Abstract
Background
Diffuse hemispheric glioma, H3 G34R/V-mutant (DHG-H3G34) is characterized by poor prognosis and lack of effective treatment options. DHG-H3G34R further harbor deactivation of alpha-thalassemia/mental retardation syndrome X-linked protein (ATRX; DHG-H3G34R_ATRX) suggesting a unique interaction of these 2 oncogenic alterations. In this study, we dissect their cell biological interplay, investigate the impact on telomere stabilization, and consequently validate a targeted therapy approach.
Methods
We characterized patient-derived primary pediatric high-grade glioma (pHGG) models for telomere-maintenance mechanisms, DNA damage stress (including protein expression, pH2AX/Rad51 foci, cell-cycle arrest) and their sensitivity towards poly-ADP ribose polymerase inhibitor (PARPi) combinations. Human induced pluripotent stem cells (iPSCs) were used for modeling the disease. The anticancer activity of PARPi combinations in vivo was studied in Chorioallantoic Membrane (CAM) and orthotopic in vivo experiments. Finally, we treated a DHG-H3G34R_ATRX patient with PARPi combination therapy.
Results
We elaborate that alternative lengthening of telomeres (ALT) is a key characteristic of DHG-H3G34R_ATRX. A dominant cooperative effect between H3G34R and ATRX loss in ALT activation also became apparent in iPSCs, which endogenously exert telomerase activity. In both, patient-derived DHG-H3G34R_ATRX models and H3G34R+/ATRX- iPSCs, the ALT-phenotype was associated with increased basal DNA damage stress, mediating synergistic susceptibility towards PARPi (talazoparib, niraparib) combinations with topoisomerase-I inhibitors (topotecan, irinotecan). In a first-of-its-kind case, treatment of a DHG-H3G34R_ATRX patient with the brain-penetrant PARP inhibitor niraparib and topotecan resulted in significant tumor reduction.
Conclusions
Our preclinical and clinical data strongly support the further development of PARPi together with DNA damage stress-inducing treatment regimens for DHG-H3G34R_ATRX.
Keywords: ATRX, DNA damage, diffuse hemispheric glioma, H3G34R, PARP inhibitor
Graphical Abstract
Graphical Abstract.
Key Points.
H3G34R mutation and loss of ATRX synergistically drive alternative lengthening of telomeres.
Concomitant H3G34R- and ATRX-mutation increase DNA damage stress.
Combination of PARP and topoisomerase inhibitors is effective against DHG-H3G34R_ATRX.
Importance of the Study.
In this study, we identify DHG-H3G34R_ATRX as an ALT-driven tumor. Our analyses in iPSCs unravel that H3G34R mutation and ATRX loss synergistically and dominantly drive replicative immortality through ALT initiation. By exacerbating the resulting intrinsic DNA damage stress, a combination of PARP inhibitors with topoisomerase or DNA repair-targeting compounds exerts synergistic activity specifically against DHG-H3G34R_ATRX. Accordingly, our study provides first-of-its-kind clinical evidence that this rational combination therapy can be translated into improved management of DHG-H3G34R_ATRX patients, struggling with an aggressive and high-risk brain tumor type.
Molecular profiling studies have disentangled distinct subtypes within pediatric high-grade glioma (pHGG). Epigenetic perturbations caused by mutations in H3F3A (histone H3.3, H3), including H3K27M or H3G34R/V, are enriched in tumors of children, as well as adolescents and young adults (AYAs), respectively, suggesting distinct modes of oncogenesis.1,2 While H3K27M mutations occur in younger patients and in midline/pontine locations (diffuse midline glioma, H3 K27-altered, DMG-H3K27M), H3G34R/V mutations are typically found in AYAs in the cerebral hemispheres.1 Patients suffering from diffuse hemispheric glioma, H3 G34-mutant (DHG-H3G34) exhibit poor therapy response and prognosis.1,3 Therefore, the development of effective treatment options remains utterly important.
Unlimited replicative potential preventing critical telomere shortening is a key characteristic of aggressive glioma, achieved by either reactivation of telomerase reverse transcriptase (TERT) or alternative lengthening of telomeres (ALT).4,5 Reactivation of telomerase induced via TERT promoter (pTERT) mutations is a predictor of negative prognosis in HGG.6–8 Interestingly, H3G34R mutations frequently coexist with loss of alpha-thalassemia/mental retardation syndrome X-linked protein (ATRX),1,9 functionally associated with ALT.10 However, the molecular mechanisms and cellular effects of H3G34R and ATRX co-mutation (H3G34R_ATRX) and potential interaction with telomere stabilization remain elusive.
Recent sequencing analyses across various pediatric cancer types have identified mutations in the double-strand break (DSB) repair pathway, involving breast cancer genes 1/2 (BRCA1/2) as potentially targetable events in brain cancers.11 These findings support the role of defective DNA repair, so-called “BRCAness,” in pHGG, suggesting poly-ADP-ribose polymerase inhibitors (PARPis) as a feasible therapeutic option. PARP is a central protein in both single-strand break (SSB) and DSB repair. Its inhibition is one mainstay in targeted treatment of breast, ovarian, and prostate tumors with impaired homologous recombination (HR) repair.12,13 Previous work has demonstrated that loss of ATRX in preclinical HGG models is associated with enhanced sensitivity towards DSB-inducing drugs.14 Additionally, DHG-H3G34 has been recently associated with DNA repair deficiencies.15 However, the interplay of H3G34R_ATRX and DNA repair remains incompletely understood.
In this study, we uncover synergistic priming of ALT by a distinct cooperative effect of H3G34R_ATRX in DHG (DHG-H3G34R_ATRX) primary patient-derived tumor cells, as well as in human induced pluripotent stem cells (iPSCs). With respect to DHG-H3G34R_ATRX therapy, enhanced DNA repair dependency causes synthetic lethality of PARPi combinations with DNA-damaging agents or DNA repair inhibitors. The clinical relevance was validated by a significant response of a DHG-H3G34R_ATRX patient to this combination treatment approach.
Materials and Methods
For detailed protocols see Supplementary Methods.
Ethics Approval and Consent to Participate
The study was approved by the institutional review board of the Medical University of Vienna (MUV, EK Nr. 1244/2016). Informed consent for study participation was obtained from patients and/or their legal representatives.
Cell Culture of Patient-Derived Cell Models and Human iPSCs
Primary tumor cell models were established from fresh tissue biopsies obtained from surgical intervention. Feeder-free iPSCs 176/1 were reprogrammed from fibroblasts of healthy donors.
Generation of ATRX-Knockout and H3G34R-Overexpressing iPSCs
Plasmids16 were modified to include genome-integrable fluorescent marker cassettes to control successful plasmid delivery through the Sleeping Beauty-transposase system (pCAG-SB100X). For ATRX-mutation constructs, an IRDR-L-CAG-GFP-IRDR-R sequence was cloned into the CRISPR–Cas9 vector pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene, 42230) containing ATRX-targeting gRNAs as described.16 For overexpression constructs, IRDR-L-CAG-X-IRDR-R (X = H3.3 WT or H3G34R) plasmids were modified to co-express a dTomato fluorophore. An overview of the constructs and experimental layouts is given in Supplementary Figures 1 A and B.
Cell Viability Assays and Analysis of Drug Effects
Cells were seeded in 96-well plates and exposed to the indicated drugs for 72 hours. Viability was measured by CellTiter-Glo assay (Promega). CalcuSyn software (Biosoft) was applied to evaluate drug combination effects,17 expressed as combination indices (CI): values <0.9 = synergism; 0.9 to 1.1 = additive effects; >1.1 = antagonism.
3D Tumoroid Assay
Cells were seeded in non-adherent U-bottom-shaped 384-well plates. After 24 hours, drugs were added for 72 hours. Thereafter, the medium was refreshed for another 72 hours of drug incubation. Sphere growth was monitored with an Incucyte system (Sartorius) and tumoroid viability was measured using CellTiter-Glo 3D.
Western Blot
Cells were seeded in 6-well plates and exposed to drug treatment for 24 hours. Protein extracts were prepared and Western blots performed as published.18Supplementary Table 1 lists all antibodies used.
Immunostaining Procedures
For immunohistochemistry (IHC), paraffin-embedded tumor sections were deparaffinized and rehydrated. Blocking and antigen retrieval were followed by antibody incubation (Supplementary Table 1) and detection using the UltraVision LP detection system (Thermo Fisher). Stainings were quantified using ImageJ (Fiji) or the histomorphometric software HALO (Indica Labs).
