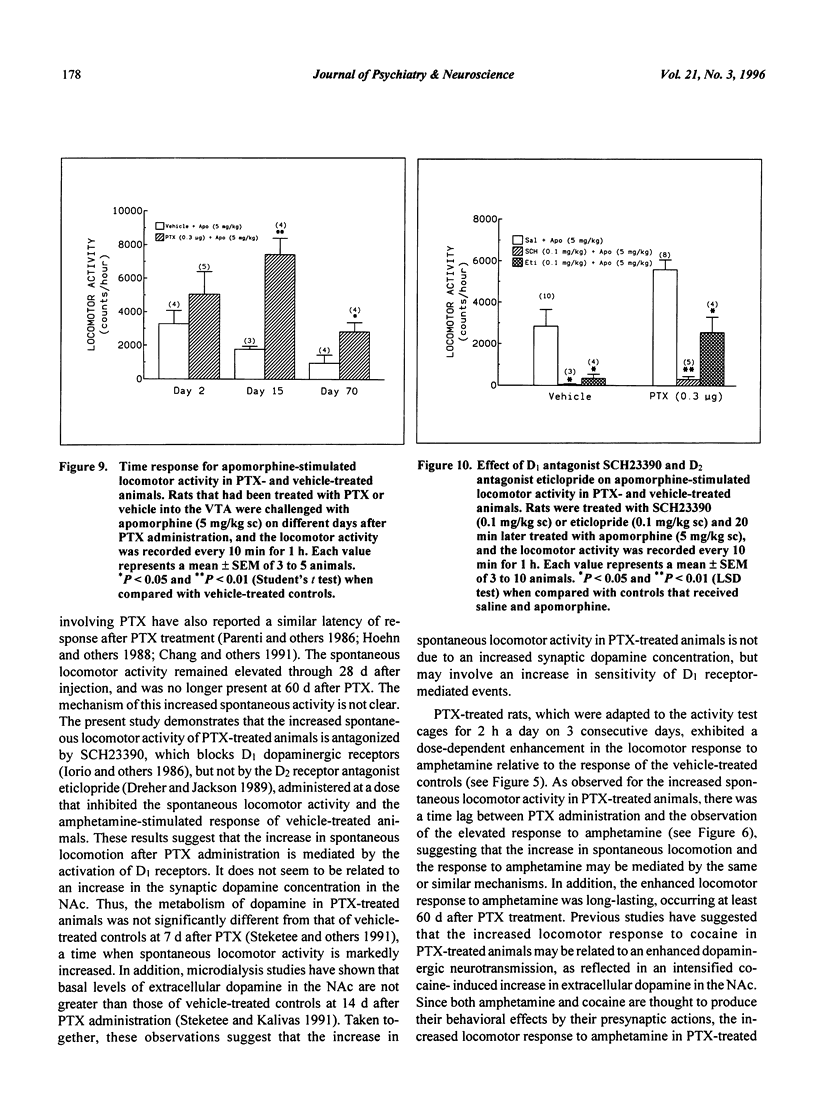
Pertussis toxin (PTX) injected into the ventral tegmental area (VTA) produces an enhanced locomotor response to amphetamine. In the present study, we have evaluated the role of dopamine receptors on spontaneous locomotor activity and the enhanced locomotor response to dopaminergic agonists after the administration of PTX into the VTA. PTX injected into the VTA of rats produced a delayed increase in spontaneous locomotor activity with a latency of 4 d. This activity was markedly increased by day 6 and remained elevated for at least 28 d after PTX treatment. This increased spontaneous locomotor activity of PTX-treated animals was antagonized by the administration of the D1 receptor antagonist SCH23390 (0.03 and 0.1 mg/kg sc), but not by the D2 receptor antagonist eticlopride (0.1 and 0.3 mg/kg sc). After adaptation to the locomotor cages, the animals showed a markedly enhanced motor response to amphetamine (0.5 mg/kg ip) and apomorphine (5 mg/kg sc). The heightened locomotor responses to these dopaminergic agonists could be elicited for at least 2 mo after PTX administration. The enhanced response to amphetamine was antagonized by the administration of SCH23390 (0.03 and 0.1 mg/kg sc), but not by eticlopride (0.1 mg/kg). The increased response to apomorphine in PTX-treated animals was inhibited by SCH23390 (0.1 mg/kg sc) and partially inhibited by eticlopride (0.1 mg/kg sc). Both of these antagonists inhibited the spontaneous and the drug-induced locomotor responses in vehicle-treated control animals. These results suggest that the administration of PTX into the VTA leads to an increase in spontaneous and drug-induced locomotor activity in which D1 receptors seem to play an important role.
