Abstract
Background
Males have a three times higher risk of a diagnosis of bladder cancer (Bca) than females. Sex hormone-binding globulin (SHBG) may be associated with Bca risk. However, the sex-specific role of SHBG in Bca remains unclear. In this study, we aimed to determine the role of SHBG in Bca.
Methods
A sex-specific univariable Mendelian randomization (MR) analysis involving 369,426 men and 395,375 women was conducted to assess the causal relationship between SHBG and testosterone and Bca risk. Sensitivity analyses and multivariable MR were conducted to confirm the robustness of our results. Linkage disequilibrium score regression assessed the genetic correlation between these diseases influenced by heredity.
Results
Univariable MR results showed that one-SD elevated SHBG was related to a low risk of Bca in males (OR: 0.60, 95% CI: 0.39–0.93; p = 0.022) but had no benefit in females. Genetically predicted BT was positively associated with Bca risk in males (OR: 1.59; 95% CI: 1.06–2.40; p = 0.027). In multivariable MR, higher SHBG levels were not related to male Bca risk after controlling for BT.
Conclusions
Our findings do not provide evidence to support a causal relationship between SHBG and Bca risk in males although an association was observed in the univariable analysis. Further research is needed to identify the underlying pathways.
Keywords: bladder cancer, sex hormone binding globulin, risk, testosterone, genome-wide association studies
1. Introduction
Bladder cancer (Bca) is a common malignant tumor of the urinary system. It is a major economic burden on the healthcare system and is well known to be associated with sex bias [1]. Sex differences in Bca incidence have been observed in epidemiological and clinical studies, with a male-to-female risk ratio of 3:1 in terms of diagnosis [2,3]. Although smoking and exposure to occupational carcinogens are factors that contribute to the sex-dependent differences in Bca incidence, Bca still occurs in males after controlling exposure to these carcinogenic factors [4–6]. Therefore, intrinsic factors are likely to play key roles in urothelial carcinogenesis. Experimental and clinical evidence suggests the involvement of androgens and androgen receptors (AR) [7,8]. However, not all studies have reached unanimous conclusions.
Sex hormones and their receptors have been posited as potential contributory factors responsible for sex-based disparities in Bca incidence [9]. Androgens are likely to contribute to sexual dimorphism in Bca by directly and indirectly influencing various cellular processes, such as the synthesis of cytokines, growth factors, and vasoactive substances. Sex hormone-binding globulin (SHBG) is involved in binding to sex hormones and regulating their biological activity [2]. Nevertheless, establishing these associations conclusively through randomized controlled trials poses considerable challenges. Observational studies face difficulties in distinguishing the effects of SHBG levels from those attributable to sex hormones, given their intricate interrelationship. Furthermore, there is a lack of research investigating the sex-specific roles of SHBG in Bca.
To address the lack of experimental evidence for SHBG involvement in Bca, we used a Mendelian randomization (MR) study design, using naturally occurring genetic variants that affect SHBG levels throughout life [10]. Genetic variants are determined at conception; therefore, using this approach establishes the role of SHBG in Bca, excluding the potential confounding effects of socioeconomic position or other factors. This research utilized linkage disequilibrium score regression (LDSC) analysis of GWAS summary statistics to investigate the genetic associations among SHBG, BT, and Bca, with a focus on hereditary contributions. We conducted a sex-specific MR analysis using published single nucleotide polymorphisms (SNPs) to predict SHBG in both males and females and controlled for bioavailable testosterone (BT) levels.
2. Materials and methods
2.1. Genetic correlation analysis
LDSC is an effective method for analyzing genetic correlations [11]. We employed LDSC to examine the shared polygenic structure between diseases, complementing MR analysis. Linkage disequilibrium (LD) scores and weights for the European population were precomputed from 1,000 Genomes data by LDSC’s original developers. The analysis utilized the R package “ldscr.”
