Abstract
Omalizumab is a biologic agent used in the management of allergic conditions, including asthma and urticaria. Although the efficacy of omalizumab has been well established, its safety profile is primarily derived from clinical trials with limited sample sizes. To conduct a comprehensive evaluation of its safety in larger populations, this study conducted an extensive analysis of data sourced from the America Food and Drug Administration’s Adverse Event Reporting System (FAERS), with the aim of elucidating adverse drug events associated with omalizumab in real-world settings. We extracted reports of adverse events associated with omalizumab from the FAERS database covering the period from the first quarter of 2004 to the second quarter of 2024. We assessed the significance of the association between omalizumab and adverse events using four distinct methods of disproportionality analysis. Furthermore, we analyzed adverse events across gender and age subgroups. We identified a total of 49,456 adverse event reports linked to omalizumab and pinpointed 357 adverse events related to omalizumab within 27 system organ classes. These adverse events encompassed several commonly reported reactions documented in the product labeling, including anaphylactic reactions (ROR: 17.28, 95%CI:16.62–17.96) and asthma (ROR:19.24, 95%CI:18.74–19.76), alongside unlisted reactions such as asthmatic crisis (ROR: 47.3, 95%CI: 43–52.03), lower respiratory tract congestion (ROR: 35.68, 95%CI: 30.42–41.84). Furthermore, the results of our analysis indicated that omalizumab-related adverse events displayed significant gender and age disparities. The median time to onset for all documented adverse events was approximately 145 days, with a substantial proportion occurring after one year of treatment. This study not only offers a significant reference for optimizing the utilization of omalizumab, enhancing its efficacy while minimizing potential side effects, but also facilitates the safe application and broader implementation of omalizumab in clinical practice.
Keywords: Omalizumab, Adverse drug event, FAERS, Real-world study
Subject terms: Drug discovery, Medical research
Introduction
Omalizumab is a humanized monoclonal antibody that specifically binds to free Immunoglobulin E (IgE), forming immune complexes1. This mechanism blocks the interaction of IgE with its receptors on mast cells and eosinophils, subsequently decreasing the release of inflammatory mediators and mitigating the allergic response2,3. Omalizumab was approved by the America Food and Drug Administration (FDA) in 2003 for the initial treatment of moderate to severe allergic asthma in both children and adults aged 6 years and older, which is mediated by IgE. The indication was expanded in 2014 to encompass chronic urticaria in individuals aged 12 years and older, and subsequently approved in 2020 for the treatment of adult nasal polyps. Recently, in 2024, it gained approval for the treatment of IgE-mediated food allergies. Furthermore, Omalizumab has shown efficacy in treating moderate to severe asthma, allergic rhinitis4, and various other allergic conditions, including atopic dermatitis5.
In recent years, alterations in environmental factors, climate conditions, and lifestyle have contributed to a substantial surge in the global incidence and prevalence of allergic diseases6. The number of diagnosed cases has significantly increased over time, impacting approximately 10–30% of the global population7–9. Asthma stands as the most prominent challenge among allergic diseases, affecting approximately 300 million individuals worldwide. Each year, over 400,000 fatalities are attributed to asthma6, highlighting its profound impact on an individual’s overall life course. Allergic diseases, exemplified by asthma, not only endanger lives but also entail substantial direct and indirect healthcare costs10. Currently, approximately 5%-10% of asthma patients suffer from severe asthma, and omalizumab is the first biologic agent recognized by the Global Initiative on Asthma (GINA) for the treatment of severe allergic asthma mediated by IgE11. The study indicated that omalizumab significantly reduces the incidence of severe asthma, enhances lung function, decreases corticosteroid usage, and enhances overall quality of life11,12. As the utilization of omalizumab increases, there has been a corresponding surge in reports of adverse drug events (ADEs) associated with its use. Previous studies have identified headache, injection site reactions, joint pain, dizziness, and upper abdominal pain as the most commonly reported ADEs associated with omalizumab13–15. These reports primarily stem from clinical trials and meta-analyses. However, due to limited sample sizes and specific selection criteria, they may not fully reflect the safety profile of omalizumab. Therefore, it is imperative to utilize larger sample sizes and real-world data for a comprehensive assessment of the safety of omalizumab. Furthermore, clinicians must possess a comprehensive understanding of these potential adverse reactions and mitigate their impact through rigorous monitoring and prompt management16.
The FDA Adverse Event Reporting System (FAERS) is a crucial tool for post-marketing drug safety surveillance17, collecting real-time ADE data submitted by healthcare professionals, consumers, and other stakeholders, with frequent updates to its database. This information is easily accessible to the public through the FDA website18. Given the scarcity of evidence concerning ADEs associated with omalizumab in real-world settings, we conducted a comprehensive study to assess its system-specific side effects, temporal distribution of occurrences, and gender-specific variations by analyzing FAERS data on ADE reports related to omalizumab from the first quarter of 2004 to the second quarter of 2024. This study aims not only to provide scientific evidence that allows clinicians and healthcare decision-makers to more accurately monitor and address potential adverse reactions associated with omalizumab, but also to offer valuable recommendations for its safe use in clinical practice, thus ensuring patient safety and promoting rational drug decision-making.
Materials and methods
Data source
The data used in this investigation were sourced from the FDA’s FAERS database, which is publicly accessible and contains de-identified information. Given the anonymized nature of the data, ethical approval was not required for this study, which began gathering information on adverse events associated with marketed drugs in 2004. The database releases new data on a quarterly basis and grants open access to the public. This resource allows the FDA to conduct post-marketing safety surveillance of drugs and therapeutic biological products. The FAERS database consists of seven distinct datasets that cover various types of information, including demographic and management details (DEMO), drug specifics (DRUG), adverse event coding (REAC), patient outcomes (OUTC), reporting sources (RPSR), treatment start and end dates for drugs (THER), as well as indications for drug usage (INDI).
Data processing
Data from the FAERS database were extracted from the first quarter of 2004 to the second quarter of 2024. The downloaded XML data package was imported into a MongoDB database and then cleaned using a dedicated program. A search was conducted using the trade name “XOLAIR” and the generic name “Omalizumab” to retrieve information specifically related to the primary suspected ADE associated with omalizumab. The ADEs reported in the study were coded and classified using the preferred term (PT) and system organ class (SOC) from the International Medical Dictionary for Regulatory Activities (MedDRA), and the SOCs of the ADE signals were analyzed19.