For multiplexed immunofluorescence analysis, tumor sections were processed using the Opal Polaris 7 Color Manual IHC Detection Kit (Akoya Biosciences) and scanned at the PhenoImager Fusion (Akoya Biosciences). Image analysis was carried out by inForm analysis software (Akoya Biosciences). Supplementary Table 1 lists all antibodies used.
For in vitro experiments, cells were seeded on 10-well polystyrene cell culture slides (CELLVIEW, 543979, Greiner bio-one) and treated for 24 hours. Pre-extraction and staining with phospho-histone H2A.X and Rad51 antibodies were performed as described in Supplementary Materials. Photographs were taken on the IXplore Spinning Disk Confocal (Olympus), and pH2AX/Rad51 foci were quantified using scanR (Olypmus) software.
Flow Cytometry for Cell-Cycle Analysis
Cells were seeded in 6-well plates and exposed to drug treatment for 24 hours. Afterward, cells were stained with propidium iodide and analyzed on an LSR Fortessa flow cytometer (BD Biosciences) using the 610/20 bandpass emission filter. Analysis was performed with FlowJo v10.06 (BD Biosciences; for gating strategy see Supplementary Figure 2).
Analysis of Telomere-Maintenance Mechanisms
HCT116/SKOV-3 and Saos-2/U2OS cells represented TERT-positive and ALT-positive controls, respectively.
Telomerase activity was analyzed by Telomerase Repeat Amplification Protocol (TRAP-assay) as previously described.19
Telomere lengths were detected on metaphase chromosome preparations following standard protocols, applying the Telomere PNA FISH Kit/FITC (K5325, Dako). Images were captured with a DMRXA fluorescence microscope (Leica).
For detection of c-circles 32 ng of DNA were used. C-circle assay (CCA) was performed using telomeric qPCR as previously described.20,21
Sequencing Experiments
Whole-exome sequencing (WES) of patient-derived cells was done on NovaSeq 6000 (Illumina) using 100 bp paired-end chemistry. Data were analyzed by alignment to the hg19 reference genome using Burrows-Wheeler Aligner (BWA MEM) and with Picard (deduplication). Variants were called with GATK Haplotype Caller and annotated with ANNOVAR.
Bulk RNA-sequencing of iPSCs was done on Illumina NovaSeq X. Reads were analyzed using genome and gene annotation for the GRCh38/hg38 assembly obtained from Homo sapiens Ensembl release 102. Reads were aligned using star v2.6.0c, processed using collapse_UMI_bam (Lexogen), and counted with featureCounts (subread v1.6.2) using strand-specific read counting (-s 1). Downstream analyses were performed in R statistical environment v4.2.0.
Irradiation Experiments
Cells seeded in 24-well plates were exposed to drug treatment combined with irradiation (2 Gy) using Maxishot (Yxlon International GmbH). After a 7-day recovery, cell clones were stained with crystal violet and quantified for color absorbance/well with the Tecan Infinite 200Pro.
In Vivo Experiments
DHG-H3G34R_ATRX patient-derived tumor cells were used for chorioallantoic membrane (CAM) and orthotopic xenografts. CAM assays were performed before hatching, and thus, did not require ethical approval.22 Briefly, tumor cells were inoculated on embryo development day (EDD) 9 onto the CAM. On EDD10, drug treatment was initiated by pipetting the drugs onto the CAM, and repeated on EDD12, EDD14, and EDD16. On EDD17, tumor volumes were measured, and tumors were embedded for IHC analysis.
For the orthotopic animal experiment, near-infrared fluorescent protein (iRFP) labeled tumor cells were implanted via the postglenoid foramen into the brains of NSG mice. Upon detection of tumor development, mice were randomized to control, topotecan monotherapy (0.5 mg/kg/day i.v.) or combination therapy with niraparib (5 mg/kg/day p.o.). The experiment was conducted in an AAALAC-accredited animal facility under permit G23/036 of the Regierungspräsidium Freiburg im Breisgau, Germany.
Case Study
Data was retrieved from medical records at the General Hospital/MUV. The diagnosis was confirmed by methylation analysis and next-generation sequencing. Treatment with niraparib and topotecan was based on off-label use under close clinical and laboratory monitoring. MRI, magnetic resonance spectroscopy (MRS), as well as PET imaging, were conducted per clinician discretion to monitor tumor response and further analyzed by central radiological assessment according to response assessment in pediatric neuro-oncology criteria.
Statistical Analysis
Appropriate statistical tests are described in the respective figure legends. The NEJM P-value style was chosen for all analyses. P-values <0.05 were considered statistically significant. *P <0.05; **P <0.01; ***P <0.001.
Results
H3G34R_ATRX Co-mutations Segregate With ALT-Phenotype in Primary Patient-Derived and Human iPSC Models
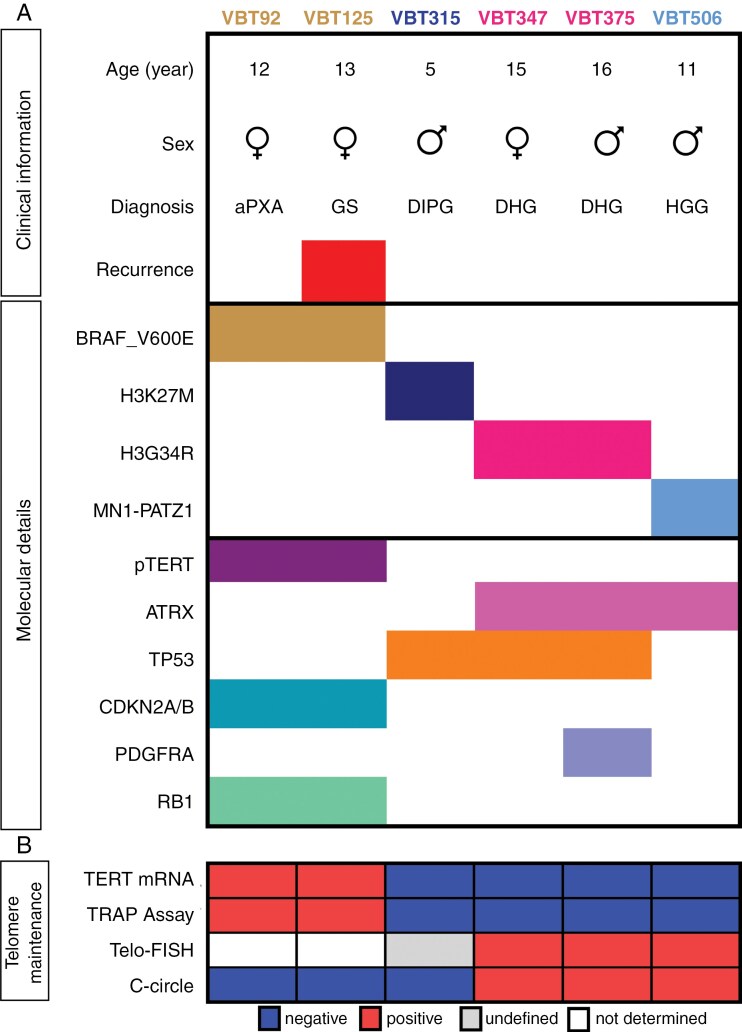
To investigate H3G34R_ATRX co-mutations, we compiled a substantial panel of pHGG cell lines (n = 6) including 2 previously published TERT-driven models7 as non-H3G34R/non-ATRX-mutated controls (Figure 1A). For the characterization of the mutational landscape, we performed WES. All 3 H3-mutated models harbored TP53 (tumor protein p53) mutations. Both H3G34R models demonstrated an ATRX mutation, while the 2 B-Raf proto-oncogene (BRAF) V600E-mutant models harbored pTERT mutations as described.7 In line with our cell models, a comprehensive cohort compiling own and published data (n = 35) confirmed concomitant ATRX and TP53 mutations in 91% of H3G34R tumors (Supplementary Figure 3A). These findings are in consonance with a previously published meta-analysis, indicating frequent co-mutations of ATRX and TP53 in DHG-H3G34 tumors.23 The ATRX mutations of our DHG-H3G34R models were located in the ATP-binding helicase domain, but upstream of the DAXX-binding domain in the MN1::PATZ1 model (Supplementary Figure 3B). Despite elevated ATRX mRNA expression in 2 models, all 3 ATRX-mutated cell lines exhibited loss of basal ATRX protein expression (Supplementary Figure 3C, D). This was further reflected by decreased H3 trimethylation at lysine 9 (H3K9me3) in all ATRX-mutated models, consistent with the well-described histone-modifying function of ATRX24–26 (Supplementary Figure 3D).