2.2. Research design
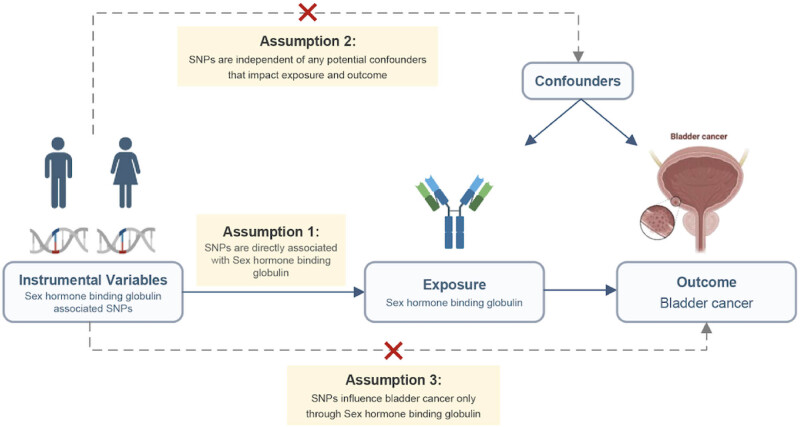
Initially, we employed univariate MR to evaluate the overall influence of SHBG and BT on Bca risk. Subsequently, we conducted a multivariable MR analysis to examine the direct and independent effects of these traits on the observed outcomes. This study followed the MR guidelines based on three key assumptions: a close relationship between genetic markers and SHBG, the independence of instrumental variables (IVs) from confounding factors, and the exclusive effect of IVs on Bca through SHBG [12] (Figure 1). The data utilized in this study were derived from publicly available genome-wide association studies (GWAS), which have been granted ethical approval and informed consent.
Figure 1.
Study design. Bca: bladder cancer; SHBG: sex hormone-binding globulin.
2.3. Data sources
2.3.1. Selection of genetic variants associated with exposure
In order to mitigate bias stemming from sample overlap, we utilized nonoverlapping datasets for each exposure-outcome pair. We selected SNPs from GWASs in the UK Biobank to serve as IVs for SHBG (GWAS ID: ieu-b-4871 for males, ieu-b-4870 for females) and BT (GWAS ID: ieu-b-4868 for males, ieu-b-4869 for females) of European ancestry [13]. SHBG genetic variations were derived from the UK Biobank’s summary sex-specific GWAS (185,221 white British males and 214,989 white British females). BT genetic variations were derived from the UK Biobank’s summary sex-specific GWAS (184,205 white British males and 180,386 white British females). We examined SNPs with genome-wide significance (p < 5 × 10−8) and utilized LD analysis to eliminate SNPs with mutual LD above the limit (LD clumping panel kb = 5,000, R 2 < 0.01) [14].
To address potential pleiotropy, we chose variables known to be involved with Bca. For instance, body mass index (BMI) might be a driver [15] or downstream factor [13] of SHBG. We used the PhenoScannerV2 (www.phenoscanner.medschl.ca.ac.uk) to exclude confounders, such as BMI, smoking, long-term exposure to industrial chemicals, arsenic contamination, chemotherapy drugs, such as cyclophosphamide, or pioglitazone for type 2 diabetes [5,16,17]. Finally, we included 48 and 66 SNPs as IVs for SHBG in males and females, respectively, and 53 and 77 SNPs as IVs for BT in males and females, respectively. To validate the strength of the identified SNPs, we produced F-statistics and conditional F-statistics for univariate and multivariable MR analyses.
2.3.2. Genetic variants associated with Bca
Summary data on the sex-specific genetic variants of Bca were obtained from FinnGen R10 comprising 175,121 European individuals (1,115 Bca cases and 174,006 controls) [18]. The GWAS analyses were adjusted for principal components.
2.3.3. Genetic variants for multivariable MR
We employed multivariable MR analysis to control for BT since SHBG was strongly related to this particular variable [13]. Genetic predictors of SHBG and BT were obtained from the UK Biobank. We excluded duplicate SNPs and those with a high correlation (r 2 > 0.05) using LDLink. The remaining SNPs were used for multivariable MR analysis.