Full text
PDF

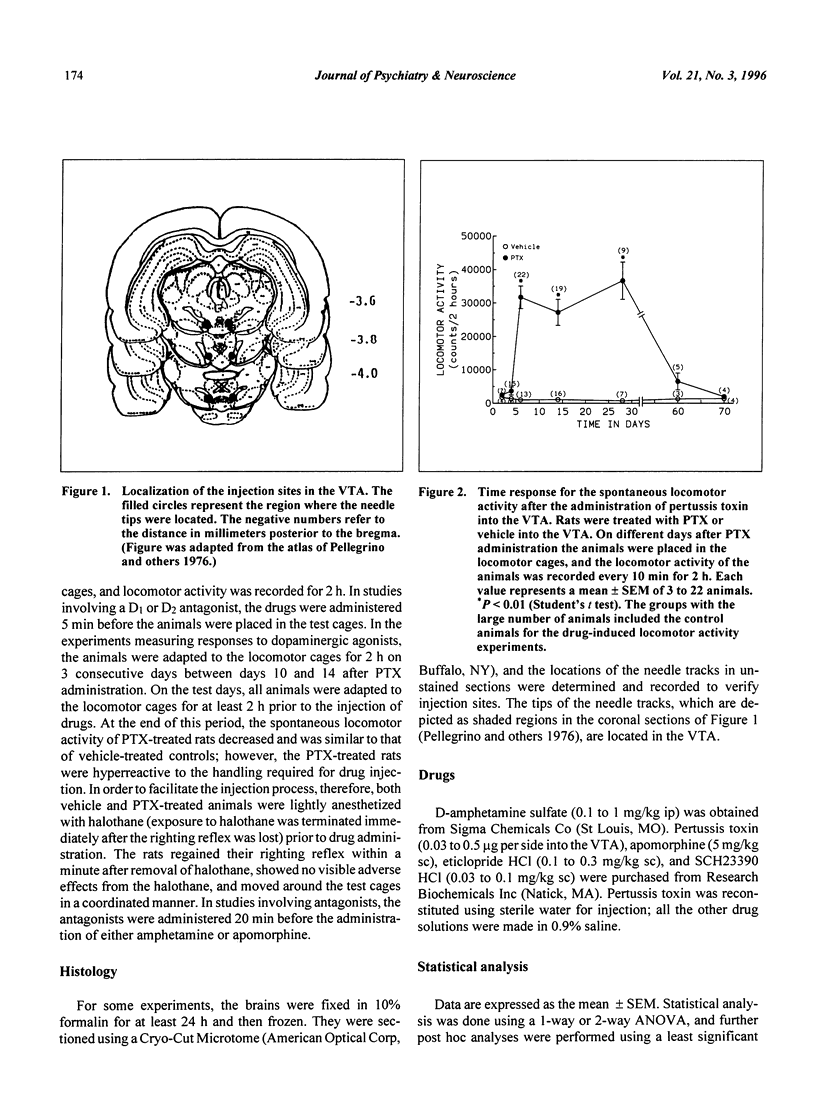
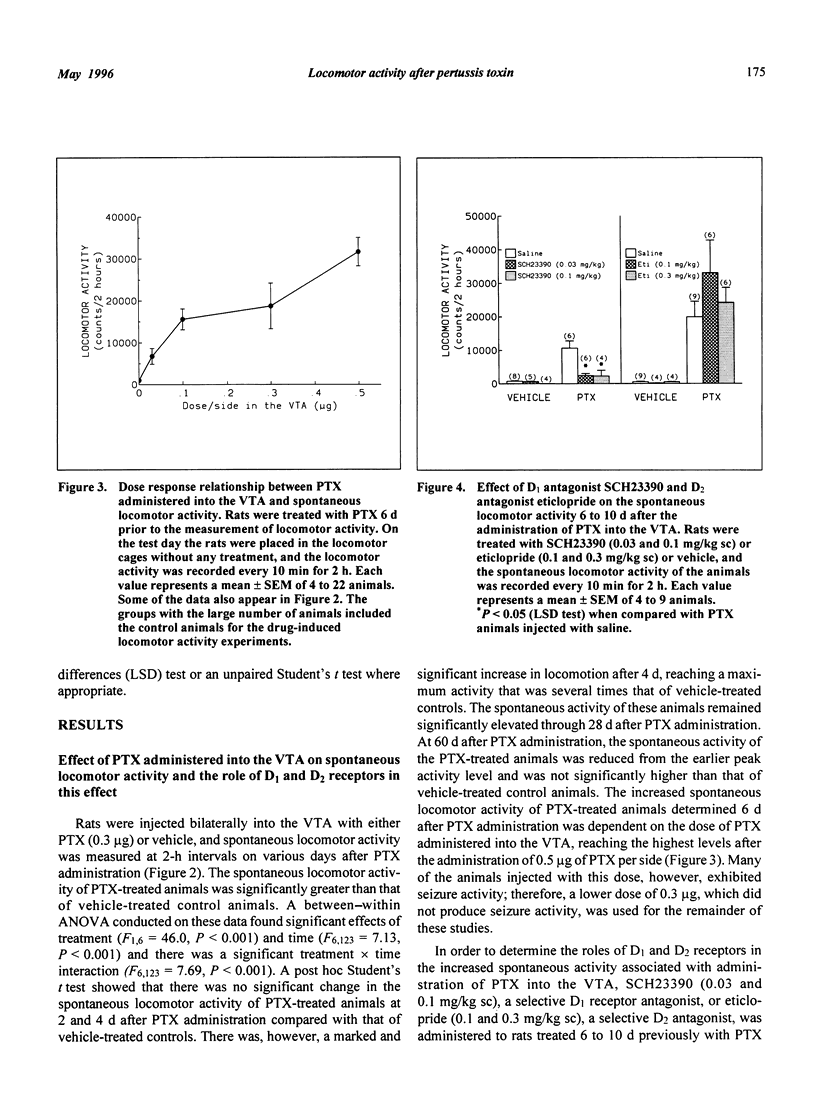
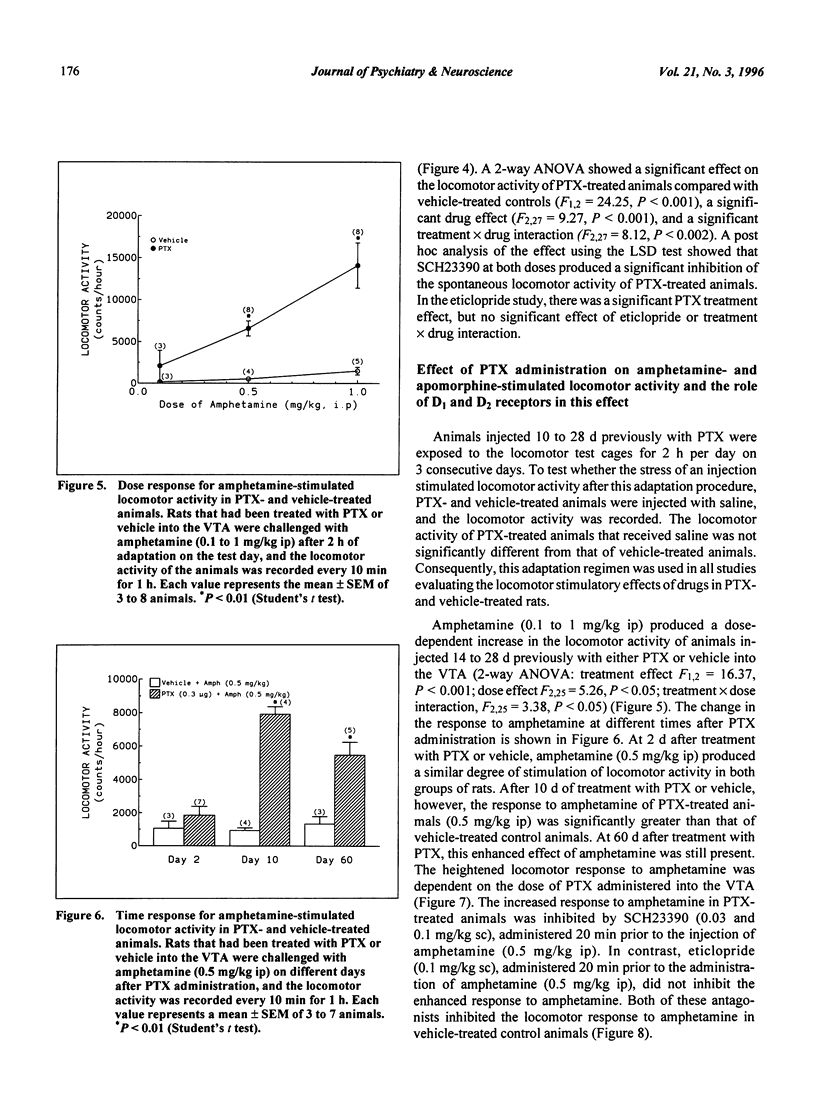
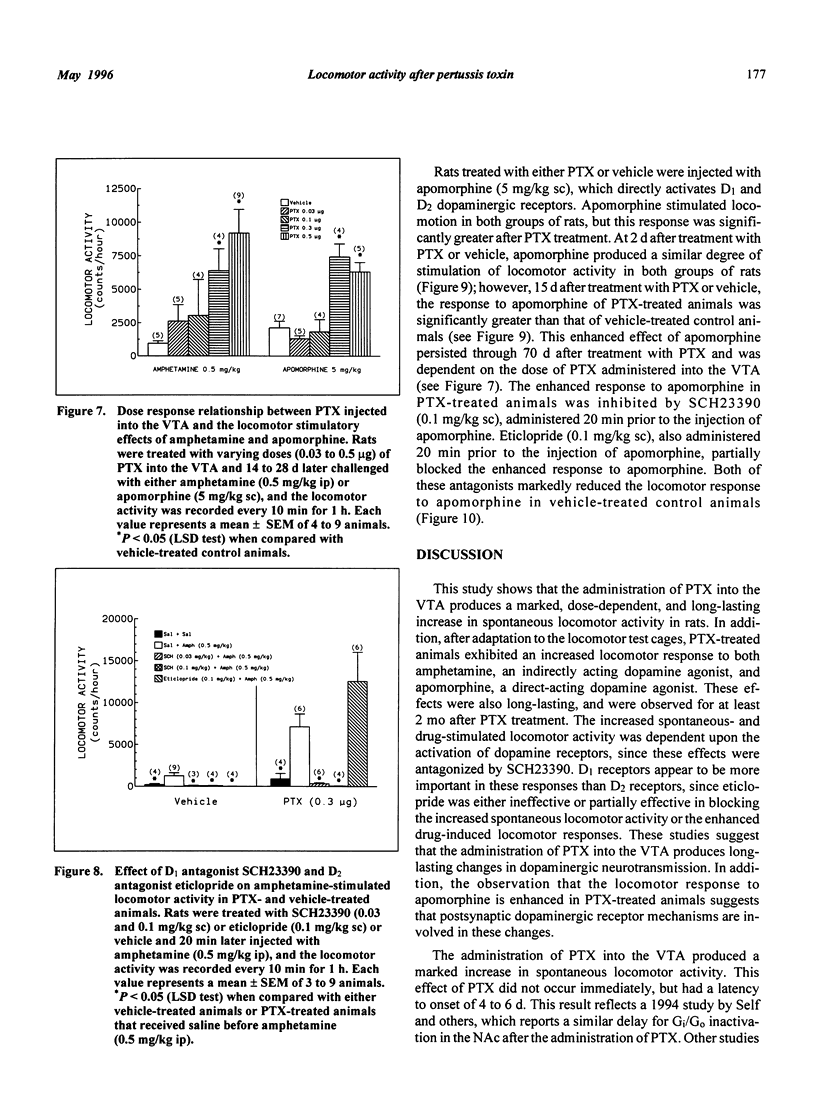


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. C., Lutfy K., Sierra V., Yoburn B. C. Dissociation of opioid receptor upregulation and functional supersensitivity. Pharmacol Biochem Behav. 1991 Apr;38(4):853–859. doi: 10.1016/0091-3057(91)90253-x. [DOI] [PubMed] [Google Scholar]
- Dreher J. K., Jackson D. M. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989 May 22;487(2):267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Hoehn K., Reid A., Sawynok J. Pertussis toxin inhibits antinociception produced by intrathecal injection of morphine, noradrenaline and baclofen. Eur J Pharmacol. 1988 Jan 27;146(1):65–72. doi: 10.1016/0014-2999(88)90487-6. