Abstract
Numerous electroencephalogram (EEG) studies have sought to elucidate the neural mechanisms of attention deficit hyperactivity disorder (ADHD), with most of the existing literature focused on children, adolescents, and adults. In this retrospective study, measures of frontal EEG power and behavioral attention of 40 5-month-old infants later diagnosed with ADHD in childhood were compared to 40 systematically matched-control infants. Compared to the control group, infants in the ADHD group exhibited longer looking fixations during an attention task. Frontal EEG power in the 6–9 Hz infant alpha band was lower in the ADHD group compared to the control group. Mean frontal EEG power was associated with visual fixations, underscoring specific attention behavior corresponding to frontal brain development in infancy. Infants later diagnosed with ADHD exhibited higher attention problems in childhood at ages 4 and 9 compared to the control group, and longer looking fixations in infancy were associated with higher childhood ADHD-related symptomatology. These findings suggest that decreased infant frontal EEG power and looking fixations as early as 5-months of age may serve as important early markers of later ADHD and can aid in building a more comprehensive model of ADHD from a developmental neuroscience approach.
Keywords: Attention, Frontal lobe, EEG, ADHD, Infancy, Childhood
Graphical Abstract
Highlights
-
•
Infants later diagnosed with ADHD show prolonged looking fixations at 5 months.
-
•
ADHD infants have lower frontal EEG alpha power compared to matched controls.
-
•
Frontal EEG activity is associated with visual fixation patterns in infancy.
-
•
Longer infant looking fixations were associated with greater attention problems at ages 4 & 9.
-
•
Early ADHD markers may include reduced frontal EEG power and longer fixations.
An estimated 11.3 % of children in the United States meet the diagnosis of attention-deficit/hyperactivity disorder (ADHD), according to the most recent report from the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (Reuben and Elgaddal, 2024). Preschoolers and children with ADHD are more likely to have difficulties in school, problems with peer relationships and learning, and a higher risk of injuries (DuPaul et al., 2001). A better understanding of how this disorder manifests in infancy could potentially help identify early attention behavior and neuromarkers of ADHD. Due to the lack of a specific test for attentional disorders, the complex nature of the symptoms, and ADHD being recognized as a developmental disorder that persists into adulthood, there has been significant interest in studying ADHD from a multimethodological developmental approach (Lenartowicz and Loo, 2014). This approach aims to utilize behavioral and neurophysiological techniques to identify ADHD characteristics and neuromarkers early in development.
Although neurodevelopmental conditions affect individuals across the lifespan, a recent review on ADHD biomarkers highlighted the need to better understand how neuromarkers vary with age, given evidence of altered brain development in children with ADHD (Parlatini, et al., 2024). ADHD has historically been classified as a disruptive behavioral disorder, often defined by patterns of inattention, hyperactivity, and impulsivity. However, recent advancements in both research and clinical practice have led to a shift in understanding ADHD as a neurodevelopmental disorder, emphasizing its roots in atypical brain development and executive function processes (Brown, 2009). This reconceptualization highlights ADHD as a disorder that extends beyond childhood, with developmental trajectories observable throughout the lifespan, including into adulthood. An important aspect of this shift involves recognizing individual differences in attention and brain development, particularly in relation to neurophysiological patterns that may emerge early in life. Despite this evolving understanding, ADHD continues to face stigma due to persistent skepticism about its legitimacy as a neurodevelopmental disorder (Mueller et al., 2012). Incorporating neurophysiological measures such as the electroencephalogram (EEG) into assessments of attention in early at-risk groups may identify more subtle atypicalities that precede or underlie later attentional difficulties (Johnson et al., 2015, Taylor and Baldeweg, 2002).
1. Infant EEG power
Atypical patterns of brain activity are a candidate factor in developmental brain disorders associated with an early-onset timeline. EEG is an efficient, non-invasive, and relatively inexpensive method for investigating different patterns in brain development. EEG power reflects the excitability of groups of neurons and ontogenetic increases in power are considered to be indicative of brain development (Hudspeth and Pribram, 1992; see Cuevas and Bell, 2022, for review). Infant EEG power computed for the 6–9 Hz frequency band is expressed as mean square microvolts and is commonly known as “infant alpha” (Marshall et al., 2002). EEG power in the alpha frequency band has been associated with infant attention, working memory, and inhibitory control (Cuevas et al., 2012, Swingler et al., 2018, Whedon et al., 2016). EEG power is positively associated with executive function performance during infancy, toddlerhood, and early childhood (Bell, 2002, Cuevas et al., 2012, Orekhova et al., 2001), with ADHD recognized in clinical practice as an impairment of executive function (Brown, 2009, Semrud-Clikeman et al., 2010). Much research has focused on determining the predominant EEG frequencies at specific ages across the lifespan, as well as the functional significance of specific frequency. The 6–9 Hz frequency alpha band has been used to examine a variety of cognitive constructs in infancy EEG research, much like the 8–13 Hz alpha band activity reflecting attentional demands and executive functioning in adulthood (Bell and Cuevas, 2012, Cuevas and Bell, 2022, Ray and Cole, 1985, Klimesch, 1999, Missonnier et al., 2006). In infancy, the alpha band is typically defined as a 6–9 Hz range (Cuevas and Bell, 2022), in contrast to the 8–13 Hz alpha band used in adult studies. This lower range is considered appropriate for infancy research, as it reflects the developing neural architecture in younger populations. Studies often use the infant alpha band to examine early cognitive and attentional constructs, much like the adult alpha band is used to explore attentional demands and executive functioning. The difference in frequency ranges between infants and adults highlights developmental changes in brain maturation, with the alpha band serving as a marker of cognitive engagement relevant to each age group. The current study focuses on the infant alpha frequency band due to prior research reporting the relationship between reduced EEG power in the alpha band frequency and ADHD symptomatology to understand if this pattern is present early in life prior to diagnosis (Debnath et al., 2021; McLaughlin et al., 2010).
1.1. Electroencephalographic studies in ADHD
EEG studies on ADHD have examined age groups from 6 to 55, with task-related EEG power offering meaningful insights into ADHD symptoms (for review, see Slater et al., 2022). EEG measures the brain’s electrical activity across different frequency bands, defined by the speed of brain wave oscillations. A common approach to EEG analysis is dividing the frequency spectrum into discrete ranges, or 'bands,' to assess the amplitude, or 'power,' of each band. These include delta, theta, alpha, and beta waves, each associated with distinct types of brain activity. Delta waves are the slowest, typically present during deep sleep. Theta waves are linked to light sleep, relaxation, and, occasionally, daydreaming. Alpha waves occur when individuals are awake, commonly reflecting attentional processes and cognitive processing, while beta waves are faster and arise during active thinking and focused tasks. EEG power represents the strength of these waves, which reflects underlying brain circuitry. Although EEG power can vary greatly between individuals, it remains relatively stable within an individual under consistent conditions (e.g., resting with eyes closed) and changes in response to task demands and developmental stages (for review, see Zietsch et al., 2007). This stability makes EEG power a valuable tool for studying brain activity across diverse tasks and populations, especially for distinguishing ADHD from typical development (Hughes and John, 1999; Zietsch et al., 2007). Theta/alpha and theta/beta ratios have also been investigated as neurophysiological indicators of ADHD, but the reliability of ratio measures is mixed and EEG power has been found to be a better neuromarker for adult ADHD compared to ratios (Kiiski, et al., 2020).