2.3.4. Statistical analysis
The primary MR approach was inverse variance weighting (IVW), employed to assess the effect of a one-standard deviation (SD) increment in genetically predicted exposure on the outcome, which was reported as odds ratio (OR) accompanied by a 95% confidence interval (CI). Other MR methods, such as MR-Egger, weighted median, and weighted mode, were also used to investigate the consistency of effect estimates.
As a first step, SNPs with a genome-wide significance level of p < 5 × 10−8 and an F-statistic >10 were carefully selected. To address potential confounding factors, we utilized the PhenoScannerV2 website to exclude genetic confounders associated with Bca. For the assessment of heterogeneity and horizontal pleiotropy, Cochran’s Q statistic was employed to quantify heterogeneity, while radial MR was utilized to eliminate outliers [19]. Additionally, MR-Egger regression and the MR-Presso method were employed to evaluate the influence of horizontal pleiotropy upon effect estimations. Sensitivity analysis was conducted through leave-one-out tests to assess the effects of individual variants on the observed associations. For multivariable MR, the test for heterogeneity and pleiotropy were similar to those described above for univariate MR [20].
R2 indicates the proportion of SHBG variation that may be attributed to SNPs.
Furthermore, the required sample size for Bca events was estimated using the log OR, which measures the patient-to-non-patient ratio. All MR analyses were performed using the two-sample MR and MR packages in R (version 4.1.0).
3. Results
3.1. Genetic correlation analysis
The results of the genetic correlation analysis are presented in Table 1. We estimated a positive genetic correlation between the following pairs of diseases: SHBG (male) and Bca (male) (rg = 0.663, p-value = 1.52 × 10−05), SHBG (female) and Bca (female) (rg = 0.697, p-value = 7.95 × 10−58), and BT (male), and Bca (male) (rg = 0.563, p-value = 1.12 × 10−39).
Table 1.
Results of genetic correlation analysis
| Trait1 | Trait2 | Genetic correlation | Standard error | p-value |
|---|---|---|---|---|
| SHBG (male) | Bca (male) | 0.663 | 0.144 | 1.52 × 10−5 |
| SHBG (female) | Bca (female) | 0.697 | 0.043 | 7.95 × 10−58 |
| BT (male) | Bca (male) | 0.563 | 0.043 | 1.12 × 10−39 |
| BT (female) | Bca (female) | −0.286 | 0.202 | 0.156 |
SHBG: sex hormone-binding globulin; BT: bioavailable testosterone; Bca: bladder cancer.
3.2. IVs
In the univariate MR analysis, we employed 48 and 66 genome-wide significant SNPs previously reported in males and females, respectively, for the assessment of SHBG. Furthermore, 53 and 77 genome-wide significant SNPs previously identified in males and females, respectively, were utilized for investigating BT. Following the methodology outlined by Burgess et al., all SNPs had an F-statistic exceeding >10, indicating the absence of weak instruments in univariate MR analysis (Table S1).
3.3. Causal effect of SHBG on Bca using univariable MR
The random-effects IVW approach was the main analytical method employed. In two sample MR, there was no indication of instrumental heterogeneity (Cochran’s Q test, p > 0.05). One SD elevated SHBG was related to a low risk of Bca in males OR: 0.60, 95% CI: 0.39–0.93; p = 0.022; Bonferroni’s correction [p = 0.05/2]). The causal effect estimation of the MR-Egger test and other methods were similar in direction and magnitude. However, genetically predicted SHBG had no association with Bca in females (OR: 1.32, 95% CI: 0.99–1.75 p = 0.061) (Figure 2).
Figure 2.
Univariable MR results of SHBG using different methods. (a) The combined forest plot of SHBG on Bca. (b) The scatter plots of SHBG on Bca in males. (c) The scatter plots of SHBG on Bca in females. The number of genetic variants, OR, 95% CI, p values, and MR methods of associations are contained. SNPs(N): the number of single-nucleotide polymorphisms used as IVs; OR: the combined causal effect; CI: confidence interval; SHBG: sex hormone binding globulin; Bca: bladder cancer.