Figure 1.
ATRX-mutated models are associated with ALT-phenotype. (A) Overview of the patient-derived pHGG cell line panel providing clinical information and molecular details. Mutations: BRAF_V600E, H3K27M, H3G34R, TERT promoter (pTERT), ATRX, TP53, PDGFRA, RB1. Fusion: MN1::PATZ1. Chromosomal loss: CDKN2A. aPXA: anaplastic pleomorphic xanthoastrocytoma, GS: gliosarcoma, DIPG: diffuse intrinsic pontine glioma, DHG: diffuse hemispheric glioma, HGG: high-grade glioma. (B) Overview of the telomere-maintenance mechanisms examined in the indicated patient-derived pHGG cell models. Results from the following assays are depicted: TERT mRNA levels analyzed by quantitative real-time PCR (TERT mRNA), telomerase activity measured with TRAP-assay, and presence of ALT assessed with Telo-FISH and c-circle assay.
To evaluate the telomere-maintenance mechanisms of our pHGG, we employed different telomerase- and ALT-specific assays (Figure 1B). Elevated TERT expression and telomerase activity were only found in BRAF_V600E and pTERT co-mutated cells (VBT92, VBT125; Figures 1B, 2A, B, Supplementary Figure 4).7 In contrast, ATRX-mutant models (VBT347, VBT375, and VBT506) showed distinct ultra-bright intranuclear telomere spots in Telo-FISH, heterogeneous length of telomeres, and the presence of extrachromosomal circular telomere repeats (Figure 2C, Supplementary Figure 5), all indicating an ALT phenotype. Together, these results suggest that TERT reactivation and ALT are mutually exclusive in pHGG. For the DMG-H3K27M cell model VBT315, no clear telomere-maintenance mechanism was identified, albeit a heterogeneous appearance of telomeres suggesting ALT (Supplementary Figure 5). Interestingly, the ALT-phenotype was more pronounced in DHG-G34R_ATRX models (VBT347, VBT375) than in the ATRX-mutated MN1::PATZ1-fused model (VBT506). Consequently, we hypothesized that H3G34R_ATRX co-mutations synergistically induce ALT.
Figure 2.
ALT parameters and DNA repair perturbations are observed in H3G34R_ATRX primary patient-derived and human iPSC models. (A) Mean mRNA expression of TERT in patient-derived pHGG cell models (n = 2 biological repeats in triplicate). The mesothelioma cell line Meso92 (Pirker, Bilecz, et al. 2020) was used as a positive control. Differences in TERT mRNA levels between TERT-positive (upper circle) and TERT-negative (lower circle) pHGG models were quantified by unpaired student’s t-test with two-tailed distribution (**P < .01). (B) Results of the TRAP-assay are presented as telomerase product generated (TPG) units (n = 2 biological repeats). Differences in telomerase activity between TERT-positive (upper circle) and TERT-negative (lower circle) pHGG models were quantified by unpaired student’s t-test with a two-tailed distribution (**P < .01). (C) Bar graph showing relative c-circle levels of patient-derived pHGG cell lines (n = 2 biological repeats in triplicate). ALT-specific telomeric c-circle content was determined by qPCR following rolling circle amplification with or without Phi polymerase and quantified relative to U2OS (ALT-positive osteosarcoma cell line). Significant differences between ALT-positive (green circle) and ALT-negative (red circle) pHGG models were evaluated by One-way ANOVA with Bonferroni post-test. *P < .05. (D) Box plot showing relative c-circle levels of iPSCs. Telomeric c-circle content was determined as described in (C; n = 2 biological repeats in triplicate). Significance levels were evaluated by unpaired student’s t-test with a two-tailed distribution. *P < .05. (E) Western blot analyses showing phosphorylation of the DNA damage marker H2AX in the patient-derived pHGG cell line panel (upper panels) and iPSCs (lower panels). β-actin served as a loading control. (F) Immunohistochemical staining of pH2AX and Rad51 in tissue sections of one BRAF_V600E and pTERT co-mutated aPXA tumor (matching cell model VBT92) and 2 DHG-H3G34R_ATRX tumors (matching cell models VBT347 and VBT375). The red arrow marks necrosis and the white arrows indicate single pH2AX-positive nuclei in the second DHG-H3G34R_ATRX tumor (VBT375). Scale bar = 20 µm.
To dissect the role of H3G34R_ATRX in telomere stabilization in a non-malignant background, we assessed the presence of telomeric c-circles in human iPSCs overexpressing either H3.3 wild type (H3.3-WT-OE) or H3G34R (H3.3-G34R-OE) with or without ATRX-knockout (ATRX-KO). We aimed to compare H3.3-G34R-OE_ATRX-KO to H3.3-WT-OE_ATRX-KO and single ATRX-KO iPSCs. In agreement with previous observations,27,28 both single ATRX-KO and H3.3-WT-OE_ATRX-KO iPSCs were not viable, in contrast to H3.3-G34R-OE_ATRX-KO. This suggests an essential pro-survival function of the H3G34R mutation in combination with loss of ATRX. The H3.3-G34R-OE_ATRX-KO iPSC model showed identically decreased levels for ATRX and H3K9me3 as the patient-derived DHG-G34R_ATRX models (compare Supplementary Figure 3E). As expected, the H3.3-G34R-OE iPSC model demonstrated an upregulated expression of H3K9me3.29 Resembling patient-derived DHG-H3G34R_ATRX, we detected c-circles solely in H3.3-G34R-OE_ATRX-KO iPSCs (Figure 2D). In addition, unsupervised transcriptome analysis revealed “DNA_Damage_Telomere_Stress_Induced_Senescence” as the most significantly enriched pathway in H3.3-G34R-OE_ATRX-KO iPSCs compared to H3.3-WT (NES: 1.88, p. adj: 0.04, Supplementary Figure 6A, B; Tables 3, 4). Furthermore, we found altered expression of DNA repair genes driven by H3.3-G34R-OE, including distinct downregulation of ATRX expression (Supplementary Figure 6C). These observations indicate that the H3G34R mutation is a prerequisite for cell survival upon ATRX inactivation, consequently allowing the development of an ALT phenotype at least in iPSCs (Figure 2D).
H3G34R_ATRX Alterations are Associated With Increased DNA Damage Stress in pHGG Tumors and Human iPSCs
Previous studies have shown the individual impact of ATRX loss or H3G34 mutations on DNA repair, but information regarding potential cooperative effects is currently lacking.14,15,24 Consequently, we analyzed the basal expression of DNA repair proteins in our pHGG cell line panel. Expression of PARP and respective protein PARylation (pADPr) were detected in all models, except VBT125, which showed low expression of all DNA repair proteins investigated (Supplementary Figure 7A, B). Likewise, the TP53-mutant DMG-H3K27M model VBT315 showed low basal DNA repair protein expression. Concerning repair of toxic DSBs, expression of Rad50, a component of the Mre11-Rad50-Nbs1 complex operating in non-homologous end-joining,30 was detected in all models except VBT125, while HR protein Rad51 expression30 was only observed in VBT92 and VBT375 cells (Supplementary Figure 7A, B). It has been shown that Rad51 is not necessary for ALT telomere stabilization in U2OS, but that loss of Rad51 increases c-circle levels.31 Indeed, the DHG-H3G34R_ATRX model VBT347 lacking Rad51 expression showed higher amounts of c-circles than Rad51-expressing VBT375 cells (Figure 2C). Furthermore, exclusively DHG-H3G34R_ATRX models exhibited elevated basal levels of phosphorylated H2AX (pH2AX), a marker for DNA damage32 (Figure 2E). Corroborating the increased levels of pH2AX observed in DHG-H3G34R_ATRX, we detected phosphorylation of H2AX in H3G34R-OE_ATRX-KO iPSCs (Figure 2E) but no changes in the expression of other DNA repair proteins (Supplementary Figure 7C).