Adults with ADHD show lower EEG power in the alpha and beta bands during attention tasks compared to controls, suggesting reduced cortical resources for attention (Hasler et al., 2016). Loo and colleagues (2009) reported that adults in a control group exhibited increased EEG power in the alpha band during the Continuous Performance Task (CPT)–a common assessment of attention–while the ADHD group maintained consistent EEG power throughout the task. This finding suggests that the control group adapted to the task, whereas the ADHD group did not. Frontal EEG power in the alpha band and clinical assessments (behavior rating inventory of executive function) have also been found to predict ADHD treatment response (AUC = 0.83) in a double-blind randomized control trial (Loo et al., 2021). These differing findings may be explained by variations in task demands, participant characteristics, or methodological differences. For instance, the observation of increased alpha power during the CPT could indicate a distinct response in individuals with ADHD to sustained attention tasks, potentially reflecting increased effort or compensatory mechanisms in attention processing (Loo et al., 2009). In contrast, studies reporting lower alpha and beta power often involve different types of tasks or resting-state measurements, which might reveal underactivity in neural circuits related to attention and cognitive control, as hypothesized in ADHD (Woltering et al., 2012). These variations highlight that EEG power changes in ADHD may not be uniform across conditions but instead could reflect differences in how individuals with ADHD respond to specific task demands (Deiber et al., 2020). Our study aims to build on this body of work by examining EEG power in a context that carefully considers task type and participant characteristics to clarify these observed patterns.
Similar findings have been shown with children and adolescents diagnosed with ADHD, who exhibit lower EEG power values compared to typically developing control groups in the alpha band (Chabot et al., 1996; Lenartowicz et al., 2018; Murias et al., 2007; Sánchez-González & García-Zapirain, 2017). A common finding is that EEG power in the alpha band is attenuated in ADHD in the frontal region, suggesting that decreased frontal alpha power may be an important neuromarker in childhood ADHD (Loo et al., 2009, Swartwood et al., 2003). McLaughlin and colleagues (2010) noted significant reductions in frontal EEG power among children with ADHD, and these EEG patterns were specific to later ADHD symptoms unrelated to depression, anxiety, or disruptive behaviors. Debnath and colleagues (2021) similarly noted children with ADHD exhibited lower EEG power in the alpha band compared to a control group, and reduced EEG power in the alpha band was associated with higher ADHD symptoms assessed by the Child Behavior Checklist (Achenbach, 2001) Attention Problems Syndrome Scale. Cortese et al., (2023) conducted a systematic review including a total of 4164 children and adolescents with ADHD and 7363 controls from 19 countries and reported alpha EEG power as the neurophysiological biomarker showing the highest number of significant effects related to ADHD across 53 studies. EEG shows promise for identifying the neuromarkers of ADHD in children, adolescents, and adults, but patterns of brain electrical activity of ADHD in infancy are understudied. EEG activity during infancy can be important for understanding the developmental trajectory of the disorder across the lifespan and prior to potential interaction effects from ADHD medication or treatments (Michelini, et al., 2022).
1.2. Attention in infancy
Attention is generally considered to comprise several systems with each network exhibiting a unique developmental trajectory and associated neurocircuitry (e.g., Colombo and Cheatham, 2006; Posner and Fan, 2008). Infant looking behavior is a complex system influenced by several factors that reflects developmental processes, with changes that have cascading effects on later attention and learning (Oakes, 2023). The orienting attention network is responsible for the selection of sensory inputs (i.e., disengaging fixation and voluntary shifts in visual attention), shows early functional capabilities between 3 and 6 months of age (Colombo, 2001, Courage et al., 2006), and continues to develop into childhood. Individual differences in infant attention and voluntary visual disengagement are thought to reflect the speed of information processing and represent one of the earliest signs of attentional control during early development (Moyano et al., 2023; Oakes, 2023).
Systematic research has revealed that infants who demonstrate brief visual fixations (i.e., short lookers) during stimulus exposure process information more efficiently than infants who demonstrate long visual fixations (i.e., long lookers; Reynolds and Romano, 2016). Short lookers encode the global features while long lookers encode the local features (i.e., holistic versus elemental encoding) and may show slower disengagement and shifting of visual attention (for review see Colombo et al., 2010). Infant short lookers exhibit higher executive function (e.g., working memory, inhibitory control, cognitive flexibility, attentional control) throughout early childhood compared to long lookers, even after controlling for verbal ability–a potential indicator of intelligence (Cuevas and Bell, 2014). Shorter peak fixation times have been linked to greater learning of the environment and are thought to be indicative of advanced levels of attentional control, demonstrated by mastery over lower level components such as shifting and disengagement (Colombo, 2001, Colombo, 2002, Conejero and Rueda, 2017, Ruff and Rothbart, 1996). Miller and colleagues (2018) in a longitudinal study spanning over a decade, observed that infants who were later diagnosed with ADHD in childhood exhibited longer looking times during infancy. This finding suggests that behavioral indicators of ADHD may manifest early in development. However, there is limited research aimed at understanding the potential neuromarkers that underlie these variations in infant looking behavior and their connection with later ADHD.
In previous research with infants in this study, we reported that when comparing the EEG activity at 5-months-old of short and long lookers, frontal EEG of short lookers during a post-distress video exhibits higher power values compared to long lookers (Diaz and Bell, 2011). Attention and cognitive processes mature during early childhood and this is thought to be driven by neurodevelopmental changes in the frontal lobe (Bell, 1998), and many common regions of the frontal lobe are recruited by cognitive demands involved in attentional processes (Duncan and Owen, 2000, Eng et al., 2022). Therefore, in the current study, we examined behavioral differences in infant attention and focused on frontal brain EEG activity, which have been found to be predictors of cognitive functions related to ADHD symptoms.
1.3. Gap: infant EEG neuromarkers of ADHD
There is a dearth of data on attention and corresponding EEG patterns in infants with later ADHD symptoms (Johnson et al., 2015). A goal of our study was to investigate the attention behavior and neural patterns in infancy of children later diagnosed with ADHD compared to a matched control group, as well as to use electrophysiology to investigate the potential brain-behavior neuromarkers of childhood ADHD. We hypothesized that children diagnosed with ADHD would exhibit longer peak looking times in infancy–indicative of less developed attention and information processing–compared to the control group. We also hypothesized that children diagnosed with ADHD would exhibit lower EEG power in infancy during an attention task at the 6–9 Hz alpha frequency bands in the frontal region.