Statistical analysis
The ADE signal detection was assessed using the method of disproportionate analysis. This tool facilitates the preliminary evaluation of potential causal relationships between a drug and an adverse reaction, necessitating subsequent comprehensive clinical assessments of the relevant case reports20. It compares the actual observed report count for a specific drug-adverse event combination with the anticipated report count, and is widely used for alertness analysis regarding adverse drug reactions within extensive spontaneous reporting databases21. The primary approaches used included the reporting odds ratio (ROR), proportional report ratio (PRR). Both the ROR algorithm and the PRR algorithm fall under the category of frequency-based algorithms. The ROR algorithm effectively mitigates bias arising from underreported specific events22, while the PRR algorithm is less susceptible to interference from omitted adverse events during processing23. Overall, frequency-based methods are noted for their computational simplicity and high sensitivity. However, in scenarios with sparse adverse event data, the risk of false positives significantly increases24. Bayesian confidence interval neural network (BCPNN), and Multi-item gamma Poisson shrinker (MGPS). BCPNN excels in integrating diverse data sources and conducting cross-validation, while MGPS has a distinct advantage in detecting signals from rare events. The Bayesian method is renowned for its robustness, as it can effectively account for the uncertainty in ratios when the number of reports is limited, thereby reducing false positives and enabling pattern recognition in more complex dimensions25. Since each algorithm has its own strengths and limitations, we combined multiple methods to broaden the scope of detection and validate the findings from diverse perspectives, aiming to produce more reliable signal detection outcomes18,26,27. We selected PTs with a minimum reporting count of ≥ 3 for initial screening22. The specific thresholds and formulas for each method are detailed in Supplementary Table 1 and Table 2.
We conducted an analysis of the time to onset (TTO) of ADEs associated with omalizumab, defined as the interval between the initiation of treatment (as indicated by START_DT in the THER file) and the documented occurrence of ADEs (recorded in EVENT_DT within the DEMO file). In our data handling process, we excluded records that contained inaccurate or missing date information, as well as those where ADEs were reported prior to the commencement of treatment. To explore temporal variations in ADE incidence, we used the Weibull test method to develop a robust analytical model. We conducted univariate and multivariate logistic regression analyses, using gender, age, weight, and ADEs onset time as exposures, and considering important medical events such as death, life-threatening events, hospitalization, and disability as outcomes. Statistical significance was set at a two-sided P-value < 0.05.
The R software (version 4.2.1) is primarily used for data processing and analysis, with the ggplot2 package being used for data visualization.
Results
Descriptive analysis
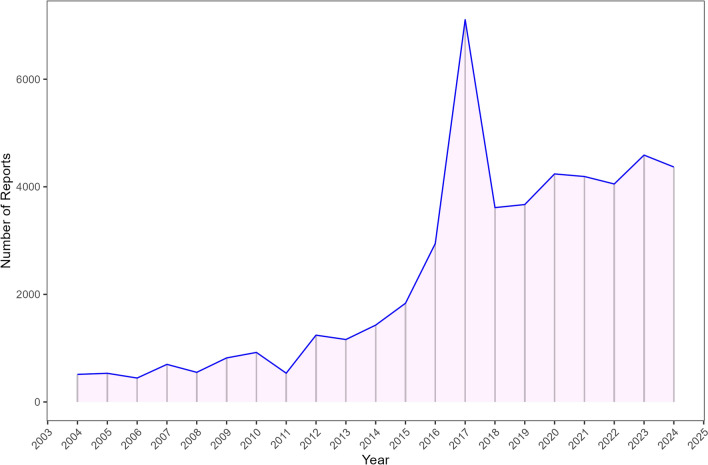
After excluding duplicate reports, a total of 49,456 ADE reports were identified, implicating omalizumab as the primary suspected drug. The selection process is illustrated in Fig. 1. From 2004 to 2024, the number of ADE reports related to omalizumab showed a steady increase, with the most recent data available from the second quarter of 2024 (Fig. 2).
Fig. 1.
The process of selecting omalizumab-associated ADEs from FAERS database.
Fig. 2.
The annual distribution of omalizumab-related ADEs reports from 2004 to 2024.
The ADE characteristics associated with omalizumab are presented in Table 1. Notably, the proportion of females affected (65.7%) significantly exceeds that of males (24.5%), indicating a gender disparity in ADE reports. The highest proportion of ADE reports is observed within the age group of 16–64.9 years (33.4%). Furthermore, the most prevalent weight range is 50–100 kg, accounting for 13% of ADE cases. Consumer reports constitute the majority of ADE notifications (43%), followed by reports from medical professionals (35.6%). Among the top five countries reporting ADEs, the United States exhibits the highest reporting rate (67.07%), followed by Canada (11.2%), Switzerland (3.2%), Japan (2.0%), and Brazil (1.5%). Regarding ADE outcomes, ‘other outcomes’ represent the largest proportion (30.11%), followed by hospitalization (16.7%), death (4.0%), life-threatening ADEs (2.4%), and disability (0.8%). Asthma stands as the most common indication for omalizumab use, accounting for 46.2% of ADE cases; additionally, records indicating unknown usage account for a further 20.9% of ADE reports.
Table 1.
Clinical characteristics of reports with omalizumab from the FAERS database.
| Characteristics | Case number, n | Case Proportion, % |
|---|---|---|
| Number of events | ||
| Gender | ||
| Female | 32,485 | 65.7 |
| Male | 12,118 | 24.5 |
| Missing | 4853 | 9.8 |
| Age | ||
| < 18 | 2048 | 4.1 |
| 18–64.9 | 16,516 | 33.4 |
| 65–85 | 4143 | 8.4 |
| > 85 | 197 | 0.4 |
| Missing | 26,552 | 53.7 |
| Weight | ||
| < 50 kg | 756 | 1.5 |
| 50–100 kg | 6440 | 13.0 |
| > 100 kg | 1707 | 3.5 |
| Missing | 40,553 | 82.0 |
| Outcomes | ||
| Death (DE) | 1954 | 4.0 |
| Hospitalization(HO) | 8249 | 16.7 |
| Disability (DS) | 380 | 0.8 |
| Life-Threatening (LT) | 1189 | 2.4 |
| Other Serious | 14,890 | 30.11 |
| Missing | 22,794 | 46.1 |
| Reported Person(Top five) | ||
| Health professional | 3485 | 7.0 |
| Consumer | 21,244 | 43 |
| Physician | 17,631 | 35.6 |
| Other health-professional | 5188 | 10.5 |
| Pharmacist | 1500 | 3.0 |
| Reported countries(Top five) | ||
| America | 33,177 | 67.08 |
| Canada | 5534 | 11.2 |
| Switzerland | 1605 | 3.2 |
| Japan | 978 | 2.0 |
| Brazil | 726 | 1.5 |
| Indications (Top five) | ||
| Asthma | 22,836 | 46.2 |
| Chronic spontaneous urticaria | 4886 | 9.9 |
| Idiopathic urticaria | 4583 | 9.3 |
| Product used for unknown | 10,331 | 20.9 |
| Urticaria | 2788 | 5.6 |
Signal of system organ class
Supplementary Table 3 presents the signal strength of ADEs associated with omalizumab based on SOCs classification. The statistical findings indicate that omalizumab’s adverse effects have impacted a total of 27 SOCs. Figure 3A illustrates the distribution of ADE reports across the 27 SOCs. Using the minimum criteria of meeting at least one method, a total of 8 SOCs were selected. We generated Fig. 3B as a forest plot based on the results of the ROR algorithm, where the SOCs meeting the positive signal criterion are highlighted in red font. They include: respiratory, thoracic and mediastinal disorders (ROR:4,ROR:4,95%CI:3.95–4.05), skin and subcutaneous tissue disorders (ROR:1.67,95%CI:1.65–1.70), infections and infestations (ROR:1.54, 95%CI:1.51–156), immune system disorders (ROR:3.61,95%CI:3.53–3.69), investigations (ROR:1.19,95%CI:1.17–1.21),general disorders and administration site conditions (ROR:1.09,95%CI:1.07–1.1) , pregnancy, puerperium and perinatal conditions (ROR:l.21,95%CI:1.14–1.29), and ear and labyrinth disorders(ROR:1.36,95%CI:1.28–1.44).