Since these in vitro data suggest that DNA damage stress is associated with DHG-H3G34R_ATRX, we assessed pH2AX- and Rad51-positive nuclei in fixed tissue samples of 2 DHG-H3G34R_ATRX cases (matching cell models VBT347 and VBT375) as compared to a TERT-driven aPXA tumor tissue (matching VBT92). Reflecting the cell models, the DHG-H3G34R_ATRX tissues generally showed moderate to strong vs. isolated nuclear pH2AX staining in VBT347 and VBT375, respectively (Figure 2F). Rad51 signals were overlapping with (VBT347) or even exceeding the pH2AX signals (VBT375). In contrast, no pH2AX signal was observed in vital tumor regions of the TERT-driven aPXA tissue (Supplementary Figure 8), except alongside necrotic regions (Figure 2F).
Combined Inhibition of PARP and Topoisomerase or the ATR Pathway Shows Synergistic Activity in pHGG and iPSCs With H3G34R_ATRX
Considering increased basal DNA damage in DHG-G34R_ATRX, we next investigated vulnerability towards DNA-damaging agents. All models showed intrinsic resistance against the frequently used DNA-damaging compound temozolomide (TMZ), with IC50 values higher than 250 µM (a common reference value in literature; Supplementary Figure 9A).33 Next, we determined the sensitivity towards single-agent PARP inhibition. We defined PARPi sensitivity by an IC50 value below 10 µM, according to a recently published talazoparib sensitivity definition for pediatric cancers.34 All models showed intrinsic resistance towards monotherapy with 7 different PARPis (Supplementary Figure 9A). Profiling of other drugs targeting DNA repair was performed according to published IC50 values of BRCA-mutated breast cancer cell lines (data were acquired from the Harvard Medical School LINCS Center, Dataset ID:20343 and Genomics of Drug Sensitivity in Cancer, Release 8.4, July 2022).35–37 All cell models were resistant against the DNA-crosslinking agent cisplatin (Supplementary Figure 9A). Concerning topoisomerase 1 inhibitors, we detected sensitivity towards the active metabolite of irinotecan, SN-38, and topotecan in VBT125 and VBT347 (Supplementary Figure 9A). This sensitivity of the BRAF_V600E and pTERT co-mutated recurrence model VBT125 might be derived from the treatment with BRAF/MEK inhibitors, recently associated with induction of a BRCAness-like state in BRAF-mutated melanoma.38
As ALT-positive cells have been shown to be sensitive towards ataxia telangiectasia and Rad3-related (ATR) inhibition,39 we tested our cell line panel for ATR pathway inhibition response. Ceralasertib reduced cell viability in all models, however, independent of their ALT status (Supplementary Figure 9A). This is of particular interest with regard to the DMG-H3K27M model VBT315, which showed selective sensitivity towards ceralasertib. Inhibitors downstream of ATR, AZD-7762, and prexasertib targeting checkpoint kinases 1 and 2 (CHK1/2), markedly reduced the viability of VBT125 and VBT375 cells (Supplementary Figure 9A). Any significant impact of the solvent dimethylsulfoxid (DMSO) could be excluded, except for TMZ in the VBT506 model (Supplementary Figure 9B).
PARPis have been shown to enhance the cytotoxic activity of multiple chemotherapeutics, including topoisomerase inhibitors, alkylating, and platinum-containing agents, as well as ionizing radiation.12,40–46 Following the detection of increased PARylation levels in DHG-H3G34R_ATRX models, we assessed the synergistic activity of PARPis with topoisomerase or ATR pathway inhibitors (Figure 3A). We selected talazoparib, exerting pronounced PARP-trapping activity,47 for combination studies. Synergistic effects between talazoparib and SN-38 were only observed for the SN-38-sensitive models VBT125 and VBT347 (Supplementary Figure 10). The combination of niraparib, the second most potent PARP-trapping agent,47 with the topoisomerase inhibitor topotecan demonstrated a clear trend towards synergistic interactions for the 2 DHG-H3G34R_ATRX models (VBT347, VBT375; Figure 3B, Supplementary Figure 11, 12A), as well as VBT125 (Figure 3C, Supplementary Figure 11, 12A), whereas antagonistic effects were observed in all other models (Figure 3C, Supplementary Figure 11, 12A). We extended the cell line panel by including 7 additional models (3 DMG-H3K27M models, one H3-WT HGG, one infant-type hemispheric glioma, and 2 NF1-altered models; Supplementary Figure 12A), but no comparable specific synergistic interactions for niraparib combined with topotecan were detected (Figure 3C, Supplementary Figure 12A, B). Concomitant targeting of PARP and ATR or CHK1/2 exerted strong synergistic effects for all 3 combinations tested selectively in DHG-H3G34R_ATRX (Figure 3D, E, Supplementary Figure 13, 14). Combined inhibition of PARP and ATR was the most effective approach for the DMG-H3K27M model VBT315 (Supplementary Figure 13). We confirmed these observed synergies of niraparib with topotecan or talazoparib in combination with ATR- or CHK1/2-inhibitors in 2 additional DHG-H3G34R_ATRX models (TP53 mutant; Supplementary Figure 15A, B). To investigate the effects of combined niraparib with topotecan treatment on multicellular tumor composition, we conducted 3D tumoroid assays of DHG-H3G34R_ATRX cell models (VBT347, VBT375) and observed reduced viability, especially concerning VBT375 tumor spheroids upon combination treatment (Supplementary Figure 16A, B, C). Concerning iPSCs, deletion of ATRX in H3G34R-OE resulted in a more predominant synergistic pattern of niraparib and topotecan, corroborating the observations in patient-derived tumor models (Figure 3F). Together, we identify that DHG-H3G34R_ATRX models are highly susceptible to PARPi-based combinations with either topoisomerase- or ATR-inhibitors, opening novel therapeutic strategies.
Figure 3.
Specific synergistic activity of PARPi combination therapies in H3G34R_ATRX patient-derived cell and iPSC models. (A) Schematic representation of a circular synergy dot-plot showing mean combination indices (CI) values. The small inner circle depicts synergistic CI values (<0.9). The middle circle represents additive CI values (0.9–1.1) and CI values >1.1 are considered antagonistic (white circle). Antagonistic CI values > 2.1 are collected in the light violet circle boundary. CI values were determined with CalcuSyn. CI < 0.9, synergism; CI = 0.9–1.1, additive effects; or CI > 1.1, antagonism. Each quarter represents one concentration of the PARPi and within each quarter increasing doses of the combined drug are shown. (B-E) Circular synergy dot-plots for niraparib in combination with topotecan (B-C), talazoparib with either ceralasertib (D) or AZD-7762 (E) are presented. Mean CI values based on the cell viability data of patient-derived pHGG cell models were calculated from n = 3 independent experiments. In case of VBT125 and VBT347 topotecan concentrations are 0.005; 0.025; 0.05 and 0.075 µM. (F) Circular synergy dot-plots for niraparib in combination with topotecan in H3.3 G34R OE and H3.3 G34R OE_ATRX KO iPSCs. Mean CI values based on the cell viability data of iPSC models were calculated from n = 3 independent experiments.