Childhood and adulthood ADHD have been characterized by lower EEG power in the frontal region of the brain relative to control groups, so we expected to find similar results in infancy during an attention task (Loo et al., 2009, Murias et al., 2007, Woltering et al., 2012). In this novel retrospective study, we compared looking behavior and brain electrical activity during an attention task at 5 months of age of 40 infants diagnosed at childhood for ADHD to those of 40 matched-control infants. We also examined the associations between behavioral attention and frontal EEG patterns in infancy in addition to developmental patterns of ADHD-related problems assessed by the CBCL (Achenbach, 2001) Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented and Attention Problems Syndrome Scales at ages 4 and 9.
2. Materials and methods
2.1. Participants
Participants were part of a longitudinal study examining the development of executive functions and emotion regulation from infancy through middle childhood (the CAP Study). The study included 410 infants across three cohorts in two research locations. Cohort 1 (n = 106) and cohort 2 (n = 105) were recruited in a rural college town (Blacksburg, VA) and cohort 3 (n = 199) was recruited in a mid-sized city (Greensboro, NC), both in the mid-Atlantic region of the United States. Infants were recruited via commercial mailing lists, newspaper birth announcements, and word of mouth.
The electrophysiological and behavioral data acquired during the initial research lab visit at 5 months were the focus of our study. Maternal report at subsequent lab visits (ages 6 or 9 years) as part of the larger longitudinal study was used to classify children into ADHD and non-ADHD categories. A question from a demographics questionnaire asked: Does your child currently have a doctor’s diagnosis of ADHD/ADD? According to mothers’ responses, 41 children had received a diagnosis. One of these children participated in the CAP Study exclusively through questionnaires across the entirety of the study and did not come in for lab visits. Because of our main focus on infant EEG and looking behavior collected in the laboratory, we excluded this child from the analyses due to missing data. Thus, our report is based on the remaining 40 children with a diagnosis.
Of the 40 children in the ADHD group (28 males and 12 females; 30 White, 6 Black or African American, 4 Multi-racial; 4 Hispanic, 36 not Hispanic), 17 were from the Blacksburg research location and 23 were from the Greensboro research location. For our focus on the 5-month data, each infant who was subsequently assigned to the ADHD group was matched with an infant from the same longitudinal study on a “matched pairs” basis to form the 40 children in the control group (see Table 1 for demographic information, by group). Care was taken in matching so that both infants in each pair were from the same research location and were of the same sex, race, and ethnicity. The infants were then matched as closely as possible for maternal education (with only one pair out of the 40 having a difference between technical school and graduate school), birth weight (in pounds), length of gestation (in weeks), age at the 5-month lab visit (in days), maternal age at the infant’s birth, paternal age at the infant’s birth, and maternal handedness. Because handedness has a heritable component (McManus, 2007) and maternal handedness was available from the larger longitudinal study, we used maternal handedness as a proxy to control for this factor. Maternal handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). A score over 5 indicates right-handedness. Mothers were right handed or had a tendency to right handedness (38 right-handed in the control group; 37 right-handed in the ADHD group).
Table 1.
Demographic Information, by Group.
| Matched Pair Variable | ADHD group (n = 40) | Control group (n = 40) |
|---|---|---|
| Research Location | 23 Greensboro, NC 17 Blacksburg, VA |
23 Greensboro, NC 17 Blacksburg, VA |
| Sex | 28 male 12 female |
28 male 12 female |
| Race | 30 White 6 African American or Black 4 Multi-racial |
30 White 6 African American or Black 4 Multi-racial |
| Ethnicity | 36 Not Hispanic 4 Hispanic |
36 Not Hispanic 4 Hispanic |
| Maternal Education Level Completed |
14 High School Diploma 3 Technical School 14 College 9 Graduate School |
14 High School Diploma 2 Technical School 14 College 10 Graduate School |
| Birth Weight (lbs) | 7.78 (1.26) | 7.78 (1.04) |
| Gestation (weeks) | 39.42 (1.52) | 39.25 (1.03) |
| Age (days) | 161.50 (8.71) | 163.43 (8.53) |
| Maternal Age (years) | 29.73 (6.31) | 30.36 (5.38) |
| Paternal Age (years) | 31.87 (6.18) | 32.50 (6.28) |
| Maternal Handedness | 8.91 (2.10) | 9.38 (1.78) |
Note: For all continuous variables, the Mean and (Standard Deviation) are reported. VA = Virginia. NC = North Carolina. lbs = pounds. Maternal Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971).
The experimental protocol was approved by the University Institutional Review Boards at each research location (Virginia Tech for the Blacksburg location; University of North Carolina at Greensboro for the Greensboro location). Data were collected in both research locations using identical protocols. Research assistants from both locations were trained together by the project’s principal investigator (final author) on protocol administration, data collection, and psychophysiological coding. To ensure that identical protocol administration and coding criteria were maintained between the labs, the Blacksburg team periodically reviewed DVD recordings and psychophysiology files collected by the Greensboro lab, provided reliability coding for all behavioral data, and verified artifact screening for psychophysiology data collected and coded by the Greensboro lab.
2.2. Procedure
Upon arrival at the research laboratory for the 5-month-old visit, infants and their mothers were greeted, procedures were described, and signed consent was obtained from the mothers. Mothers were paid for participation in the study. Infants were seen no later than three weeks after their 5-month birth date. The infant sat on the mother's lap while an electrode cap was placed on the scalp. EEG was recorded during the entire lab visit and the session was videotaped for off-line coding purposes. Baseline physiology was recorded and then the infant participated in a series of cognitive, affective, and mother-infant interaction tasks that are not part of this report. Associated with this report is the attention task that occurred after the cognitive tasks and before any of the affective and interaction tasks (e.g., Blankenship et al., 2019; Morasch and Bell, 2012; Whedon et al., 2020).
2.3. EEG recording and processing
EEG was recorded from 16 scalp locations (international 10–20 configuration), referenced to Cz, using an Electro-Cap (Eaton, OH: E-1 series cap). After the EEG cap was placed on the head, a small amount of abrasive gel was inserted into each electrode and the scalp gently rubbed. Afterwards, a small amount of conductive gel was inserted, and electrode impedances were measured and accepted if they were below 10 K ohms. The electrical activity from each EEG cap electrode was individually amplified with James Long Company Bioamps (James Long Company, Caroga Lake, NY). During data collection, the high pass filter was a single pole RC filter with a 0.1 Hz cutoff (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two pole Butterworth type with a 100 Hz cutoff (3 dB or half-power point) and 12 dB octave roll-off. Activity for each lead was displayed on the monitor of an acquisition computer. The EEG signal was digitized at 512 Hz to eliminate the effects of aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp., Southfield, MI). Prior to recording EEG for each child, a 10-Hz, 50 uV peak-to-peak sine wave was input through each amplifier and this calibration signal digitized for 30 s. Spectral analysis of the calibration signal and computation of power at the 9–11 Hz frequency band was used to calibrate the power derived from the subsequent spectral analysis of the EEG. After calibration, EEG data were examined and analyzed using the EEG Analysis software that was developed by the James Long Company. First, EEG data were re-referenced via software to an average reference configuration to eliminate concerns that power values at each active site reflect interelectrode distance as much as they reflect electrical potential. Then, average reference EEG data were artifact-scored for eye movements by using electrodes Fp1 and Fp2 to examine peak-to-peak criterion of 100 μV or greater. The EEG data also were artifact-scored for gross motor movements by using a peak-to-peak criterion of 200 μV or greater. Only artifact-free data were used in subsequent analyses. The data were then analyzed with a discrete Fourier transformation, using a Hanning window of 1-s width and 50 % overlap. EEG power was computed for the 6–9 Hz alpha frequency band, which is the dominant band from 5 to 51 months (Marshall, et al., 2002). EEG power was expressed as mean square microvolts, and the data were transformed by using the natural log (ln) to normalize the distribution. EEG power recorded from the frontal electrodes (Fp1, Fp2, F3, F4, F7, F8) during the attention task was averaged to compute frontal brain activity.