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Aghajanian G. K. Pertussis toxin blocks autoreceptor-mediated inhibition of dopaminergic neurons in rat substantia nigra. Brain Res. 1987 May 12;411(1):139–143. doi: 10.1016/0006-8993(87)90690-1. [DOI] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Billard W., Gold E. H. Benzazepines: structure-activity relationships between D1 receptor blockade and selected pharmacological effects. Adv Exp Med Biol. 1986;204:1–14. doi: 10.1007/978-1-4684-5191-7_1. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988 Jun;245(3):1095–1102. [PubMed] [Google Scholar]
- Nestler E. J., Terwilliger R. Z., Walker J. R., Sevarino K. A., Duman R. S. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990 Sep;55(3):1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Parenti M., Tirone F., Giagnoni G., Pecora N., Parolaro D. Pertussis toxin inhibits the antinociceptive action of morphine in the rat. Eur J Pharmacol. 1986 May 27;124(3):357–359. doi: 10.1016/0014-2999(86)90240-2. [DOI] [PubMed] [Google Scholar]
- Paulson P. E., Robinson T. E. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequent intra-accumbens amphetamine challenge in rats. Psychopharmacology (Berl) 1991;104(1):140–141. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. E., Becker J. B. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986 Jun;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Self D. W., Terwilliger R. Z., Nestler E. J., Stein L. Inactivation of Gi and G(o) proteins in nucleus accumbens reduces both cocaine and heroin reinforcement. J Neurosci. 1994 Oct;14(10):6239–6247. doi: 10.1523/JNEUROSCI.14-10-06239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee J. D., Kalivas P. W. Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region. J Pharmacol Exp Ther. 1991 Nov;259(2):916–924. [PubMed] [Google Scholar]
- Steketee J. D., Striplin C. D., Murray T. F., Kalivas P. W. Possible role for G-proteins in behavioral sensitization to cocaine. Brain Res. 1991 Apr 5;545(1-2):287–291. doi: 10.1016/0006-8993(91)91299-g. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Koob G. F. The neural substrates of apomorphine-stimulated locomotor activity following denervation of the nucleus accumbens. Life Sci. 1984 Dec 17;35(25):2537–2544. doi: 10.1016/0024-3205(84)90440-5. [DOI] [PubMed] [Google Scholar]
- Wolf M. E., White F. J., Nassar R., Brooderson R. J., Khansa M. R. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993 Jan;264(1):249–255. [PubMed] [Google Scholar]
- van der Ploeg I., Cintra A., Altiok N., Askelöf P., Fuxe K., Fredholm B. B. Limited distribution of pertussis toxin in rat brain after injection into the lateral cerebral ventricles. Neuroscience. 1991;44(1):205–214. doi: 10.1016/0306-4522(91)90261-l. [DOI] [PubMed] [Google Scholar]