Combined Inhibition of PARP and Topoisomerase-I Causes Replication Stress and Persistent DNA Damage in ATRX-Mutated DHG-H3G34R Models
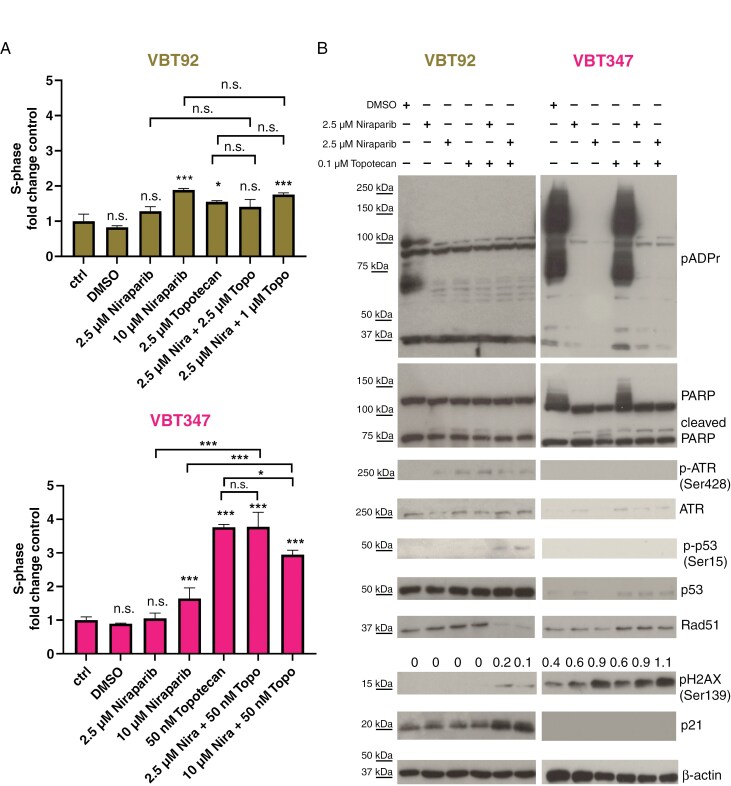
Next, the cellular effects of PARPi combinations on cell-cycle distribution were analyzed. We observed induction of an S-phase arrest by topotecan in DHG-H3G34R_ATRX (VBT347) but not aPXA (VBT92) cells, albeit this effect was reduced when niraparib was added (Figure 4A, Supplementary Figure 17A). In addition, we tested the combination of talazoparib with SN-38, resulting in an increased S-phase arrest in DHG-H3G34R_ATRX (VBT375) but not in aPXA cells (VBT92, Supplementary Figure 17B). Expression of PARP and PARylation levels were selectively reduced by niraparib in DHG-G34R_ATRX cells (VBT347; Figure 4B). We further detected increased levels of pH2AX, that were highest in the combination of topotecan with high-dose niraparib and more pronounced in DHG-G34R_ATRX (VBT347) compared to aPXA (VBT92) cells. Furthermore, expression of the DNA damage signaling protein ATR, involved in stalled replication forks signaling,48 and activation/upregulation of p53 or p21 were missing in DHG-G34R_ATRX (VBT347) compared to aPXA (VBT92) cells (Figure 4B). We further investigated the effect of combined niraparib with topotecan in our iPSC models. Unsupervised transcriptome analysis revealed the reactome pathway “Response_of_EIF2AKI_HRI_to_heme_deficiency” (heme-regulated inhibitor (HRI)-induced protein translation inhibition), related to cell death induction,49,50 as top significantly enriched pathway in H3.3-G34R-OE_ATRX-KO iPSCs upon combination treatment compared to H3.3-WT (NES: 2.07, P.adj: 0.005; Supplementary Figure 18A, B, Tables 5 and 6). On the protein level, we found massively increased cleavage of PARP, accompanied by an increase in pH2AX protein levels specifically in H3.3-G34R-OE_ATRX-KO iPSCs (Supplementary Figure 18C). Furthermore, we observed massive downregulation of protein PARylation by H3G34R overexpression (Supplementary Figure 18C). Corroborating these results, we detected increased pH2AX and decreased Rad51 foci in untreated DHG-G34R_ATRX (VBT347) cells when compared to aPXA (VBT92, compare Figures 2E and 5A and B, Supplementary Figures 7A and 19). Moreover, VBT92 cells showed a significant surplus of Rad51 (Supplementary Figure 19). While treatment increased pH2AX foci formation in both cell models, only in VBT92 cells pH2AX foci were outnumbered by Rad51 foci (Figure 5A and B, Supplementary Figure 19). Taken together, concomitant treatment with niraparib and topotecan induces increased replication stress and impairs the repair of DNA damage causing persistent DSB in DHG-H3G34R_ATRX cells.
Figure 4.
Combined inhibition of PARP and topoisomerase causes cell-cycle perturbations and exacerbates DNA damage stress in DHG-H3G34R_ATRX models. (A) The impact of niraparib combined with topotecan at the indicated concentrations for 24 hours on the proportion of cells in S-phase of the cell cycle of VBT92 and VBT347 cells was analyzed with PI-based flow cytometry. Data of n = 3 independent experiments in triplicate are given and normalized to the respective untreated controls. Results are represented as mean ± SD of the respective untreated control. Significance levels between untreated and treated samples, as well as single-drugs and combinations, were evaluated by One-way ANOVA with Bonferroni post-test. *P <0.05; ***P <0.001; n. s. not significant. (B) Western blot analyses of VBT92 and VBT347 cell models treated for 24 hours with niraparib alone or in combination with topotecan with the indicated concentrations. Expression, cleavage, and phosphorylation of the indicated proteins involved in DNA repair are shown. β-actin served as a loading control. Densitometric quantification insets show phosphorylation of H2AX normalized to β-actin.
Figure 5.
The combination of niraparib and topotecan drives DNA damage and stemness marker loss in DHG-H3G34R_ATRX models. (A) Immunofluorescence staining of pH2AX (DNA damage, right) and Rad51 (DNA repair, middle) in VBT92 (pTERT-mutated aPXA) and VBT347 (DHG-H3G34R_ATRX) DAPI-stained nuclei (left). Cells were untreated (control) or treated with vehicle (DMSO), 10 µM niraparib or/and 0.1 µM topotecan for 24 h. Images were captured on an Olympus spinning disk confocal microscope (40× objective). Representative images of one experiment are shown. (B) Quantification of pH2AX foci per nucleus of VBT92 and VBT347 cells as depicted in (A), represented as a percentage of foci-positive cells. A minimum of 1500 cells per condition were quantified and analyzed with ScanR (n = 3 independent experiments). Error bars represent mean ± SEM. (C) Tumor cell density was measured in DHG-H3G34R_ATRX PDX brain tissues of control, topotecan monotherapy, or topotecan combination therapy with niraparib-treated mice. One sample of the combination treatment group showed no tumor in the brain parenchyma, except alongside the injection channel, and was therefore excluded from this analysis. Statistical significance was calculated with unpaired two-tailed student’s t-test and two-way ANOVA with Dunnett post-test. *P <0.05; **P <0.01; ***P <0.001; ns not significant. (D) Quantification of nestin (stemness)-positive cells. 2.2 million H3G34R-positive tumor cells were phenotyped. Profiling was performed on all samples containing bulk tumors. Statistical significance between untreated and treated was evaluated by unpaired student´s t-test. (E) Representative immunofluorescence images for the stemness marker (nestin). Scale bar = 100 µm.
Anti-Cancer Effect of Niraparib and Topotecan Therapy in Vivo
In order to study the anti-cancer effect of niraparib combined with topotecan in a multicellular model, we conducted CAM assays with the DHG-H3G34R_ATRX model VBT347. However, no significant changes in tumor volume were found between all groups (Supplementary Figure 20A). Nevertheless, we observed a significantly reduced expression of the human glial marker GFAP, indicating reduced tumor cell density (Supplementary Figure 20 B and C). Furthermore, we investigated the combination treatment in a DHG-H3G34R_ATRX orthotopic PDX model (Supplementary Figure 21A). Mice treated with the combination showed a trend toward a delayed onset of accelerated tumor growth and longer survival (Supplementary Figure 21 B, C, and D). Due to the model-inherent highly variable time to tumor progression in all experimental groups, these data did not reach statistical significance. Nevertheless, histo-pathological analysis revealed a significantly reduced tumor cell density in the combination group (Figure 5C, Supplementary Figure 22). As a next step, we sought to interrogate the impact of therapy on intra-tumoral heterogeneity and cell states. Multiplexed immunofluorescence phenotyping revealed a significant decrease of stemness (nestin) and protein translation (p4EBP1) markers in the combination therapy arm (Figure 5D, E, Supplementary Figure 23 A and B). Vice versa, markers for interneuronal maturation (TUBB3, DCX) were significantly increased in the treatment groups (Supplementary Figure 23B).