One SD increase in BT was associated with a significantly increased Bca risk in males (OR: 1.59; 95% CI: 1.06–2.40; p = 0.027). However, the result did not reach statistical significance after Bonferroni’s correction (p = 0.05/2), indicating a suggestive causal association. MR analyses, such as MR-Egger, weighted median, and weighted mode, were similar in direction and magnitude. In females, genetically predicted BT was unrelated to Bca (OR: 1.38, 95% CI: 0.94–2.02; p = 0.098) (Figure 3). The scatter plots of univariable MR analyses performed on FinnGen data using different methods showing the effect of exposure on Bca in male and female. The single MR effect of univariable MR analyses showing the effect of exposure on Bca is shown in Figure S1.
Figure 3.
Univariable MR results of BT using different methods. (a) The combined forest plot of BT on Bca. (b) The scatter plots of BT on Bca in males. (c) The scatter plots of BT on Bca in females. The number of genetic variants, OR, 95% CI, p values, and MR methods of associations are contained. SNPs(N): the number of single-nucleotide polymorphisms used as IVs; OR: the combined causal effect; CI: confidence interval; Bca: bladder cancer.
The MR-Egger intercept test was employed to assess pleiotropy. There was no directional pleiotropy found using MR-Egger regression or MR-PRESSO analysis (Table 2). The results of a leave-one-out sensitivity analysis demonstrated that no one SNP was responsible for the overall impact of sex hormones on Bca (Figure S2). The MR funnel diagram was symmetrical (Figure S3).
Table 2.
Sensitive analyses result in univariable MR for Bca
| Outcome | Exposure | IVW- Q test | MR-Egger | MR-Presso Global test | ||
|---|---|---|---|---|---|---|
| p-value | Intercept | SE | p-value | p-value | ||
| Bca | ||||||
| Male | SHBG | 0.987 | −0.017 | 0.017 | 0.317 | 0.994 |
| BT | 0.849 | 0.011 | 0.015 | 0.463 | 0.816 | |
| Female | SHBG | 0.250 | −0.003 | 0.009 | 0.779 | 0.312 |
| BT | 0.663 | 0.016 | 0.017 | 0.343 | 0.576 | |
IVW: inverse-variance weighted; SE: standard error; SHBG: sex hormone-binding globulin; BT: bioavailable testosterone; Bca: bladder cancer.
3.4. Causal effect of SHBG on Bca using multivariable MR
After adjusting for BT in the multivariable MR for males, genetically predicted increased SHBG was not associated with the risk of Bca (OR: 1.08; 95% CI: 0.65–1.81; p = 0.763). After controlling for SHBG, the causal relationship between BT and Bca did not remain statistically significant (OR: 0.85; 95% CI: 0.63–1.15; p = 0.301) (Figure 4). The conditional F-statistics for the SHBG and BT were 40.2 and 95.0%, respectively. The conditional F-statistics indicated the absence of weak instruments in multivariable MR. Cochran’s Q statistic testing indicated the absence of heterogeneity in multivariable MR. Horizontal pleiotropy was not observed (Table 3). In direction and magnitude, the estimations were stable to the multivariable in MR-Egger, MR-Lasso, and MR-Presso approaches.
Figure 4.
Multivariable MR results of SHBG and BT. The number of genetic variants, OR, 95% CI, p values, and MR methods of associations are contained. SNPs(N), the number of single-nucleotide polymorphisms used as IVs; OR, the combined causal effect; CI, confidence interval; p value, p value of the causal estimate; SHBG: sex hormone-binding globulin; Bca: bladder cancer; IVW: inverse variance weighted.
Table 3.
Conditional F-statistics and sensitive analyses result in multivariable MR for Bca in males
| Outcome | Exposure | F-statistics | Q-test | MR-Egger | MR-Presso Global test | |||
|---|---|---|---|---|---|---|---|---|
| Q-statistic | Q_p | Intercept | SE | p-value | p-value | |||
| Bca | ||||||||
| SHBG | 40.177 | 59.574 | 0.976 | −0.006 | 0.008 | 0.431 | 0.977 | |
| BT | 95.046 | |||||||
SE: standard error; SHBG: sex hormone-binding globulin; BT: bioavailable testosterone; Bca: bladder cancer.