2.4. Infant attention task
During the attention task, infants sat on mothers’ laps approximately 1.5 m from a television (45 cm diagonal screen). Infants were shown 45 seconds of a video clip depicting a visually dynamic musical segment from Sesame Street (Cecile - Up Down, in Out, Over and Under). The video was presented after the cognitive tasks and before any affective and interaction tasks of the larger study (Blankenship et al., 2019, Morasch and Bell, 2012, Whedon et al., 2020). In our study, we focus on a more global form of attentional orienting toward dynamic stimuli by measuring the length of the longest look, also referred to as peak fixation (Courage et al., 2006, Frick et al., 1999; Richards, 1997). While static stimuli have been used to assess peak fixation in prior research, individual infant looking patterns have been found to be consistent across various types of visual stimuli, including dynamic Sesame Street clips, and can reliably distinguish between short-looking and long-looking infants (Reynolds et al., 2013). A video camera above the monitor recorded the infant's behavior throughout the segment. Using Video Coding System software developed by James Long Company, shifts in gaze during the video segment were coded off-line by trained research assistants. Each eye shift was judged as either toward or away from the video screen and the durations of the resulting looks toward the video were analyzed to calculate the peak look. Inter-rater reliability (Cronbach's α ≥.90) was established for at least 20 % for all tasks of the entire sample.
2.5. Childhood ADHD-related symptomatology
The Child Behavior Checklist (CBCL; Achenbach, 2001) was mailed to mothers at 4 years of age (cohorts 2 and 3) and 9 years of age (all 3 cohorts) as part of the larger longitudinal study. Maternal ratings were obtained on 99 problem items, plus descriptions of problems, disabilities, what concerns the mother had most about the child, and the strengths of the child. Ratings were obtained on a three-point Likert scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). We used the CBCL DSM-oriented Attention-Deficit/Hyperactivity Disorder Scale and Attention Problems Syndrome Scale total scores in our study.
3. Results
To investigate whether there were differences in attention task performance and EEG power between the ADHD and the matched-control groups, two-sided matched paired-samples t-tests were performed, with group (ADHD; matched-control) as the independent variable and attention task performance and frontal EEG power as dependent variables. A within-subjects analysis was utilized because the statistical assumption that the independent variable consisting of two categorical, related groups was met due to participants being matched-pairs. Analyses were conducted using R (R Core Team, 2024). Alpha was set at .05 for all tests. Cohen’s d and 95 % confidence intervals (CIs) were reported for effect size estimates. Because of our matched controls procedure, there were no differences in age at the 5-month lab visit, birth weight, gestation, maternal handedness, or maternal age at birth between the infants in the ADHD and control groups (for all measures t < 1.04, p > .304, Cohen’s d < 0.17).
3.1. Infant attention behavior
Behavioral data on the attention task were not available for three out of the 80 infants: two due to video recording equipment failure and one due to strabismus. Infants’ mean peak fixation time was longer in the ADHD group (M = 21.16 s, SD = 13.87 s) compared to the control group (M = 12.67 s, SD = 7.65 s), paired-sample t = 3.29; Cohen’s d = .76, 95 %CI [.38, 1.13], p = .002. On average, looking duration was 8.50 seconds (SE = 2.58) longer during the attention task in the ADHD group compared to the control group, 95 %CI [3.26, 13.74]. There were four outliers (i.e., average looking duration that deviated >3 SD away from the group mean). With the removal of these outliers, there was still evidence of a main effect of group on mean looking duration, paired-sample t = 4.14; Cohen’s d = 1.02, 95 %CI [.59, 1.44], p = .00024. These results indicate that infants in the ADHD group exhibited longer looking durations compared to infants in the control group (see Fig. 1, for paired box plot).
Fig. 1.
Paired box plot of looking duration in the ADHD and control groups. Boxplot center line identifies the median, the upper whiskers extend from the 75th percentile to the 75th percentile + 1.5 interquartile range, the lower whiskers extend from the 25th percentile to the 25th–1.5 interquartile range. Note: Outliers not displayed (R Core Team, 2024).
We used a logistic regression to investigate whether children’s later ADHD diagnosis (ADHD vs. No ADHD) could be significantly predicted by infants’ mean peak fixation time at 5-months-old. This analysis was performed in R using the MASS Package for logistic regression (Venables and Ripley, 2002). The logistic regression analysis revealed that looking time in infancy is a significant predictor of whether children were later diagnosed with ADHD or not. The logistic regression output revealed a significant coefficient for looking time (β = 0.08, 95 %CI [.03, .14]). The associated Z-value of 2.92 (SE = .026), p = .003 suggests that the effect of longer looking times in infancy on the likelihood of having a later childhood ADHD diagnosis is statistically significant. The logistic model correctly classified a childhood ADHD diagnosis 23 times and without an ADHD diagnosis 17 times, for a total of 40 correct predictions. The logistic regression correctly predicted the classification of ADHD and non ADHD groups 65 % of the time. However, it should be noted that logistic regression coefficients are estimated through an iterative process that requires a sufficient amount of data to converge on stable values. With small samples, estimates can become highly variable or even fail to converge, and these results should be cautiously interpreted as logistic regression should be carefully interpreted with small samples because they can lack the statistical power needed to detect meaningful effects.
3.2. Infant EEG frontal activity
EEG data were not available for six of the 80 infants: two due to excessive artifacts, two due to equipment failure, and two due to EEG recording errors. Infants’ mean frontal EEG activity during the attention task was lower in the ADHD group (M = 1.86, SD = 0.41) compared to the control group (M = 2.12, SD = 0.42), paired-sample t = 2.91; Cohen’s d = 0.50, 95 %CI [0.14, 0.85], p = .006. There were no outliers (i.e., average frontal EEG power that deviated >3 SD away from the group mean). These results indicate that infants in the ADHD group exhibited lower frontal EEG power values during the attention task compared to infants in the control group (see Fig. 2 for paired box-plot).
Fig. 2.
Paired box plot of the mean frontal EEG activity during the attention task for the alpha band, by group. Boxplot center line identifies the median, the upper whiskers extend from the 75th percentile to the 75th percentile + 1.5 interquartile range, the lower whiskers extend from the 25th percentile to the 25th–1.5 interquartile range.