Fig. 3.
Signals detection at the SOC level. (A) The bar chart displays the reported cases of ADEs at each SOC level. (B) Forest plot of SOC level signal detection. ADEs, adverse drug events;SOC, System Organ Class; ROR, reporting odds ratio.
Signal of preferred terms and subgroup analysis
To minimize the false positive rate, we incorporated results from all four assessment methods that yielded positive outcomes, culminating in a total of 357 ADEs (Supplementary Table 4) associated with omalizumab, which corresponded to 21 SOCs. Table 2 presents the results of PT signals occurring at least 100 times in association with omalizumab, encompassing a total of 74 PTs, which correspond to 12 SOCs. Furthermore, we identified specific ADEs associated with omalizumab that are not explicitly stated in the product labeling.
Table 2.
Signal strength of reports of omalizumab at the PT level in the FAERS database.
| Soc name | PTs | Case Numbers |
ROR (95%Cl) |
PRR χ2 |
EBGM (EBGM05) |
IC(IC025) |
|---|---|---|---|---|---|---|
| Ear and labyrinth disorders | Ear pain | 235 |
3.68 (3.24–4.19) |
3.68 (452.21) |
3.64 (3.27) |
1.86 (0.2) |
| Gastrointestinal disorders | Lip swelling* | 439 |
4.05 (3.68–4.45) |
4.04 (989.59) |
3.99 (3.69) |
2 (0.33) |
| Paraesthesia oral* | 161 |
3.45 (2.95–4.03) |
3.45 (276.24) |
3.42 (3) |
1.77 (0.11) |
|
| General disorders and administration site conditions | No adverse event | 5316 |
10.82 (10.52–11.13) |
10.56 (44,336.67) | 10.19 (9.95) |
3.35 (1.68) |
| Chest discomfort | 1459 |
4.48 (4.25–4.72) |
4.45 (3848.43) |
4.4 (4.21) |
2.14 (0.47) |
|
| Sensitivity to weather change* | 355 |
23.88 (21.42–26.62) |
23.84 (7121.04) | 21.94 (20.03) |
4.46 (2.79) |
|
| Secretion discharge* | 263 |
7.11 (6.29–8.04) |
7.1 (1343.03) |
6.94 (6.27) |
2.8 (1.13) |
|
| Temperature intolerance* | 127 |
3.93 (3.3–4.68) |
3.92 (272.73) |
3.88 (3.35) |
1.96 (0.29) |
|
| Concomitant disease aggravated* | 110 |
5.24 (4.34–6.33) |
5.24 (369.99) |
5.16 (4.4) |
2.37 (0.7) |
|
| Immune system disorders | Anaphylactic reaction | 2768 |
17.28 (16.62–17.96) |
17.05 (39,302.94) | 16.07 (15.56) |
4.01 (2.34) |
| Hypersensitivity | 1992 |
3.3 (3.15–3.45) |
3.27 (3116.19) |
3.25 (3.13) |
1.7 (0.03) |
|
| Anaphylactic shock | 434 |
5.42 (4.93–5.96) |
5.41 (1528.68) |
5.32 (4.91) |
2.41 (0.75) |
|
| Seasonal allergy* | 325 |
7.71 (6.9–8.61) |
7.7 (1839.68) |
7.5 (6.84) |
2.91 (1.24) |
|
| Food allergy | 192 |
9.32 (8.07–10.76) |
9.31 (1375.05) |
9.02 (8) |
3.17 (1.51) |
|
| Serum sickness | 116 |
12.81 (10.63–15.43) |
12.8 (1203.12) | 12.25 (10.48) |
3.61 (1.95) |
|
| Anaphylactoid reaction* | 107 |
7.94 (6.55–9.62) |
7.93 (629.35) |
7.73 (6.58) |
2.95 (1.28) |
|
| Perfume sensitivity* | 101 |
78.15 (62.58–97.6) |
78.11 (5923.55) | 60.41 (50.16) |
5.92 (4.25) |
|
| Infections and infestations | Nasopharyngitis | 1971 |
3.35 (3.21–3.51) |
3.33 (3182.6) |
3.3 (3.18) |
1.72 (0.06) |
| Influenza* | 1344 |
3.91 (3.7–4.12) |
3.89 (2846.17) |
3.85 (3.68) |
1.94 (0.28) |
|
| Sinusitis | 1103 |
3.28 (3.09–3.48) |
3.27 (1715.39) |
3.24 (3.08) |
1.7 (0.03) |
|
| Bronchitis | 1094 |
4.39 (4.13–4.66) |
4.37 (2799.34) |
4.31 (4.1) |
2.11 (0.44) |
|
| Lower respiratory tract infection* | 616 |
4.49 (4.14–4.86) |
4.48 (1636.87) |
4.42 (4.13) |
2.14 (0.48) |
|
| Respiratory tract infection | 341 |
4.22 (3.79–4.7) |
4.21 (822.69) |
4.16 (3.81) |
2.06 (0.39) |
|
| Rhinitis* | 153 |
5.91 (5.03–6.94) |
5.9 (609.71) |
5.8 (5.07) |
2.54 (0.87) |
|
| Injury, poisoning and procedural complications | Intercepted product storage error* | 181 |
23.38 (20.08–27.23) |
23.36 (3557.75) | 21.53 (18.96) |
4.43 (2.76) |
| Intercepted medication error* | 162 |
10.03 (8.57–11.74) |
10.02 (1267.67) |
9.69 (8.5) |
3.28 (1.61) |
|
| Scratch* | 139 |
4.63 (3.91–5.47) |
4.62 (387.86) |
4.56 (3.96) |
2.19 (0.52) |
|
| Ligament sprain* | 132 |
4.17 (3.51–4.95) |
4.17 (312.61) |
4.12 (3.56) |
2.04 (0.38) |
|
| Maternal exposure timing unspecified* | 102 |
9.79 (8.03–11.92) |
9.78 (775.21) |
9.47 (8.02) |
3.24 (1.58) |
|
| Investigations | Heart rate increased* | 1115 |
3.43 (3.24–3.64) |
3.42 (1888.25) |
3.39 (3.23) |
1.76 (0.1) |
| Forced expiratory volume decreased* | 792 |
132.61 (121.74–144.45) |
132.08 (68,517.51) | 88.17 (82.08) |
6.46 (4.8) |
|
| Blood pressure systolic increased | 699 |
11.12 (10.31–11.99) |