Treatment of a Radiotherapy-Resistant DHG-H3G34R_ATRX Case With Concomitant Inhibition of PARP and Topoisomerase
Based on our preclinical findings, we decided to treat a 15-year-old girl (harboring the VBT347 tumor) diagnosed with TP53-mutated DHG-H3G34R_ATRX with a PARPi and a topoisomerase inhibitor, after failed first-line therapy. Initially, the patient presented due to tonic-clonic seizure and received near-total tumor resection (Figure 6A), followed by local radiotherapy with 60 Gy and concomitant TMZ (75 mg/m2). MRI 4 weeks after completion of radiotherapy showed a local tumor progression within the radiation field (Figure 6B). MRS showed increased choline/NAA ratios, further favoring progression over pseudoprogression or radiation necrosis (Figure 6B, Supplementary Figure 24). Radiotherapy resistance was confirmed in the respective VBT347 cell model. Combination with niraparib reversed radioresistance in VBT347 but not in VBT92 cells (Supplementary Figure 25). Considering the early in-field recurrence, treatment with niraparib and concomitant topotecan was initiated 7 weeks after radiotherapy completion. Niraparib was initiated at 300 mg/day with topotecan 1.5 mg/m2 on days 1–3 in 21-day cycles and adapted following hematological side effects (details in Supplementary Methods). Regular follow-up with MRI showed a continuous decrease of tumor size (representative MRI after 8 months, Figure 6C) and partial tumor response. In general, treatment was well tolerated, with good quality of life. In total, 13 cycles were administered. However, thirteen months after combination treatment initiation, MRI revealed progressive disease with a new tumor manifestation precentral, in the right thalamus (Figure 6D).
Figure 6.
Targeting DHG-H3G34R with PARPi and topoisomerase-I inhibitors is applicable in the clinical setting. MRI and f-18-fluor ethyl tyrosine positron emission (FET-PET) displaying the course of disease and therapy of a pediatric patient diagnosed with a DHG-H3G34R (corresponding to the VBT347 cell model). (A) Axial FLAIR image and contrast-enhanced (CE) T1-weighted MR image prior to primary surgery and postoperative axial CE T1-weighted MRI. (B) Axial FLAIR image and CE T1-weighted MR image at disease progression following irradiation (RTX) with concomitant temozolomide (TMZ) 3 months after initial diagnosis. The third image shows the color map of the choline/NAA ratios of a multivoxel MR spectroscopy (CSI; TE = 135 ms) at the level of one of the enhancing components. The colors red and green represent the voxels with high ratios. (C) Axial FLAIR image and CE T1-weighted MR image following 8 months of niraparib/topotecan treatment. In the third image (FLAIR) no signal changes are seen in the location of the subsequent recurrence. (D) Axial FLAIR image and CE T1-weighted MR image showing disease progression 13 months after initiation of niraparib/topotecan treatment, with a non-enhancing tumor manifestation rostral to the initial tumor sight with increased amino acid tracer uptake in an axial FET-PET/MR-fusion color map. The timeline starts with the presentation of the patient to the clinic (time point zero) and indicates further therapeutic interventions and therapy duration. The red arrows indicate tumor manifestation, and the green arrows point to areas of regressing tumor components.
Discussion
Despite advances in the molecular understanding of the underlying biology, treatment of pHGG remains challenging. While the epigenetic effects of the key pHGG H3 mutations H3K27M or H3G34R/V have been extensively studied, translation into clinically approved therapeutic regimens is still missing. Here, we demonstrate that DHG-H3G34R tumors with TP53 mutation and loss of ATRX constitutively exert ALT-driven telomere maintenance. ALT is associated with the collapse of the ATRX-DAXX histone chaperone complex,26 and mutations of ATRX and DAXX occur mutually exclusive.26 We also found ATRX loss in one MN1::PATZ1-fused case lacking H3F3A or TP53 alterations, exhibiting signs of ALT activation, however, less pronounced as observed for the DHG-H3G34R_ATRX models. This fits a previous study in mouse embryonic stem cells (mESCs) describing the combined loss of ATRX, p53, and TERT, together with either H3G34R or IDH mutation as necessary for full ALT initiation.51 By genetic modification of human iPSC models, we show for the first time that H3G34R_ATRX directly cooperates to induce a viable cellular ALT phenotype. This is of particular interest, as H3 and ATRX cooperatively maintain chromatin structures. H3 is regulated by methylation of diverse lysine residues, subsequently influencing chromatin accessibility (hetero- or euchromatin) and gene expression.52 The H3G34R mutation exerts a dual epigenetic function, as it increases both H3K9me3 (repressive mark) and H3K36me3 (activating mark), reflected also in our transcriptome analyses of iPSC models.29,52 ATRX is well known to preferably deposit H3 WT to H3K9me3-enriched (silenced) regions, maintaining pericentromeric heterochromatin and telomeres in a transcriptionally inactive state.25,26 Moreover, in a previous study, the transcriptionally active 3´ exonic regions of zinc finger genes (ZNFs) with elevated levels of H3K36me3 and H3K9me3 have been identified as binding targets for ATRX.53 Thus, the epigenetic perturbations caused by H3G34R may interact with ATRX recruitment to specific regulatory chromatin regions. Indeed, we found that the elevation of H3K9me3 by H3G34R expression was reversed upon additional knockout of ATRX.
Interestingly, the H3G34R mutation causes perturbed DNA damage repair in yeast cells as well as reduced proliferation in eukaryotic cells.15,54 However, overexpression of H3G34R in iPSCs in our study did neither induce enhanced phosphorylation of H2AX nor p53 overexpression and was compatible with cell viability. In contrast, knockout of ATRX in a wild-type H3F3A background was not viable, which is in agreement with the above-mentioned mESC study.51 Strikingly, the H3G34R mutation caused decreased ATRX expression and was necessary to allow survival of ATRX-inactivated iPSCs. Thus, H3G34R_ATRX appears to be essential for immortalization by activation of ALT in DHG-H3G34R tumors. With regard to DHG-H3G34R, it is important to mention that, in line with published data,1,2,55 the 2 cell line-forming DHG-H3G34R cases in our study also harbor TP53 mutations in addition to ATRX loss. This strongly suggests that for the full process of tumorigenesis, ALT must be accompanied by an impaired DNA damage response, allowing tumor cells to survive enhanced DNA damage stress associated with the ALT phenotype. Regarding iPSCs, it needs to be considered, that analyses were performed in a telomerase-positive stem cell background. ALT induction by H3G34R_ATRX in the presence of active telomerase proposes ALT activation to be an autonomous process, not requiring selection due to telomere crisis. Comparable observations for ALT activation have recently been reported by co-mutation of ATRX, TP53, RB, and CDKN2A.56
H3, a major histone variant necessary for DNA repair, is deposited to sites of UV-induced damage and DSB.57 Consequently, H3G34R mutation and ATRX loss have individually been shown to interfere with DNA damage response in HGG,15,24 but the cooperative effect has not been studied yet. Recently, increased tolerance of ALT-driven pHGG to DNA damage accumulation was reported,58 well in accordance with frequent TP53 co-mutation. However, despite the loss of p53, our H3G34R_ATRX models exhibit clear signs of enhanced DNA damage stress compared to telomerase-driven pHGG subtypes. The H3G34R mutation was reported to be responsible for the reduced DNA repair capacity of a genetically modified DHG-H3G34R mouse model with ATRX and TP53 loss.15 Accordingly, our iPSC data reveal enhanced DNA damage induced by H3G34R_ATRX, while no alterations were found after a single H3G34R mutation. Together, these observations suggest a cooperative role of both genomic alterations in the survival of cells under enhanced DNA damage responses. Supporting the in vitro data, clinical DHG-H3G34R_ATRX specimens showed increased DNA damage stress.