4. Discussion
Understanding the influence of sex differences on the incidence, prevalence, and severity of Bca has become an increasingly important area of research. Sex should be regarded as a crucial biological variable to be included in future Bca research [21]. To the best of our knowledge, this study is the first to examine the sex-specific risk of Bca from a genetic perspective. We investigated the relationship between SHBG and Bca using univariate and multivariable MR analyses.
The glycoprotein SHBG is predominantly synthesized in the liver and exists as a homodimeric protein with a molecular weight of 90–100 kDa. Its binding ability extends to all androgens and estrogens, except for dehydroepiandrosterone sulfate and androstenedione. The free hormone hypothesis suggests that because binding proteins are the gatekeepers of steroid action, the biological activity of a hormone is best represented by the concentration of the free hormone rather than its total concentration. Notably, SHBG contributes considerably to the balance between free and protein-bound components of plasma and serves as a transporter of steroid hormones, facilitating their transport from the point of synthesis to their target location. Particularly for androgens, interactions with SHBG and albumin determine the delicate balance between bioavailability and total testosterone, thereby controlling tissue exposure. This balance can be influenced by factors such as aging, genetics, and various pathological conditions that affect target tissue hormone exposure [22]. Although the precise mechanism is yet unknown, it is conceivable that the regulatory function of SHBG in modulating the levels of free/BT in the bloodstream contributes to this protective association.
Additionally, SHBG may play a role in male Bca through other pathways. The development of Bca is influenced by inflammation in several ways. Chronic inflammation can cause DNA damage and mutations in bladder epithelial cells, thereby increasing the risk of Bca [23]. Bca cells can trigger an “inflammatory cytokine storm,” stimulating the secretion of tumor growth-promoting factors and weakening the cytotoxic function of immune cells. Cytokines and growth factors released by these cells may stimulate Bca cell proliferation [24]. Increased levels of reactive oxygen species, lipid peroxidation products, pro-inflammatory cytokines, and pro-angiogenic factors may induce inflammatory responses, ultimately activating Bca angiogenesis [25]. SHBG inhibits inflammation in vitro, which is not altered by co-supplementation with testosterone or estradiol [26], supporting a pathway through inflammation. Further investigation into potential pathways, especially those associated with sex-specific responses to SHBG and Bca, would be beneficial. Similarly, a meta-analysis of previous clinical trials showed that androgen deprivation therapy (ADT) may have antitumor effects on Bca [8]. However, these findings should be interpreted with caution. The relationship between ADT and Bca in previous observational studies has been inconsistent, and clinical trials to validate their efficacy are still lacking [7]. The efficacy of abiraterone and other approved anticancer agents for urothelial cancer in the treatment of Bca may be further elucidated through an ongoing clinical trial [27].
This study design has several strengths that contribute to the validity of our findings. This study adheres strictly to the assumptions of MR so that potential confounding factors and reverse causality are minimized, and an independent correlation between SHBG and Bca risk can be established. Moreover, LDSC aids in distinguishing true polygenic effects from confounding factors such as implicit associations and population stratification. Notably, this study is the first to comprehensively and systematically analyze prospective studies to evaluate the role of SHBG in Bca risk. We also employed MR-Egger, which provides robustness against pleiotropy, a potential limitation of MR. Replication with larger samples is required in such cases. Furthermore, the high F-statistics of the genetic instruments employed in this study mitigates concerns regarding the presence of weak instruments that could potentially introduce bias into our findings.
Despite its novelty, this study has several limitations. First, the research might have been influenced by survivorship bias and competing risks, which preclude the occurrence of Bca. We adjusted the common confounders of Bca. Second, compensatory processes or feedback mechanisms may dilute genetic effects, resulting in skewed MR estimations. However, this did not explain the observed beneficial association between SHBG and Bca. A common limitation is that non-muscle-invasive Bca is not distinguished from muscle-invasive disease. We could not stratify Bca across diagnostic types. Third, it is crucial to recognize a fundamental limitation of LDSC: while it indicates overall positive, negative, or absent genetic correlations, it does not account for mixed directional effects among shared genetic variants. We agree that the potential pleiotropic nature of the included variants and the shared genetic risk between SHBG and BT could distort the results. Consequently, additional research is needed to provide a more detailed quantification of polygenic overlap and deepen insights into the genetic interconnections between these diseases [28].