11.08 (6153.54) | 10.67 (10.02) |
3.42 (1.75) |
|
| Body temperature decreased* | 496 |
15.22 (13.9–16.66) |
15.18 (6211.62) | 14.4 (13.35) |
3.85 (2.18) |
|
| Blood immunoglobulin E increased* | 443 |
71.89 (64.72–79.87) |
71.74 (24,261.78) | 56.54 (51.78) |
5.82 (4.15) |
|
| Respiratory rate increased* | 349 |
12.33 (11.08–13.73) |
12.31 (3465.31) | 11.81 (10.79) |
3.56 (1.9) |
|
| Breath sounds abnormal* | 215 |
14.33 (12.49–16.44) |
14.31 (2524.77) | 13.62 (12.15) |
3.77 (2.1) |
|
| Pulmonary function test decreased* | 166 |
11.55 (9.89–13.5) |
11.54 (1531.33) |
11.1 (9.74) |
3.47 (1.81) |
|
| Eosinophil count increased | 129 |
4.78 (4.01–5.69) |
4.77 (378.05) |
4.71 (4.07) |
2.23 (0.57) |
|
| Forced vital capacity decreased* | 105 |
65.11 (52.58–80.63) |
65.08 (5307.36) | 52.33 (43.76) |
5.71 (4.04) |
|
| Pregnancy, puerperium and perinatal conditions | Pregnancy | 389 |
5.57 (5.03–6.15) |
5.56 (1423.93) |
5.46 (5.02) |
2.45 (0.78) |
| Product issues | Needle issue | 287 |
3.49 (3.1–3.92) |
3.49 (502.1) |
3.45 (3.13) |
1.79 (0.12) |
| Psychiatric disorders | Middle insomnia* | 579 |
9.94 (9.15–10.8) |
9.91 (4472.6) |
9.59 (8.94) |
3.26 (1.6) |
| Respiratory, thoracic and mediastinal disorders | Asthma | 5952 |
19.24 (18.74–19.76) |
18.7 (93,230.94) | 17.52 (17.13) |
4.13 (2.47) |
| Cough | 3013 |
3.38 (3.26–3.51) |
3.35 (4922.23) |
3.32 (3.22) |
1.73 (0.06) |
|
| Wheezing* | 2199 |
12.63 (12.1–13.19) |
12.5 (22,231.25) | 11.98 (11.56) |
3.58 (1.92) |
|
| Productive cough* | 1163 |
8.09 (7.63–8.58) |
8.05 (6969.12) |
7.84 (7.46) |
2.97 (1.3) |
|
| Oropharyngeal pain | 980 |
3.25 (3.05–3.46) |
3.24 (1497.77) |
3.21 (3.04) |
1.68 (0.02) |
|
| Nasal congestion | 913 |
4.99 (4.67–5.33) |
4.97 (2843.22) |
4.9 (4.63) |
2.29 (0.63) |
|
| Rhinorrhoea* | 732 |
3.56 (3.31–3.83) |
3.56 (1327.58) |
3.52 (3.31) |
1.82 (0.15) |
|
| Sputum discoloured* | 611 |
21.33 (19.64–23.17) |
21.27 (10,918.71) | 19.75 (18.43) |
4.3 (2.64) |
|
| Obstructive airways disorder* | 602 |
17.39 (16.01—18.89) |
17.34 (8696.77) | 16.33 (15.24) |
4.03 (2.36) |
|
| Dyspnoea exertional* | 532 |
4.48 (4.12–4.89) |
4.48 (1412.64) |
4.42 (4.11) |
2.14 (0.48) |
|
| Asthmatic crisis* | 500 |
47.3 (43–52.03) |
47.19 (19,157.24) | 40.14 (37.06) |
5.33 (3.66) |
|
| Respiratory disorder* | 489 |
5.03 (4.59–5.5) |
5.02 (1543.23) |
4.94 (4.58) |
2.3 (0.64) |
|
| Throat tightness* | 477 |
5.43 (4.96–5.94) |
5.42 (1683.89) |
5.33 (4.94) |
2.41 (0.75) |
|
| Respiratory tract congestion* | 386 |
7.72 (6.97–8.54) |
7.7 (2188.48) |
7.51 (6.9) |
2.91 (1.24) |
|
| Bronchospasm | 350 |
7.4 (6.65–8.23) |
7.39 (1880.26) |
7.21 (6.6) |
2.85 (1.18) |
|
| Sneezing* | 284 |
3.98 (3.54–4.48) |
3.98 (623.4) |
3.93 (3.56) |
1.98 (0.31) |
|
| Sinus congestion* | 269 |
6.09 (5.39–6.87) |
6.08 (1116.43) |
5.97 (5.39) |
2.58 (0.91) |
|
| Pulmonary congestion | 210 |
4.94 (4.31–5.67) |
4.94 (648) |
4.87 (4.34) |
2.28 (0.62) |
|
| Pharyngeal swelling* | 196 |
7.36 (6.39–8.48) |
7.35 (1046.89) |
7.18 (6.38) |
2.84 (1.18) |
|
| Pharyngeal oedema | 195 |
3.42 (2.97–3.94) |
3.42 (330) |
3.39 (3.01) |
1.76 (0.1) |
|
| Hypoventilation* | 195 |
18.06 (15.62–20.88) |
18.04 (2936.56) |
16.94 (15) |
4.08 (2.42) |
|
| Nasal polyps | 192 |
20.08 (17.34–23.26) |
20.06 (3230.5) | 18.71 (16.54) |
4.23 (2.56) |
|
| Rales* | 187 |
9.79 (8.46–11.33) |
9.78 (1421.06) |
9.46 (8.38) |
3.24 (1.58) |
|
| Lower respiratory tract congestion* | 172 |
35.68 (30.42–41.84) |
35.65 (5098.91) |
31.5 (27.57) |
4.98 (3.31) |
|
| Aphonia* | 170 |
3.79 (3.26–4.42) |
3.79 (344.58) |
3.75 (3.31) |
1.91 (0.24) |
|
| Rhinitis allergic* | 145 |
10.3 (8.72–12.16) |
10.29 (1170.41) |
9.94 (8.65) |
3.31 (1.65) |
|
| Upper-airway cough syndrome* | 139 |
5.44 (4.6–6.44) |
5.44 (493.51) |
5.35 (4.65) |
2.42 (0.7) |
|
| Bronchiectasis* | 100 |
4.91 (4.03–5.98) |
4.9 (305.12) |
4.83 (4.09) |
2.27 (0.61) |
|
| Skin and subcutaneous tissue disorders | Urticaria | 4885 |
9.7 (9.42–9.98) |
9.49 (35,885.67) |
9.19 (8.97) |
3.2 (1.53) |
| Angioedema | 906 |
6.41 (6–6.84) |
6.38 (4016.22) |
6.25 (5.92) |
2.64 (0.98) |
|
| Chronic spontaneous urticaria | 229 |
127.61 (108.97–149.45) |
127.47 (19,333.82) | 86.09 (75.43) |
6.43 (4.76) |
|
| Skin plaque* | 217 |
7.79 (6.8–8.91) |
7.78 (1245.47) |
7.59 (6.77) |
2.92 (1.26) |
*, ADEs that are not mentioned in the drug label. PT, Preferred Terms.