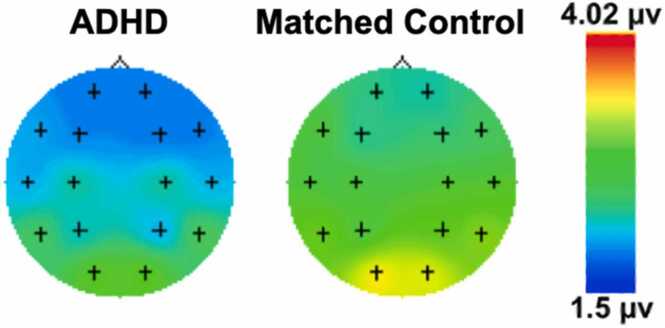
On average, frontal EEG power was 0.26 (SE = 0.09); 95 % CI [0.08, 0.43] higher in the control group compared to the ADHD group (see Fig. 3 for topographic EEG maps detailing group-averaged EEG power between groups for the alpha frequency band).
Fig. 3.
Topographic EEG maps detailing group-averaged EEG Power (referenced to F3 and F4 for the left and right hemisphere, respectively) for the Alpha 6–9 Hz frequency band during the attention task. Legend is shown by the color bar. Note: μV = microvolts.
3.3. Association between behavioral attention task performance and EEG data
We then examined the association between infants’ attention behavior and frontal EEG power. Increased frontal alpha power, r = -.29, 95 % CI[-.06, −.48], p = .014 was negatively associated with attention task looking time (see Fig. 4). In other words, infants who exhibited prolonged looking of visual fixation during the attention task tended to have lower frontal EEG activity.
Fig. 4.
Scatterplot of correlation between behavioral attention task performance and EEG power. Longer looking durations were negatively associated with mean EEG power in the alpha frequency band. Shaded region represents the 95 % confidence interval of the prediction line.
3.4. Developmental patterns of later childhood attention behavior
CBCL questionnaires were completed by 74 mothers at later childhood visits at 4 and 9 years of age in the larger longitudinal study. Data on childhood attention behavior (assessed by the CBCL DSM-oriented Attention-Deficit/Hyperactivity Disorder Scale and Attention Problems Syndrome Scale total scores) were available for 38 children with ADHD and 36 children from the matched-control group (53 participants at 4 years of age and 71 participants at 9 years of age, with data from 49 of these participants at both 4 and 9 years of age). At 4 years of age, childhood attention problems were higher in the ADHD group (M = 9.33, SD = 4.67) compared to the control group (M = 6.25, SD = 4.07), paired-sample t = 2.66; Cohen’s d = 0.54, 95 %CI [0.11, 0.97], p = .014. At 9 years of age, childhood attention problems were higher in the ADHD group (M = 17.00, SD = 5.28) compared to the control group (M = 5.69, SD = 5.31), paired-sample t = 9.04; Cohen’s d = 1.60, 95 %CI [1.07, 2.12], p < .0001 (see Fig. 5 for paired boxplots, by age).
Fig. 5.
Paired box plots of attention problems assessed by the scores of the Child Behavior Checklist (Achenbach, 2001) Attention Problems Syndrome Scale and DSM-oriented and Syndrome Scale at ages 4 and 9, by group. Boxplot center line identifies the median, the upper whiskers extend from the 75th percentile to the 75th percentile + 1.5 interquartile range, the lower whiskers extend from the 25th percentile to the 25th–1.5 interquartile range. Data points were jittered in R by 0.02 to prevent overplotting (R Core Team, 2024).
Longer looking times in infancy during the attention task were positively associated with childhood ADHD-related Symptomatology at age 4, r = 0.39, 95 % CI [.120, .603], p = .006 and childhood ADHD-related Symptomatology at age 9, r = 0.26, 95 % CI [.025, .473], p = .031. In other words, participants who exhibited more attention problems in childhood tended to have longer visual fixations in infancy at 5 months of age.
4. Discussion
Children with a later ADHD diagnosis exhibited decreased frontal EEG activity and longer visual fixations at 5 months of age compared to a matched group controlling for research location, sex, race, ethnicity, maternal education, birth weight, length of gestation, age at lab visit, parental age at child’s birth, and maternal handedness. This finding underscores the valuable role of psychophysiological measures such as EEG and looking behavior in infancy in elucidating the neuromarkers of ADHD. Our findings highlight early neuromarkers–specifically infant look duration and associated frontal EEG power–that may reflect underlying attentional mechanisms potentially relevant to ADHD.
Frick and colleagues (1999) foundational work demonstrated that infants with longer look durations to static stimuli are slower to disengage their visual attention. The authors theorize that developmental and individual differences in look duration are linked to the development of neural attentional systems controlling the ability to disengage visual fixation. While much of the early research on infant visual attention focused on peak look duration during exposure to novel static stimuli (e.g., Colombo, 1995; Frick et al., 1999), subsequent studies have expanded on this work. For example, Reynolds et al., (2011) examined both static and dynamic stimulus types and reported that peak look duration to static stimuli–such as those used by Colombo (1995) to classify short and long lookers–correlated with peak look duration to dynamic stimuli, including the dynamic Sesame Street stimulus used in the current study. This finding bridges earlier work on static stimuli with more recent investigations of dynamic stimuli, which may be particularly ecologically relevant given infants' increasing exposure to screen-based stimuli in the modern digital era. The current study builds on this prior research by demonstrating that individual differences in infant visual attention, as measured by peak look duration to dynamic stimuli, are associated with reduced frontal EEG activity, later childhood attentional problems, and longer looking behavior in infants who are later diagnosed with ADHD. These behavioral findings align with prior reviews stating that individual differences in infant visual fixations may serve as important early markers for deficits in executive attention and risk for attention disorders (Conejero and Rueda, 2017).
A large scale systematic review reported electrophysiological markers of reduced Error-Related Negativity (ERN) and P300 (Pe) amplitudes were associated with behavioral performance (higher error rates) in children and adults with ADHD compared to controls, suggesting altered electrophysiological correlates of underactive monitoring can cause difficulties in self-regulating behavior, particularly when task demands require internal voluntary attention and external cues are not provided (Bellato et al., 2021). Interestingly, the pooled effect sizes were not moderated by age, suggesting that underactive internal performance monitoring mechanisms persist across developmental stages in ADHD. The results of our study are aligned with this framework: infants later diagnosed with ADHD exhibited reduced frontal EEG activity that was associated with longer peak fixation times, behavior linked to lower attentional control (Miller et al., 2018). Alpha power has been connected with cortical excitability and inhibition balance, an aspect increasingly recognized as altered in individuals with ADHD (e.g., Lenartowicz et al., 2014). Following these infants longitudinally, we found differences in later childhood attention behaviors between the groups at ages 4 and 9. Furthermore, infant looking behavior was associated with later childhood ADHD-related symptomatology at both ages, with infants who exhibited longer visual fixations at 5 months of age tending to exhibit more attention problems in childhood at ages 4 and 9.