Regardless of the consistent and beneficial associations observed in this study, caution should be exercised when interpreting our findings [29]. Instead of focusing on the immediate results of an exogenous exposure, MR considers the long-term consequences of an endogenous one. Consequently, the impact on male Bca may not be significant in terms of factors that regulate SHBG.
Our findings do not provide evidence to support a causal relationship between SHBG and Bca risk in males. While an association was observed in the univariable analysis, this is likely influenced by shared genetic risk variants and the pleiotropic effects of the included variants. Further studies, including formal mediation analyses, are required to explore the potential interplay between SHBG, BT, and Bca risk.
Abbreviations
- ADT
androgen deprivation therapy
- AR
androgen receptor
- Bca
bladder cancer
- BMI
body mass index
- CI
confidence interval
- GWAS
genome-wide association study
- IVs
instrumental variables
- IVW
inverse variance-weighted
- MR
Mendelian randomization
- OR
odds ratio
- SHBG
sex hormone-binding globulin
- SNP
single nucleotide polymorphism
Supplementary Material
Acknowledgements
The authors acknowledge the efforts of the genome-wide association study consortia (GWAS) to provide high-quality resources for researchers.
Footnotes
Funding information: This work was supported by the Beijing Natural Science Foundation (Z230014), the Key Clinical Projects of Peking University Third Hospital (BYSYFY2021046), and CSCO Clinical Oncology Research Foundation of Beijing (Y-tongshu2021/ms-0072).
Author contributions: Conceptualization: J.Y.O., H.B., S.D.Z., J.F.Y.; search and evaluation: J.Y.O., H.B.Z., H.B.; data analysis: J.Y.O., H.B.Z., P.C.D., Z.Y.Z., Z.Z.Z., Z.X.X.; writing – original draft and visualization: J.Y.O., H.B.Z., P.C.D., Z.Y.Z., H.M.Y.; writing – review and revision: J.Y.O., S.D.Z., J.F.Y.; supervision and project management: S.D.Z., H.B., X.J.T.; resources: S.D.Z., H.B., J.F.Y., X.J.T.
Conflict of interest: The authors declare that they have no conflicts of interest to disclose. This includes no financial or personal relationships with other people or organizations that could inappropriately influence the author’s work, including but not limited to employment, affiliation, grants, consultancies, honoraria, stock ownership, options, expert testimony, royalties, or patents.
Data availability statement: The datasets for this study can be found in the GWAS Catalog [https://www.ebi.ac.uk/gwas/], FinnGen [https://www.finngen.fi/en], and Neale lab [http://www.nealelab.is/uk-biobank]. GWAS ID details can be found in methods or Supplementary Material.
Contributor Information
Jianfei Ye, Email: jamesyeh@126.com.
Shudong Zhang, Email: zhangshudong@medmail.com.cn.