We conducted subgroup analyses stratified by gender and age to reduce the influence of demographic characteristics on our findings28. In the subgroup analysis, we listed the top 15 ADEs. Our findings revealed that asthma was the most frequently reported PT event for both males and females. Notably, unique ADEs in males comprised cough, nasopharyngitis, hypertension, hypersensitivity reactions, and an elevation in heart rate, whereas, unique ADEs in females encompassed bronchitis, angioedema, lower respiratory tract infections, an increase in diastolic blood pressure, and changes in sputum color (Supplementary Figure S1).
In the age stratified subgroup analysis, we found that asthma was the most prevalent ADE among children, adults, and the elderly. Unique ADEs observed in children comprised dyspnea, discomfort, hypotension, asthma crisis, and nasal congestion (Supplementary Figure S2). Understanding both the similarities and differences among these subgroup analyses is crucial for optimizing clinical management and can provide valuable insights to clinical decision makers, enabling them to tailor treatment strategies based on the characteristics of specific subgroups.
Time to onset of omalizumab associated ADE
Figure 4 illustrates the temporal progression of ADEs associated with omalizumab. The final dataset comprised a total of 9,502 ADEs with precise start and end timestamps. The median duration for omalizumab-related ADEs was determined to be 145 days, with an IQR of 33–520 days. During the initial month of omalizumab administration, there were 2,230 reported ADEs, representing 23.47% of the overall total. In contrast, the incidence and proportion of ADEs in the second month (N = 975, 10.27%) were significantly lower than those observed in the first month. Furthermore, both the frequency and percentage of ADEs exhibited a continuous decline from months three through six, with the rate of ADEs reaching its nadir at six months. It is important to highlight that 31.80% of the population continued to experience ADEs one year post-medication.
Fig. 4.
Time to onset of omalizumab-related ADEs.
To assess whether the incidence of ADEs associated with omalizumab would increase over time, we performed a Weibull distribution analysis on a population with comprehensive data and various subgroups. The findings are presented in Table 3, where the shape parameter (β) for the overall population was determined to be 0.61, 95%CI:0.60–0.62, suggesting that the occurrence of ADEs related to omalizumab diminished over time. Additionally, the Weibull distribution analysis conducted on other subgroups revealed that all curves exhibited characteristics indicative of early failure.
Table 3.
Time-to-onset analysis for signals.
| TTO (day) | Weibull distribution | Failure type | |||
|---|---|---|---|---|---|
| Variable | Case, N | Median (IQR) | α(95%CI) | β(95%CI) | |
| Total | 9502 | 145(33–520) | 292.68(282.52–302.84) | 0.61(0.60–0.62) | Early failure |
| Male | 2835 | 148(35–560) | 303.97(284.70–323.24) | 0.61(0.60–0.63) | Early failure |
| Females | 6578 | 144(32–504) | 287.68(275.66–299.70) | 0.61(0.60–0.62) | Early failure |
| Children | 707 | 95(28–383) | 203.79(178.13–229.45) | 0.62(0.58–0.65) | Early failure |
| Adult | 5912 | 140(32–489) | 281.32(268.91–293.73) | 0.61(0.60–0.62) | Early failure |
| Elderly | 1563 | 182(40–732) | 379.06(345.86–412.26) | 0.60(0.57–0.62) | Early failure |
Logistic regression for the occurrence of important medical events
Table 4 presents the findings from both univariate and multivariate logistic regression analyses. In the univariate analysis, we found that adverse drug reactions occurring between 180–360 days were not significantly associated with the outcome (P > 0.05), while other findings showed significant associations (P < 0.05). In the multivariate analysis, it was noted that individuals aged 18–65 years and those who experienced adverse drug reactions between 180–360 days did not exhibit significant associations with the outcome (P > 0.05), whereas other results were significantly associated (P < 0.05).
Table 4.
Results of Logistic Regression Model.
| Logistics regression | ||||
|---|---|---|---|---|
| Univariate | Multivariate† | |||
| Variable | OR (95%CI) | P-value | OR (95%CI) | P-value |
| Gender | ||||
| Female | 1(ref) | 1(ref) | ||
| Male | 1.29(1.12–1.49) | < 0.001 | 1.23(1.06–1.43) | 0.006 |
| Age | ||||
| < 18 | 1(ref) | 1(ref) | ||
| 18–65 | 0.75(0.61–0.93) | 0.008 | 1(0.78–1.28) | 0.976 |
| > 65 | 1.41(1.10–1.82) | 0.008 | 1.75(1.32–2.31) | < 0.001 |
| Weight | ||||
| < 50 kg | 1(ref) | 1(ref) | ||
| 50–100 kg | 0.60(0.48–0.75) | < 0.001 | 0.59(0.46–0.76) | < 0.001 |
| > 100 kg | 0.57(0.44–0.73) | < 0.001 | 0.56(0.42–0.75) | < 0.001 |
| Time | ||||
| < 180 days | 1(ref) | 1(ref) | ||
| 180–360 days | 1.08(0.87–1.34) | 0.494 | 1.07(0.86–1.33) | 0.544 |
| > 360 days | 1.40(1.20–1.64) | < 0.001 | 1.38(1.18–1.62) | < 0.001 |
†Three variables in addition to the independent variables are used as covariates to adjust.