In the context of ADHD, our findings may indicate an early divergence in the neural substrates that support attentional regulation. This divergence could represent an early imbalance in excitation and inhibition processes, a mechanism hypothesized to contribute to the characteristic attentional and executive function challenges seen in ADHD. While these measures are non-specific and associated with multiple domains of cognitive development, when observed in conjunction with behavioral markers for ADHD, they may represent early neural profiles that predispose individuals to ADHD-related difficulties. By identifying these patterns early, this study contributes to our understanding of possible developmental trajectories that precede ADHD diagnosis, supporting a neurodevelopmental model of ADHD.
Although the reported associations between lower EEG power and looking behavior in infancy are promising and the control group was systematically matched as closely as possible to the ADHD group, this study is not without limitations. First, the sample was not intentionally recruited for ADHD and diagnosis was based on maternal reports of a doctor diagnosis. Second, ADHD is a heterogeneous disorder. The DSM-V maintains there is no single test to diagnose ADHD and there are three presentations (previously called subtypes) of ADHD: predominantly inattentive (ADHD-IN), predominantly hyperactive-impulsive (ADHD-HI), and a combined presentation of displaying both inattentive and hyperactive-impulsive symptoms (ADHD-COM). Slater and colleagues (2022) systematic review with children and adults report resting state and task-related modulation of alpha, beta and theta power, and the event-related potential (ERP) N2 and P3 components may be useful in providing insight into EEG neuromarkers of varying ADHD presentations and symptom severity. However, the results reported here still convey valuable information within a unique developmental sample, potentially aiding in distinguishing shared from unique features based on neuroimaging profiles and understanding how the neural patterns are associated with individual differences in attention behavior in infancy.
Finally, although EEG alpha power has been a predominant metric reported in ADHD studies, other EEG measures have also been investigated, such as ERPs, mean frequency, peak frequency, coherence, aperiodic exponent, and laterality (Lenartowicz et al., 2018). For example, one metric reported to achieve 100 % replication in children and adolescents when comparing ADHD to controls is N2 amplitude–the size of an ERP waveform typically observed between 200 and 350 ms after the presentation of a stimulus, with larger N2 amplitude indicating stronger neural responses to task-relevant stimuli and a smaller amplitude associated with challenges in attention–during commonly used executive function tasks (Parlatini et al., 2024). Karalunas and colleagues (2021) conducted one of the few infant EEG studies on ADHD with 1-month-old infants with a family history of ADHD compared to a control group of infants with no family history of ADHD. The researchers found that infants at risk for ADHD had a larger aperiodic exponent, which indicates a steeper decline in power across frequencies when plotted on a log-log scale and relatively more low-frequency power compared to high-frequency power. A larger aperiodic exponent is indicative of relatively more low-frequency power compared to high-frequency power. These findings are theorized to reflect differences in the balance of excitatory and inhibitory processes, with at-risk infants requiring more neural resources to maintain attention, leading to decreased power in specific bands as these resources are utilized (Karalunas et al., 2021). Examining ERN, N2 and Pe amplitudes with developmentally appropriate executive function tasks with infants at familial risk for ADHD in addition to aperiodic exponent with 5-month-old infants would be important next steps for future research endeavors. Another promising direction for future research is using eye-tracking technology to capture more detailed eye gaze patterns, including moment-by-moment shifts toward or away from task stimuli.
Our study offers empirical evidence supporting the conceptualization of ADHD as a neurodevelopmental disorder, emphasizing the focus on individual differences in attention and brain development. While ADHD is now known to extend into adulthood, our research highlights neurophysiological and behavioral patterns observable from infancy. By addressing the stigma stemming from skepticism about the legitimacy of ADHD as a disorder (Mueller et al., 2012), findings from our study further support the classification of ADHD as a neurodevelopmental disorder with behavioral and neural differences found between groups as young as infancy.
5. Conclusion
This novel retrospective study contributes to our understanding of ADHD through an early development neurocognitive lens, and aids in building a more comprehensive model to supplement existing genetic, environmental, and neurocognitive risk factors of ADHD (Faraone et al., 2015). As ADHD manifests early in development and can have cascading detrimental effects if left undiagnosed or untreated, it is highly valuable to report attention behavior and neuromarkers early on. Our study provides insight into neuromarkers of ADHD–particularly during infancy–a developmental stage that is relatively understudied in relation to the disorder. The findings from our study pave the way for future research on reproducibility and may eventually help in detecting or confirming the presence of ADHD using neuromarkers and behavioral attention early on. Frontal EEG power and longer looking fixations to dynamic stimuli in infancy may contribute to developing a more comprehensive model of ADHD from a developmental neuroscience perspective.
Funding sources
This research was supported by grants R01 HD049878 and R03 HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH. Cassondra M. Eng was supported by grant R25GM066534 from the National Institutes of Health (NIH) Virginia Tech Postbaccalaureate Research Education Program (PREP) awarded to Ed Smith and by training grant T32MH019908 from the NIH awarded to Allan L. Reiss.
CRediT authorship contribution statement
Eng Cassondra M.: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Patton Leslie A.: Project administration, Investigation, Data curation. Bell Martha Ann: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the many families in Blacksburg, VA and Greensboro, NC for their long-term participation in the CAP Study, as well as Susan D. Calkins and her team at the University of North Carolina at Greensboro for their contributions to this project.
References
- Achenbach, T.M., 2001. Manual for ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth & Families..
- Bell, M.A. (1998). Frontal lobe function during infancy: Implications for the development of cognition and attention.