Consequently, we evaluated, if the enhanced DNA damage might represent an Achilles heel for improved therapy of DHG-H3G34R_ATRX. As PARP family proteins are crucial for maintaining genome integrity and have a central role in SSB and DSB repair, we aimed to specifically target DHG-H3G34R_ATRX with PARPis.12,13 Supporting this therapeutic strategy, PARP1 becomes activated in ATRX-mutated cells to protect stalled replication forks from degradation,26 and BRCA1 is a top regulator of the cycling DHG-G34 tumor cell population.59 Screening a pHGG patient-derived cell line panel, we observed insensitivity towards single agent PARPi. However, combinations with either topoisomerase or ATR/CHK1 inhibitors showed enhanced activity specifically in DHG-H3G34R_ATRX. Indeed, a synergistic effect of niraparib combined with topotecan was also induced by ATRX-KO in H3G34R-OE iPSCs, despite their telomerase-positive background. This was also reflected in CAM and PDX experiments. High variability and instability in tumor-take and progression concerning both in vivo models reflected general difficulties in modeling DHG-H3G34R_ATRX in a preclinical setting. Nevertheless, we observed in both models a significant reduction in tumor cell density. In the PDX model, loss of stemness characteristics was accompanied by induction of mature interneuronal markers, indicative of loss of DHG-H3G34R aggressiveness.59
Regarding the current standard of care, some DHG-H3G34R tumors have been shown to harbor O6-methylguanine DNA methyltransferase (MGMT) promoter methylation,60 suggesting TMZ sensitivity. Furthermore, DHG-H3G34R tumors have been described as responsive to irradiation in a preclinical setting.15 The patient presented in this study, diagnosed as MGMT promoter-methylated, TP53-mutated DHG-H3G34R_ATRX, progressed immediately after irradiation, with an in-field recurrence. Based on our promising preclinical data, we decided to treat the patient (corresponding to VBT347) with off-label niraparib and topotecan combination. This decision was supported by 2 studies indicating the potential of implementing PARPis in therapeutic regimens for pHGG; however, without considering the impact of DHG-H3G34R_ATRX background.61,62 In our DHG-H3G34R_ATRX case, the niraparib/topotecan regimen resulted in a partial response lasting for thirteen months. Importantly, this implies that niraparib can cross the blood-brain barrier at least in the malignant glioma tissue, in accordance with data published for brain metastases of endometrial and ovarian cancers.63,64 Considering the relapse, other PARPis with a better PARP trapping potency, not available in time for the described case, might even exert enhanced responses in this combination setting. For sure, molecular mechanisms underlying resistance development and relapse need to be further investigated. Nevertheless, this responsive case provides the first preliminary evidence for the successful clinical use of PARPi combinations with DNA-damaging agents at least for standard therapy-resistant DHG-H3G34R_ATRX. Thus, clinical trials further investigating this combination strategy specifically in DHG-H3G34R_ATRX are warranted.
Supplementary material
Supplementary material is available online at Neuro-Oncology (https://academic.oup.com/neuro-oncology).
Acknowledgments
We kindly thank the patients and their families for generously donating tumor tissue, giving their permission to include samples and clinical data in this study, and their valuable time during this hard period of their life. Without their contributions, this study would not have been possible. We also want to express our gratitude to the clinical staff supporting studies and patient care. We thank Doris Mejri for her assistance in c-circle assay, Mirjana Stojanovic, Sarah Machreich, Johannes Reisecker, Jennifer Hsu and Georg Schröckenfuchs for excellent technical assistance, and Joanna Loizou for valuable discussion. We further would like to thank Barbara Kapeller for generously providing us with access to an automatic rotary egg turner as well as her expertise in CAM assays.
Contributor Information
Anna Laemmerer, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria; Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Christian Lehmann, Vienna BioCenter (VBC), PhD Program, Doctoral School of the University of Vienna and Medical University of Vienna, Vienna, Austria; IMBA - Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Vienna BioCenter (VBC), Vienna, Austria.
Lisa Mayr, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Katharina Bruckner, Department of Neurosurgery, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria; Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Lisa Gabler, Department of Neurosurgery, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria; Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Daniel Senfter, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Philipp Meyer, Charles River Laboratories Germany GmbH, Freiburg, Germany.
Theresa Balber, Joint Applied Medicinal Radiochemistry Facility, University of Vienna, Medical University of Vienna, Vienna, Austria; Division of Nuclear Medicine, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria.
Christine Pirker, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Carola N Jaunecker, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Dominik Kirchhofer, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Petra Vician, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Michelle Griesser, Department of Neurosurgery, Kepler University Hospital GmbH, Johannes Kepler University, Linz, Austria.
Sabine Spiegl-Kreinecker, Department of Neurosurgery, Kepler University Hospital GmbH, Johannes Kepler University, Linz, Austria.
Maria T Schmook, Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria.
Tatjana Traub-Weidinger, Division of Nuclear Medicine, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria.
Peter Kuess, Department of Radiation Oncology, Medical University of Vienna, Vienna, Austria.
Franziska Eckert, Department of Radiation Oncology, Medical University of Vienna, Vienna, Austria.
Aniello Federico, National Center for Tumor Diseases (NCT), Heidelberg, Germany; Division of Pediatric Neurooncology, German Cancer Research Center (DKFZ) and German Cancer Consortium (DKTK), Heidelberg, Germany; Hopp Children’s Cancer Center (KiTZ), Heidelberg, Germany.
Sibylle Madlener, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Natalia Stepien, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Bernhard Robl, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Alicia Baumgartner, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Johannes A Hainfellner, Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Karin Dieckmann, Department of Radiation Oncology, Medical University of Vienna, Vienna, Austria.
Christian Dorfer, Department of Neurosurgery, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Karl Roessler, Department of Neurosurgery, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Nina S Corsini, IMBA - Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Vienna BioCenter (VBC), Vienna, Austria.
Klaus Holzmann, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Wolfgang M Schmidt, Division of Cell and Developmental Biology, Center for Anatomy and Cell Biology, Medical University of Vienna, Vienna, Austria.
Andreas Peyrl, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Amedeo A Azizi, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Christine Haberler, Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Alexander Beck, Center for Neuropathology, Ludwig-Maximilians-University, Munich, Germany.
Stefan M Pfister, Department of Pediatric Hematology and Oncology, Heidelberg University Hospital, Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Division of Pediatric Neurooncology, German Cancer Research Center (DKFZ) and German Cancer Consortium (DKTK), Heidelberg, Germany; Hopp Children’s Cancer Center (KiTZ), Heidelberg, Germany.
Julia Schueler, Charles River Laboratories Germany GmbH, Freiburg, Germany.
Daniela Lötsch-Gojo, Department of Neurosurgery, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Jürgen A Knoblich, Department of Neurology, Medical University of Vienna, Vienna, Austria; IMBA - Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Vienna BioCenter (VBC), Vienna, Austria.
Walter Berger, Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Johannes Gojo, Department of Pediatrics and Adolescent Medicine, Comprehensive Cancer Center and Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria.
Funding
This study was supported by the Austrian Science fund (TRANSCAN-2 “BRCAddict” project #I 4164 to JG, project #T 906-B28 to DLG) and the German National Funding Organization BMBF (“BRCAddict” project #TRS-2018-00000714, under the umbrella of the TRANSCAN-2 call #JTC 2017). The project was supported by the ITCC-P4 consortium, an Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 116064. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations. Further, the Physician Researcher Pathway scholarship of the Medical University of Vienna (granted to LM), Oncomine Clinical Research Grant 2020 (S.M.), the “Verein unser_kind” and the “Forschungsgesellschaft für Zerebrale Tumore” and the “City of Vienna Fund for Innovative Interdisciplinary Cancer Research” (project #21128 to LG) supported this study. Work in J.A.K.’s laboratory is supported by a Research Program of the Austrian Science Fund FWF (SFBF78 Stem Cell, F 7803-B).
Authorship statement
Conceptualization and study design: A.L., W.B., and J.G.; methodology: A.L., C.L., T.B., S.S.K., N.S.C., K.H., D.L.G., A.B., J.S., J.A.K., W.B., and J.G.; formal analysis and investigation: A.L., C.L., L.M., K.B., L.G., D.S., P.M., T.B., C.P., C.N.J., D.K., P.V., M.G., A.F., S.M., N.S.C., K.H., A.B., D.L.G., J.A.K., W.B., and J.G.; sequencing data analysis: LG (RNAseq), WMS (WES); radiological validation: M.T.S., T.T.W., P.K., and F.E.; neuropathological description: J.A.H. and C.H.; supporting material: N.S., B.R., A.B., K.D., C.D., A.P., A.A.A., and S.M.P.; writing—original draft preparation, A.L., C.L., L.M., W.B., and J.G. (with input from all coauthors); supervision: J.A.K., W.B., J.G.; funding acquisition: L.M., S.M., D.L.G., J.A.K., and J.G.; resources: S.S.K., K.H., J.S., J.A.K., W.B., and J.G..
Competing interests
A.L. is consultant and advisory board member for CCIE (not relevant to this study). A.A. is consultant and advisory board participant for Novartis (not relevant to this study). F.E. is advisory board participant for Servier and received honoraria from Dr. Sennewald Medizintechnik (not relevant to this study). S.M.P is consultant for Epignostix GbmH and BioSkryb (not relevant to this study). W.B. received honoraria from BMS, Novartis, and Roche (not relevant to this study). J.G. is consultant, advisory board participant and received honoraria for Novartis and Roche (not relevant to this study).