Signal of preferred terms gender difference risk
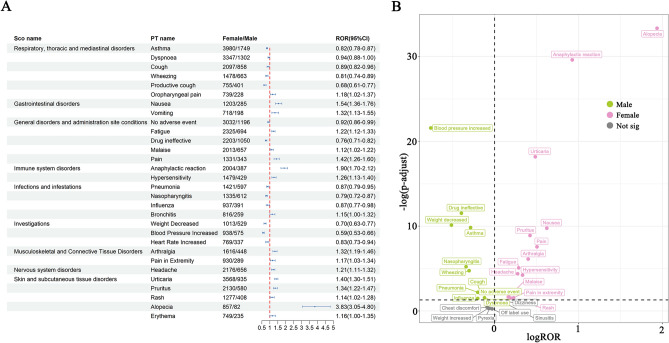
We used the ROR method to analyze the effect of gender on ADEs related to omalizumab. As shown in Fig. 5A, we gave the top 30 PTs with unequal ADEs incidence rates between female and male patients, sorted by SOC. Certain ADEs are more prevalent in men, including asthma, dyspnoea, cough, wheezing, productive coughs, no adverse event, drug ineffective, pneumonia, nasopharyngitis, influenza, weight decreased, blood pressure increased, and heart rate increased. Common ADEs in women encompass oropharyngeal pain, nausea, vomiting, fatigue, malaise, pain, anaphylactic reaction, hypersensitivity, bronchitis, arthralgia, pain in extremity, headache, urticaria, pruritus, rash, alopecia and erythema. Additionally, we constructed a volcano plot to offer a more nuanced visual representation of the gender differences in ADEs associated with omalizumab (Fig. 5B).
Fig. 5.
Analysis of gender difference risk signals in omalizumab. (A) Reporting odds ratios (ROR) with 95% CI for all positive gender-related ADEs. (B) Gender difference risk signal volcano. plot for omalizumab.
Sensitivity analysis
Omalizumab is typically administered in conjunction with glucocorticoids and loratadine. Upon excluding reports related to concurrent drug use, we identified a total of 48,372 reports encompassing 351 distinct PTs, the five most prevalent being asthma, absence of adverse drug events, urticaria, cough, and anaphylaxis necessitating immediate intervention. Detailed information regarding these events can be found in the Supplementary table 5.
Discussion
In recent years, the drug labeling of omalizumab has undergone continuous revisions based on adverse risk information collected from post-marketing clinical use. Paying attention to adverse information associated with omalizumab usage is crucial, as it enhances patient safety in medication administration, improves healthcare quality, and fosters ongoing drug innovation and improvement29. A meta-analysis indicated that ADEs associated with omalizumab were elevated (RR:1.27, 95%CI:0.93–1.72), with a potential increase in serious ADEs among both adolescents and adults (RR: 1.62, 95%CI:0.76–3.45). However, due to inherent limitations in the included studies, confidence in the reported ADEs remains low11. Our investigation into ADEs associated with omalizumab, using the FAERS database, represents the most comprehensive pharmacovigilance study conducted to date. This study provides a thorough and systematic overview of global ADEs related to omalizumab.
The findings of the study indicate a rising trend in ADEs associated with omalizumab, potentially attributable to the broadened indications outlined in its usage guidelines and an increased volume of omalizumab administration, resulting in a higher incidence of reported adverse reactions. The baseline data from the study revealed that the incidence of ADEs associated with omalizumab was significantly higher in women (65.7%) than in men (24.5%). Omalizumab is primarily indicated for the treatment of allergic conditions such as asthma and urticaria. Epidemiological studies suggest that the prevalence of asthma and urticaria in women is significantly higher than in men30. Both conditions are closely associated with the immune system, with female sex hormones exerting an inductive effect on immune responses, thereby amplifying inflammatory processes. In contrast, male sex hormones exhibit anti-inflammatory and protective characteristics31. Furthermore, women with asthma have a higher population of type 2 innate lymphocytes32, and an increase in these lymphocyte levels may lead to exacerbated allergic airway inflammation, complicating the effective treatment of asthma symptoms29. Given the larger population size and inherent gender differences, it follows that women may experience a higher incidence of ADEs.
The liver and kidney functions in children are not yet fully mature, which may alter the metabolism and excretion of drugs, leading to a higher risk of drug accumulation and subsequent adverse reactions. Additionally, variations in immune function among different age groups may account for the observed disparities in ADEs. Studies have shown that age is a major factor influencing immune cell composition, marked by a decrease in the proportion of naive cells and an increase in inflammation-associated cells33. In childhood, the immune system is in a state of development, resulting in increased drug sensitivity and a higher susceptibility to allergic reactions. In adulthood, the immune cell composition stabilizes, demonstrating enhanced adaptability and resilience. Even after immune challenges like infections or vaccinations, the immune cell composition can quickly revert to an individual’s baseline state. In old age, thymic involution results in a decrease in naive T cells, leading to immune senescence34. Furthermore, older adults frequently have multiple comorbidities and are on multiple medications, further elevating their risk of ADEs. However, it is crucial to recognize that the significant disparities in population size and disease prevalence among different age groups may play a role in the age-related differences in the incidence of ADEs linked to omalizumab.
The common adverse reactions associated with omalizumab, such as ear pain, chest discomfort, anaphylactic reactions, asthma, urticaria, respiratory infections, serum sickness, and sinusitis, were also identified as positive signals in our study, thus further validating the accuracy of our research findings. Despite the consistency in identifying common ADEs, our study also revealed several notable differences compared to previous research. Firstly, by analyzing a larger and more diverse population, our study detected a broader spectrum of ADEs associated with omalizumab. This includes ADEs that were not explicitly mentioned in the product labeling, such as asthmatic crisis (ROR: 47.3, 95%CI: 43–52.03), lower respiratory tract congestion (ROR: 35.68, 95%CI: 30.42–41.84), forced vital capacity decrease (ROR:65.11, 95%CI:52.58–80.63), and increased blood immunoglobulin E (ROR:71.89, 95%CI:64.72–79.87). This suggests that the potential risks encountered in real-world clinical applications may extend beyond the scope of understanding derived from clinical trial data.
Our study offers an in-depth examination of the temporal distribution of ADEs associated with omalizumab. We found that the median onset time for all reported ADEs was approximately 145 days, with a significant proportion occurring after one year of treatment. This finding highlights the importance of long-term monitoring for assessing the safety of omalizumab, a point that has received limited attention in previous studies, which have primarily focused on short-term safety outcomes. Furthermore, our subgroup analysis revealed notable gender and age differences in the incidence of ADEs associated with omalizumab. For instance, females exhibited a higher susceptibility to ADEs such as anaphylactic reactions and urticaria, whereas certain ADEs, such as cough and nasopharyngitis, were more common in males. These findings emphasize the necessity of considering patient-specific factors when evaluating the safety of omalizumab and devising personalized treatment strategies. Although the efficacy of omalizumab in managing allergic conditions like asthma and urticaria is well-recognized, our findings underscore the importance of vigilant monitoring for potential ADEs, especially during the early stages of treatment and long-term follow-up. Furthermore, incorporating real-world evidence into clinical decision-making can aid in optimizing the safety and efficacy of omalizumab, thereby guaranteeing that patients receive the most suitable and safe treatment.