- Bell M.A. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1111/1469-8986.3940450. [DOI] [PubMed] [Google Scholar]
- Bell M.A., Cuevas K. Using EEG to study cognitive development: issues and practices. J. Cogn. Dev.:official J. Cogn. Dev. Soc. 2012;13(3):281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellato A., Norman L., Idrees I., Ogawa C.Y., Waitt A., Zuccolo P.F.…Shephard E. A systematic review and meta-analysis of altered electrophysiological markers of performance monitoring in Obsessive-Compulsive Disorder (OCD), Gilles de la Tourette Syndrome (GTS), Attention-Deficit/Hyperactivity disorder (ADHD) and Autism. Neurosci. Biobehav. Rev. 2021;131:964–987. doi: 10.1016/j.neubiorev.2021.10.018. [DOI] [PubMed] [Google Scholar]
- Blankenship T.L., Slough M.A., Calkins S.D., Deater-Deckard K., Kim-Spoon J., Bell M.A. Attention and executive functioning in infancy: Links to childhood executive function and reading achievement. Dev. Sci. 2019;22(6) doi: 10.1111/desc.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.E. ADD/ADHD and impaired executive function in clinical practice. Curr. Atten. Disord. Rep. 2009;1(1):37–41. doi: 10.1007/s11920-008-0065-7. [DOI] [Google Scholar]
- Chabot R.J., Merkin H., Wood L.M., Davenport T.L., Serfontein G. Sensitivity and specificity of QEEG in children with attention deficit or specific developmental learning disorders. Clin. EEG (Electroencephalogr. 1996;27(1):26–34. doi: 10.1177/155005949602700105. [DOI] [PubMed] [Google Scholar]
- Colombo J. On the neural mechanisms underlying developmental and individual differences in visual fixation in infancy: two hypotheses. Dev. Rev. 1995;15(2):97–135. doi: 10.1006/drev.1995.1005. [DOI] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annu. Rev. Psychol. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant attention grows up: the emergence of a developmental cognitive neuroscience perspective. Curr. Dir. Psychol. Sci. 2002;11(6):196–200. doi: 10.1111/1467-8721.00199. [DOI] [Google Scholar]
- Colombo J., Cheatham C.L. The emergence and basis of endogenous attention in infancy and early childhood. Adv. Child Dev. Behav. 2006;34:283–322. doi: 10.1016/s0065-2407(06)80010-8. [DOI] [PubMed] [Google Scholar]
- Colombo J., Kapa L., Curtindale L.M. Oxford University Press; 2010. Varieties of Attention in Infancy. [DOI] [Google Scholar]
- Conejero A., Rueda M.R. Early development of executive attention. J. Child Adolesc. Behav. 2017;05(02) doi: 10.4172/2375-4494.1000341. [DOI] [Google Scholar]
- Cortese S., Solmi M., Michelini G., Bellato A., Blanner C., Canozzi A.…Correll C.U. Candidate diagnostic biomarkers for neurodevelopmental disorders in children and adolescents: a systematic review. World Psychiatry. 2023;22(1):129–149. doi: 10.1002/wps.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage M.L., Reynolds G.D., Richards J.E. Infants' attention to patterned stimuli: developmental change from 3 to 12 months of age. Child Dev. 2006;77(3):680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. Infant attention and early childhood executive function. Child Dev. 2014;85(2):397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. EEG frequency development across infancy and childhood. Oxf. Handb. Hum. EEG Freq. Anal. 2022:293–323. doi: 10.1093/oxfordhb/9780192898340.013.13. [DOI] [Google Scholar]
- Cuevas K., Hubble M., Bell M.A. Early childhood predictors of post-kindergarten executive function: behavior, parent-report, and psychophysiology. Early Educ. Dev. 2012;23(1):59–73. doi: 10.1080/10409289.2011.611441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R., Miller N.V., Morales S., Seddio K.R., Fox N.A. Investigating brain electrical activity and functional connectivity in adolescents with clinically elevated levels of ADHD symptoms in alpha frequency band. Brain research. 2021;1750:147142. doi: 10.1016/j.brainres.2020.147142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber M.-P., Hasler R., Colin J., Dayer A., Aubry J.-M., Baggio S., Perroud N., Ros T. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG Neurofeedback. NeuroImage: Clin. 2020;25 doi: 10.1016/j.nicl.2019.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A., Bell M.A. Information processing efficiency and regulation at five months. Infant Behav. Dev. 2011;34(2):239–247. doi: 10.1016/j.infbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- DuPaul G.J., McGoey K.E., Eckert T.L., VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(5):508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Eng C.M., Fulton V., Moron S., Pocsai M., Thiessen E., Fisher A. Longitudinal investigation of executive function development employing task-based, teacher reports, and fNIRS multimethodology in 4-to 5-year-old children. Dev. Sci. 2022;25(6) doi: 10.1111/desc.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V., Asherson P., Banaschewski T., Biederman J., Buitelaar J.K., Ramos-Quiroga J.A., Rohde L.A., Sonuga-Barke E.J., Tannock R., Franke B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Frick J.E., Colombo J., Saxon T.F. Individual and developmental differences in disengagement of fixation in early infancy. Child Dev. 1999;70(3):537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Hasler R., Perroud N., Meziane H.B., Herrmann F., Prada P., Giannakopoulos P., Deiber M.P. Attention-related EEG markers in adult ADHD. Neuropsychologia. 2016;87:120–133. doi: 10.1016/j.neuropsychologia.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Hudspeth W.J., Pribram K.H. Psychophysiological indices of cerebral maturation. Int. J. Psychophysiol.: Off. J. Int. Organ. Psychophysiol. 1992;12(1):19–29. doi: 10.1016/0167-8760(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Hughes J.R., John E.R. Conventional and quantitative electroencephalography in psychiatry. J. Neuropsychiatry Clin. Neurosci. 1999;11(2):190–208. doi: 10.1176/jnp.11.2.190. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Gliga T., Jones E., Charman T. Annual research review: Infant development, autism, and ADHD--early pathways to emerging disorders. J. Child Psychol. Psychiatry, Allied Discip. 2015;56(3):228–247. doi: 10.1111/jcpp.12328. [DOI] [PubMed] [Google Scholar]
- Karalunas S.L., Antovich D., Goh P.K., Martel M.M., Tipsord J., Nousen E.K., Nigg J.T. Longitudinal network model of the co-development of temperament, executive functioning, and psychopathology symptoms in youth with and without ADHD. Dev. Psychopathol. 2021;33(5):1803–1820. doi: 10.1017/S0954579421000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiski H., Bennett M., Rueda-Delgado L.M., Farina F.R., Knight R., Boyle R., Roddy D., Grogan K., Bramham J., Kelly C., Whelan R. EEG spectral power, but not theta/beta ratio, is a neuromarker for adult ADHD. Eur. J. Neurosci. 2020;51(10):2095–2109. doi: 10.1111/ejn.14645. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 1999;29(2-3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., Loo S.K. Use of EEG to diagnose ADHD. Curr. Psychiatry Rep. 2014;16(11):498. doi: 10.1007/s11920-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A., Mazaheri A., Jensen O., Loo S.K. Aberrant modulation of brain oscillatory activity and attentional impairment in attention-deficit/hyperactivity disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3(1):19–29. doi: 10.1016/j.bpsc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S.K., Hale T.S., Macion J., Hanada G., McGough J.J., McCracken J.T., Smalley S.L. Cortical activity patterns in ADHD during arousal, activation and sustained attention. Neuropsychologia. 2009;47(10):2114–2119. doi: 10.1016/j.neuropsychologia.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S.K., Salgari G.C., Ellis A., Cowen J., Dillon A., McGough J.J. Trigeminal nerve stimulation for attention-deficit/hyperactivity disorder: cognitive and electroencephalographic predictors of treatment response. J Am Acad Child Adolesc Psychiatry & Adolescent Psychiatry. 2021;60(7):856–864. doi: 10.1016/j.jaac.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Fox N.A., Zeanah C.H., Sheridan M.A., Marshall P., Nelson C.A. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(4):329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, I.C. (2007). The inheritance of left-handedness. In Ciba Foundation Symposium 162-Biological Asymmetry and Handedness: Biological Asymmetry and Handedness: Ciba Foundation Symposium 162 (pp. 251-281). Chichester, UK: John Wiley & Sons, Ltd. 10.1002/9780470514160.ch15. [DOI] [PubMed]