References
- [1].Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017 Jan;71(1):96–108. [DOI] [PubMed]
- [2].Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016 Feb;69(2):300–10. [DOI] [PubMed]
- [3].Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. [DOI] [PubMed]
- [4].Siegel RL, Jacobs EJ, Newton CC, Feskanich D, Freedman ND, Prentice RL, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015 Sep;175(9):1574–6. [DOI] [PubMed]
- [5].Hemelt M, Yamamoto H, Cheng KK, Zeegers MPA. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer. 2009 Jan;124(2):412–9. [DOI] [PubMed]
- [6].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed]
- [7].Martínez-Rojo E, Berumen LC, García-Alcocer G, Escobar-Cabrera J. The role of androgens and androgen receptor in human bladder cancer. Biomolecules. 2021 Apr;11(4):594. [DOI] [PMC free article] [PubMed]
- [8].Creta M, Celentano G, Napolitano L, La Rocca R, Capece M, Califano G, et al. Inhibition of androgen signalling improves the outcomes of therapies for bladder cancer: results from a systematic review of preclinical and clinical evidence and meta-analysis of clinical studies. Diagnostics (Basel). 2021 Feb;11(2):351. [DOI] [PMC free article] [PubMed]
- [9].Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. 2014 Jun;33(25):3225–34. [DOI] [PubMed]
- [10].Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004 Feb;33(1):30–42. [DOI] [PubMed]
- [11].Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015 Mar;47(3):291–5. [DOI] [PMC free article] [PubMed]
- [12].Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020 Jan 17;11(1):376. [DOI] [PMC free article] [PubMed]
- [13].Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020 Feb;26(2):252–8. [DOI] [PMC free article] [PubMed]
- [14].Ou J, Zou L, Wu Y, Zhang Q, Fang Y, Qiu M, et al. Causal inference between rheumatoid arthritis and prostate cancer. Clin Exp Med. 2023 Dec;23(8):4681–94. 10.1007/s10238-023-01151-9. [DOI] [PubMed]
- [15].Maggio M, Lauretani F, Basaria S, Ceda GP, Bandinelli S, Metter EJ, et al. Sex hormone binding globulin levels across the adult lifespan in women – the role of body mass index and fasting insulin. J Endocrinol Invest. 2008 Jul;31(7):597–601. [DOI] [PMC free article] [PubMed]
- [16].Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018 Dec;74(6):784–95. [DOI] [PubMed]
- [17].Fernández MI, López JF, Vivaldi B, Coz F. Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure. J Urol. 2012 Mar;187(3):856–61. [DOI] [PubMed]
- [18].Risteys FinnGen R10 - C3_BLADDER [Internet]. [cited 2023 Mar 27]. https://risteys.finngen.fi/endpoints/C3_BLADDER#dialog-table-case-counts.
- [19].Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018 Dec;47(6):2100. [DOI] [PMC free article] [PubMed]
- [20].Ou J, Zhen K, Wu Y, Xue Z, Fang Y, Zhang Q, et al. Systemic lupus erythematosus and prostate cancer risk: a pool of cohort studies and Mendelian randomization analysis. J Cancer Res Clin Oncol. 2023 Sep;149(12):9517–28. [DOI] [PMC free article] [PubMed]
- [21].Theodorescu D, Li Z, Li X. Sex differences in bladder cancer: emerging data and call to action. Nat Rev Urol. 2022 Aug;19(8):447–9. [DOI] [PMC free article] [PubMed]
- [22].Narinx N, David K, Walravens J, Vermeersch P, Claessens F, Fiers T, et al. Role of sex hormone-binding globulin in the free hormone hypothesis and the relevance of free testosterone in androgen physiology. Cell Mol Life Sci. 2022 Oct 7;79(11):543. [DOI] [PMC free article] [PubMed]
- [23].Michaud DS. Chronic inflammation and bladder cancer. Urol Oncol. 2007;25(3):260–8. [DOI] [PubMed]
- [24].Gakis G. The role of inflammation in bladder cancer. Adv Exp Med Biol. 2014;816:183–96. [DOI] [PubMed]
- [25].Wigner P, Grębowski R, Bijak M, Saluk-Bijak J, Szemraj J. The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int J Mol Sci. 2021 Apr;22(9):4483. [DOI] [PMC free article] [PubMed]
- [26].Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, Nakatsu Y, et al. Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018;2018:3062319. [DOI] [PMC free article] [PubMed]
- [27].Study Record | Beta ClinicalTrials.gov [Internet]. [cited 2023 Apr 18]. https://beta.clinicaltrials.gov/study/NCT02788201?distance=50&cond=NCT02788201&rank=1.
- [28].Frei O, Holland D, Smeland OB, Shadrin AA, Fan CC, Maeland S, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019 Jun 3;10(1):2417. [DOI] [PMC free article] [PubMed]
- [29].Neale lab [Internet]. [cited 2024 Nov 19]. UK Biobank. http://www.nealelab.is/uk-biobank.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.