In the context of immune system disorders, adverse reactions primarily manifest as various forms of allergies, with anaphylactic reaction (ROR: 17.28, 95%CI: 16.62–17.96) being the most common, ranking fifth among all ADEs. Previous research has primarily focused on the anaphylactic reactions associated with omalizumab. The majority of these reactions occur within 2 h following the first three injections, with an incidence rate ranging from approximately 0.1%-0.2%35, common clinical manifestations encompass bronchospasm, hypotension, syncope, urticaria, and angioedema affecting the larynx or tongue36. Research has shown that the incidence of anaphylactic shock among patients allergic to peanuts increased after treatment with omalizumab37, while common adverse reactions in those allergic to milk or peanuts typically involve mild allergic reaction and flushing37.A retrospective analysis has shown that the majority of anaphylactic shocks associated with omalizumab occurred in women aged 18 to 44 years, with life-threatening reactions being more common among asthma patients compared to those with chronic urticaria38. Early allergic reactions typically manifest within seconds to minutes following injection, reaching a peak around 15–30 min. Consequently, the European Academy of Allergy and Clinical Immunology has issued guidelines for biological agents in severe asthma, recommending close monitoring for allergic reactions post-omalizumab injection. It is advised that patients be observed for 60 min after the first three injections. However, physicians may adjust this observation period based on individual patient responses or previous adverse reaction histories39.
The precise mechanism underlying omalizumab-induced allergic reactions remains elusive. Certain studies suggest that specific excipients, notably polyethylene glycol, which aids in the rapid dissolution of the drug in aqueous solutions, may be accountable for these allergic reactions40. Reports indicate that polyethylene glycol has the potential to elicit hypersensitivity reactions41,42. A study was conducted on two patients who experienced anaphylactic shock after one year of omalizumab treatment, encompassing both in vitro and in vivo immunological assessments. The ultimate conclusion inferred that the polyethylene glycol present in the excipient was probably involved in triggering these adverse reactions42. Given that omalizumab is a biological agent capable of modulating the immune system while providing therapeutic benefits, and requires long-term treatment for patients, it is crucial to emphasize and conduct a thorough assessment of any potential long-term immunological risks that may emerge43. Among these risks, immunogenicity emerges as a pivotal factor deserving attention, it relates to the possibility that the therapeutic protein and its metabolic byproducts may elicit an immune response in the patient or trigger immune-related events, potentially leading to ADEs such as hypersensitivity reactions and allergic manifestations44,45.
Omalizumab was initially developed as a targeted therapy against IgE for allergic asthma. However, among all SOCs, it was associated with the highest incidence of respiratory system-related ADEs, totaling 103 cases. Based on the analysis of signal frequency, the five most prominent ADEs, arranged in descending order, consist of asthma, cough, wheezing, productive cough, and oropharyngeal pain, each of these manifests as symptoms related to the respiratory system. It is crucial to further distinguish whether these adverse reactions are attributed to disease progression or the drug itself. Therefore, a comprehensive understanding of the drug’s approved indications for use is imperative. A substantial portion of adverse reactions can be traced back to off-label use of medications. Additionally, the drug’s label explicitly warns against its use as a treatment for acute asthma attacks12. It is also crucial to conduct necessary laboratory tests on patients, with a particular emphasis on closely monitoring any abnormalities in laboratory parameters after drug administration. These diagnostic measures are essential for evaluating the drug’s effects on patients and can aid in the timely detection and management of potential ADEs.
Our study is not without limitations. Firstly, the absence of complete patient information and the scarcity of comprehensive clinical data pose challenges for controlling confounding variables and quantifying risk. Secondly, although discriminant analysis can identify statistical associations, it falls short in establishing causality or enabling the calculation of ADE incidence. Thirdly, another limitation of our study is the potential bias introduced by the high proportion of consumer reports in our dataset (43%). Consumer reports may differ in quality and level of detail compared to those submitted by healthcare professionals, which could impact the accuracy and reliability of our findings. Consequently, cautious interpretation of FAERS data is necessary, and further clinical studies are needed to validate these associations.
Conclusion
In conclusion, this comprehensive pharmacovigilance study emphasizes the necessity for continuous monitoring and personalized approaches to omalizumab therapy. Our findings suggest integrating real-world evidence into clinical practice to improve the safety and efficacy of omalizumab. Future research should focus on elucidating the molecular mechanisms underlying omalizumab-related ADEs, conducting long-term follow-up studies to assess long-term safety and efficacy, and exploring personalized medicine approaches to optimize treatment regimens. For clinical practice, we recommend close monitoring of patients during initial and long-term treatment, patient education about potential ADEs, individualized treatment plans based on patient characteristics, and enhanced reporting of ADEs to improve pharmacovigilance.
Supplementary Information
Acknowledgements
We would like to thank everyone who participated in this study.
Abbreviations
- FDA
Food and Drug Administration
- FAERS
Administration’s Adverse Event Reporting System
- ADE
Adverse drug events
- TTO
Time to onset
- PT
Preferred term
- SOC
System organ class
- ROR
Reporting odds ratio
- PRR
Proportional report ratio
- BCPNN
Bayesian confidence interval neural network
- MGPS
Multi-item gamma Poisson shrinker
- MedDRA
Medical Dictionary for Regulatory Activities
- TTO
Time to onset
Author contributions
YFS and YJW conceived and designed the study. YFS and ZW collected the data. ZW and NW extracted data. NW and XFX conducted statistical analysis and interpretation of the data. XFX and TSZ conducted the subgroup analysis and optimized the figure. YFS and YJW contributed to manuscript writing and revised the manuscript. All authors have thoroughly reviewed and endorsed this manuscript, thereby consenting to its publication.
Funding
This study was funded by the Jilin Provincial Scientific and Technological Development Program (No. 20220401062YY).
Data availability
The data utilized in this study are entirely publicly accessible and were sourced from the FAERS database, which can be accessed at https: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Declarations
Competing interests
The authors declare no competing interests.
Ethics
This study utilized anonymous data sourced from an open-access database, thereby obviating the need for institutional ethical approval and written informed consent from participants or their legal guardians/relatives.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-91463-5.