- Michelini G., Norman L.J., Shaw P., Loo S.K. Treatment biomarkers for ADHD: Taking stock and moving forward. Transl. Psychiatry. 2022;12(1):444. doi: 10.1038/s41398-022-02207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Iosif A.M., Young G.S., Hill M.M., Ozonoff S. Early detection of adhd: insights from infant siblings of children with autism. J. Clin. Child Adolesc. Psychol.: Off. J. Soc. Clin. Child Adolesc. Psychol., Am. Psychol. Assoc., Div. 2018;47(5):737–744. doi: 10.1080/15374416.2016.1220314. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missonnier P., Deiber M.P., Gold G., Millet P., Gex-Fabry Pun M., Fazio-Costa L., Giannakopoulos P., Ibáñez V. Frontal theta event-related synchronization: comparison of directed attention and working memory load effects. J. Neural Transm. (Vienna, Austria.: 1996) 2006;113(10):1477–1486. doi: 10.1007/s00702-005-0443-9. [DOI] [PubMed] [Google Scholar]
- Morasch K.C., Bell M.A. Self-regulation of negative affect at 5 and 10 months. Dev. Psychobiol. 2012;54(2):215–221. doi: 10.1002/dev.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano S., Rico-Picó J., Conejero Á., Hoyo Á., de los Ángeles Ballesteros-Duperón M., Rueda M.R. Influence of the environment on the early development of attentional control. Infant Behav. Dev. 2023;71 doi: 10.1016/j.infbeh.2023.101842. [DOI] [PubMed] [Google Scholar]
- Mueller A.K., Fuermaier A.B., Koerts J., Tucha L. Stigma in attention deficit hyperactivity disorder. Atten. deficit Hyperact. Disord. 2012;4(3):101–114. doi: 10.1007/s12402-012-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M., Swanson J.M., Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb. Cortex. 2007;17(8):1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes L.M. The development of visual attention in infancy: a cascade approach. Adv. Child Dev. Behav. 2023;64:1–37. doi: 10.1016/bs.acdb.2022.10.004. [DOI] [PubMed] [Google Scholar]
- Oakes L.M. The cascading development of visual attention in infancy: learning to look and looking to learn. Curr. Dir. Psychol. Sci. 2023;32(5):410–417. doi: 10.1177/09637214231178744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. Edinburgh handedness inventory. J. Abnorm. Psychol. 1971 doi: 10.1016/0028-3932(71)90067-4. [DOI] [Google Scholar]
- Orekhova E.V., Stroganova T.A., Posikera I.N. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin. Neurophysiol.: Off. J. Int. Fed. Clin. Neurophysiol. 2001;112(5):740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Parlatini V., Bellato A., Gabellone A., Margari L., Marzulli L., Matera E., Petruzzelli M.G., Solmi M., Correll C.U., Cortese S. A state-of-the-art overview of candidate diagnostic biomarkers for Attention-deficit/hyperactivity disorder (ADHD) Expert Rev. Mol. Diagn. 2024;24(4):259–271. doi: 10.1080/14737159.2024.2333277. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Fan J. Attention as an organ system. Top. Integr. Neurosci. 2008:31–61. doi: 10.1017/CBO9780511541681.005. [DOI] [Google Scholar]
- Ray W.J., Cole H.W. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Reuben, C.E., & Elgaddal, N. (2024). Attention-Deficit/Hyperactivity Disorder in Children Ages 5-17 Years: United States, 2020-2022. NCHS Data Brief, no 499, Centers for Disease Control and Prevention National Center for Health Statistics. https://doi.org/10.15620/cdc/148043.
- Reynolds G.D., Romano A.C. The development of attention systems and working memory in infancy. Front. Syst. Neurosci. 2016;10:00015. doi: 10.3389/fnsys.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.D., Guy M.W., Zhang D. Neural correlates of individual differences in infant visual attention and recognition memory. Infancy.: Off. J. Int. Soc. Infant Stud. 2011;16(4):368–391. doi: 10.1111/j.1532-7078.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.D., Zhang D., Guy M.W. Infant attention to dynamic audiovisual stimuli: Look duration from 3 to 9 months of age. Infancy. 2013;18(4):554–577. doi: 10.1111/j.1532-7078.2012.00134.x. [DOI] [Google Scholar]
- Richards J.E. Effects of attention on infants' preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Dev. Psychol. 1997;33(1):22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Ruff H., Rothbart M. Oxford University Press; New York: 1996. Attention in Early Development: Themes and Variations. [DOI] [Google Scholar]
- Sánchez-González A., García-Zapirain B. Electroencephalography Mu rhythm suppression analysis during observation-execution tasks in children with attention-deficit/hyperactivity disorder. J Med. Imag. Health. 2017;7(5):1005–1012. doi: 10.1166/jmihi.2017.2129. [DOI] [Google Scholar]
- Semrud-Clikeman M., Walkowiak J., Wilkinson A., Butcher B. Executive functioning in children with Asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. J. Autism Dev. Disord. 2010;40(8):1017–1027. doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Slater J., Joober R., Koborsy B.L., Mitchell S., Sahlas E., Palmer C. Can electroencephalography (EEG) identify ADHD subtypes? A systematic review. Neurosci. Biobehav. Rev. 2022;139 doi: 10.1016/j.neubiorev.2022.104752. [DOI] [PubMed] [Google Scholar]
- Swartwood J.N., Swartwood M.O., Lubar J.F., Timmermann D.L. EEG differences in ADHD-combined type during baseline and cognitive tasks. Pediatr. Neurol. 2003;28(3):199–204. doi: 10.1016/s0887-8994(02)00514-3. [DOI] [PubMed] [Google Scholar]
- Swingler M.M., Isbell E., Zeytinoglu S., Calkins S.D., Leerkes E.M. Maternal behavior predicts neural underpinnings of inhibitory control in preschoolers. Dev. Psychobiol. 2018;60(6):692–706. doi: 10.1002/dev.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Baldeweg T. Application of EEG, ERP and intracranial recordings to the investigation of cognitive functions in children. Dev. Sci. 2002;5(3):318–334. doi: 10.1111/1467-7687.00372. [DOI] [Google Scholar]
- Team, R.C. (2024). R: A Language and Environment for Statistical Computing. 〈http://www.r-project.org/index.html〉.
- Venables, W.N., & Ripley, B.D. (2002). MASS: Modern Applied Statistics with S [R package]. 〈https://cran.r-project.org/web/packages/MASS/MASS.pdf〉.
- Whedon M., Perry N.B., Calkins S.D., Bell M.A. Changes in frontal EEG coherence across infancy predict cognitive abilities at age 3: The mediating role of attentional control. Dev. Psychol. 2016;52(9):1341–1352. doi: 10.1037/dev0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whedon M., Perry N.B., Bell M.A. Relations between frontal EEG maturation and inhibitory control in preschool in the prediction of children's early academic skills. Brain Cogn. 2020;146 doi: 10.1016/j.bandc.2020.105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering S., Jung J., Liu Z., Tannock R. Resting state EEG oscillatory power differences in ADHD college students and their peers. Behav. Brain Funct. 2012;8(1):1. doi: 10.1186/1744-9081-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietsch B.P., Hansen J.L., Hansell N.K., Geffen G.M., Martin N.G., Wright M.J. Common and specific genetic influences on EEG power bands Delta, Theta, alpha, and beta. Biol. Psychol. 2007;75(2):154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]