References
- 1.Pelaia, C. et al. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis.12, 1753466618810192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert, M. et al. IgE-mediated multimorbidities in allergic asthma and the potential for omalizumab therapy. J. Allergy Clin. Immunol. Pract.7(5), 1418–1429 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Chow, T. G., Franzblau, L. E. & Khan, D. A. Adverse reactions to biologic medications used in allergy and immunology diseases. Curr. Allergy Asthma Rep.22(12), 195–207 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma, T., Wang, H. & Wang, X. Effectiveness and response predictors of omalizumab in treating patients with seasonal allergic rhinitis: A real-world study. J. Asthma Allergy.14, 59–66 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, S., Cornelius, V., Cro, S., Harper, J. I. & Lack, G. Treatment effect of omalizumab on severe pediatric atopic dermatitis: The ADAPT randomized clinical trial. JAMA Pediatr.174(1), 29–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin, Y. H. et al. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy.78(8), 2232–2254 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce, N. et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. ISAAC steering committee and the European community respiratory health survey. International study of asthma and allergies in childhood. Eur. Respir. J.16(3), 420–426 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Alavinezhad, A. & Boskabady, M. H. The prevalence of asthma and related symptoms in Middle East countries. Clin. Respir. J.12(3), 865–877 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Koo, M. J. et al. National trends in the prevalence of allergic diseases among Korean adolescents before and during COVID-19, 2009–2021: A serial analysis of the national representative study. Allergy.78(6), 1665–1670 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Yaghoubi, M., Adibi, A., Safari, A., FitzGerald, J. M. & Sadatsafavi, M. The projected economic and health burden of uncontrolled asthma in the United States. Am. J. Respir. Crit. Care Med.200(9), 1102–1112 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agache, I. et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy.75(5), 1043–1057 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Agache, I. et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy.75(5), 1023–1042 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Maurer, M. et al. The XTEND-CIU study: Long-term use of omalizumab in chronic idiopathic urticaria. J. Allergy Clin. Immunol.141(3), 1138–9.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Hanania, N. A. et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: A randomized trial. Ann. Intern. Med.154(9), 573–582 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Deschildre, A. et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur. Respir. J.46(3), 856–859 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Peng, M. et al. A real-world study of adverse drug reactions of two isocitrate dehydrogenase inhibitor based on the US FDA adverse event reporting system and VigiAccess databases. Front. Pharmacol.15, 1489045 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, Z. et al. Real-world safety of PCSK9 inhibitors: A pharmacovigilance study based on spontaneous reports in FAERS. Front. Pharmacol.13, 894685 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci.10(7), 796–803 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ke, H. et al. Characteristics of adverse reactions of three anti-glioma drugs in WHO-VigiAccess. Front. Pharmacol.15, 1485067 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caster, O., Aoki, Y., Gattepaille, L. M. & Grundmark, B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: Recommendations for limiting false-positive associations. Drug Saf.43(5), 479–487 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montastruc, J. L., Sommet, A., Bagheri, H. & Lapeyre-Mestre, M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br. J. Clin. Pharmacol.72(6), 905–908 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf.13(8), 519–523 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf.10(6), 483–486 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Wu, Y., Wei, M. & Zhang, J. A real-world pharmacovigilance analysis of FDA adverse event reporting system database for upadacitinib. Front. Pharmacol.14, 1200254 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang, S., Wu, Z., Xu, L., Wen, Q. & Zhang, X. Adverse reaction signals mining and hemorrhagic signals comparison of ticagrelor and clopidogrel: A pharmacovigilance study based on FAERS. Front. Pharmacol.13, 970066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi, Y. et al. Signals of gastroesophageal reflux disease caused by incretin-based drugs: A disproportionality analysis using the Japanese adverse drug event report database. J. Pharm. Health Care Sci.4, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, Q. et al. Adverse events of epidiolex: A real-world drug safety surveillance study based on the FDA adverse event reporting system (FAERS) database. Asian J. Psychiatr.90, 103828 (2023). [DOI] [PubMed] [Google Scholar]
- 28.de Vries, S. T., Denig, P., Ekhart, C., Mol, P. G. M. & van Puijenbroek, E. P. Sex differences in adverse drug reactions of metformin: A longitudinal survey study. Drug Saf.43(5), 489–495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou, F. et al. A real-world pharmacovigilance study of mepolizumab in the FDA adverse event reporting system (FAERS) database. Front. Pharmacol.14, 1320458 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciprandi, G. & Gallo, F. The impact of gender on asthma in the daily clinical practice. Postgrad. Med.130(2), 271–273 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Sirufo, M. M., De Pietro, F., Ginaldi, L. & De Martinis, M. Sex, Allergic diseases and omalizumab. Biomedicines. 10.3390/biomedicines10020328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cephus, J. Y. et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep.21(9), 2487–2499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr, E. J. et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol.17(4), 461–468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer, D. B. The effect of age on thymic function. Front. Immunol.4, 316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox, L. et al. American academy of allergy, asthma & immunology/american college of allergy, asthma and immunology joint task force report on omalizumab-associated anaphylaxis. J. Allergy Clin. Immunol.120(6), 1373–1377 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Bonini, M. et al. Aqua allergy questionnaire for athletes. Development and validation. Med. Sci. Sports Exerc.41(5), 1034–1041 (2009). [DOI] [PubMed] [Google Scholar]
- 37.MacGinnitie, A. J. et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J. Allergy Clin. Immunol.139(3), 873–81.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, L. et al. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin. Transl. Allergy.11(4), e12038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agache, I. et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy.76(1), 14–44 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Kozutsumi, D. et al. PS80 interferes with the antiallergic effect of Cry-consensus peptide, a novel recombinant peptide for immunotherapy of Japanese cedar pollinosis, at very low concentration through modulation of Th1/Th2 balance. Immunology.118(3), 392–401 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steele, R. H., Limaye, S., Cleland, B., Chow, J. & Suranyi, M. G. Hypersensitivity reactions to the polysorbate contained in recombinant erythropoietin and darbepoietin. Nephrol. (Carlton).10(3), 317–320 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Price, K. S. & Hamilton, R. G. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc.28(3), 313–319 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Eyerich, S., Metz, M., Bossios, A. & Eyerich, K. New biological treatments for asthma and skin allergies. Allergy.75(3), 546–560 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Vahle, J. L. Immunogenicity and immune complex disease in preclinical safety studies. Toxicol. Pathol.46(8), 1013–1019 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Pera, V. et al. Parasitic infections related to anti-type 2 immunity monoclonal antibodies: A disproportionality analysis in the food and drug administration’s adverse event reporting system (FAERS). Front. Pharmacol.14, 1276340 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized in this study are entirely publicly accessible and were sourced from the FAERS database, which can be accessed at https: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.