Data availability
WES data of pHGG patient-derived cell models will be available upon request to the corresponding authors. Upon publication data will be deposited on the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). Bulk RNA seq data of iPSCs will be available upon request to the corresponding authors.
References
- 1. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 3. Haase S, Nunez FM, Gauss JC, et al. Hemispherical pediatric high-grade glioma: Molecular basis and therapeutic opportunities. Int J Mol Sci . 2020;21(24):9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minasi S, Baldi C, Gianno F, et al. Alternative lengthening of telomeres in molecular subgroups of paediatric high-grade glioma. Childs Nerv Syst. 2021;37(3):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA.. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 6. Ebrahimi A, Korshunov A, Reifenberger G, et al. Pleomorphic xanthoastrocytoma is a heterogeneous entity with pTERT mutations prognosticating shorter survival. Acta Neuropathol Commun. 2022;10(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gabler L, Lotsch D, Kirchhofer D, et al. TERT expression is susceptible to BRAF and ETS-factor inhibition in BRAF(V600E)/TERT promoter double-mutated glioma. Acta Neuropathol Commun. 2019;7(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiegl-Kreinecker S, Lötsch D, Ghanim B, et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015;17(9):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daub JT, Amini S, Kersjes DJE, et al. A systematic analysis of genetic interactions and their underlying biology in childhood cancer. Commun Biol. 2021;4(1):1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lovejoy CA, Li W, Reisenweber S, et al. ; ALT Starr Cancer Consortium. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8(7):e1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gröbner SN, Worst BC, Weischenfeldt J, et al. ; ICGC PedBrain-Seq Project. The landscape of genomic alterations across childhood cancers. Nature. 2018;555(7696):321–327. [DOI] [PubMed] [Google Scholar]
- 12. Curtin NJ, Szabo C.. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat Rev Drug Discov. 2020;19(10):711–736. [DOI] [PubMed] [Google Scholar]
- 13. Lord CJ, Ashworth A.. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koschmann C, Calinescu AA, Nunez FJ, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med. 2016;Vol 8(328):328ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haase S, Banerjee K, Mujeeb AA, et al. H3.3-G34 mutations impair DNA repair and promote cGAS/STING-mediated immune responses in pediatric high-grade glioma models. J Clin Invest. 2022;132(22):e154229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bian S, Repic M, Guo Z, et al. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15(8):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ting-Chao Chou PT. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;Volume 22:27–55. [DOI] [PubMed] [Google Scholar]
- 18. Lotsch D, Kirchhofer D, Englinger B, et al. Targeting fibroblast growth factor receptors to combat aggressive ependymoma. Acta Neuropathol. 2021;142(2):339–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pirker C, Bilecz A, Grusch M, et al. Telomerase reverse transcriptase promoter mutations identify a genomically defined and highly aggressive human pleural mesothelioma subgroup. Clin Cancer Res. 2020;26(14):3819–3830. [DOI] [PubMed] [Google Scholar]
- 20. Henson JD, Lau LM, Koch S, et al. The C-Circle Assay for alternative-lengthening-of-telomeres activity. Methods. 2017;114:74–84. [DOI] [PubMed] [Google Scholar]
- 21. Bicanova L, Kreilmeier-Berger T, Reifinger M, Holzmann K, Kleiter M.. Prevalence and potentially prognostic value of C-circles associated with alternative lengthening of telomeres in canine appendicular osteosarcoma. Vet Comp Oncol. 2021;19(2):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benčurová K, Friske J, Anderla M, et al. CAM-xenograft model provides preclinical evidence for the applicability of [68Ga]Ga-pentixafor in CRC imaging. Cancers. 2022;14(22):5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crowell C, Mata-Mbemba D, Bennett J, et al. Systematic review of diffuse hemispheric glioma, H3 G34-mutant: Outcomes and associated clinical factors. Neurooncol. Adv.. 2022;4(1):vdac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han B, Cai J, Gao W, et al. Loss of ATRX suppresses ATM dependent DNA damage repair by modulating H3K9me3 to enhance temozolomide sensitivity in glioma. Cancer Lett. 2018;419:280–290. [DOI] [PubMed] [Google Scholar]
- 25. Valenzuela M, Amato R, Sgura A, Antoccia A, Berardinelli F.. The multiple facets of ATRX protein. Cancers (Basel). 2021;13(9):2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haase S, Garcia-Fabiani MB, Carney S, et al. Mutant ATRX: Uncovering a new therapeutic target for glioma. Expert Opin Ther Targets. 2018;22(7):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funato K, Smith RC, Saito Y, Tabar V.. Dissecting the impact of regional identity and the oncogenic role of human-specific NOTCH2NL in an hESC model of H3.3G34R-mutant glioma. Cell Stem Cell. 2021;28(5):894–905.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrick D, Sharpe JA, Arkell R, et al. Loss of Atrx affects trophoblast development and the pattern of X-inactivation in extraembryonic tissues. PLoS Genet. 2006;2(4):e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voon HPJ, Udugama M, Lin W, et al. Inhibition of a K9/K36 demethylase by an H3.3 point mutation found in paediatric glioblastoma. Nat Commun. 2018;9(1):3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson SP, Bartek J.. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang JM, Yadav T, Ouyang J, Lan L, Zou L.. Alternative lengthening of telomeres through two distinct break-induced replication pathways. Cell Rep. 2019;26(4):955–968.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carusillo A, Mussolino C.. DNA Damage: From threat to treatment. Cells. 2020;9(7):1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3(3):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keller KM, Koetsier J, Schild L, et al. The potential of PARP as a therapeutic target across pediatric solid malignancies. BMC Cancer. 2023;23(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Database issue):D955–D961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim HJ, Min A, Im SA, et al. Anti-tumor activity of the ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int J Cancer. 2017;140(1):109–119. [DOI] [PubMed] [Google Scholar]
- 37. Lee KJ, Wright G, Bryant H, et al. EGFR signaling promotes resistance to CHK1 inhibitor prexasertib in triple negative breast cancer. Cancer Drug Resist. 2020;3(4):980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maertens O, Kuzmickas R, Manchester HE, et al. MAPK pathway suppression unmasks latent dna repair defects and confers a chemical synthetic vulnerability in BRAF-, NRAS-, and NF1-Mutant Melanomas. Cancer Discov. 2019;9(4):526–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flynn RL, Cox KE, Jeitany M, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347(6219):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Norris RE, Adamson PC, Nguyen VT, Fox E.. Preclinical evaluation of the PARP inhibitor, olaparib, in combination with cytotoxic chemotherapy in pediatric solid tumors. Pediatr Blood Cancer. 2014;61(1):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Augustine T, Maitra R, Zhang J, Nayak J, Goel S.. Sensitization of colorectal cancer to irinotecan therapy by PARP inhibitor rucaparib. Invest New Drugs. 2019;37(5):948–960. [DOI] [PubMed] [Google Scholar]
- 42. George SL, Lorenzi F, King D, et al. Therapeutic vulnerabilities in the DNA damage response for the treatment of ATRX mutant neuroblastoma. EBioMedicine. 2020;59:102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plummer R, Jones C, Middleton M, et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14(23):7917–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jain MR, Mohapatra J, Bandhyopadhyay D, et al. Identification and preclinical characterization of a novel and potent poly (ADP-ribose) polymerase (PARP) inhibitor ZYTP1. Cancer Chemother Pharmacol. 2018;82(4):635–647. [DOI] [PubMed] [Google Scholar]
- 45. Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105(44):17079–17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Vuurden DG HE, Meijer OL, Wedekind LE, et al. PARP inhibition sensitizes childhood high grade glioma, medulloblastoma and ependymoma to radiation. Oncotarget. 2011;2(12):984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas A, Murai J, Pommier Y.. The evolving landscape of predictive biomarkers of response to PARP inhibitors. J Clin Invest. 2018;128(5):1727–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kruger K, Geist K, Stuhldreier F, et al. Multiple DNA damage-dependent and DNA damage-independent stress responses define the outcome of ATR/Chk1 targeting in medulloblastoma cells. Cancer Lett. 2018;430:34–46. [DOI] [PubMed] [Google Scholar]
- 49. Burwick N, Aktas BH.. The eIF2-alpha kinase HRI: A potential target beyond the red blood cell. Expert Opin Ther Targets. 2017;21(12):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eytan K, Versano Z, Oren R, et al. Pediatric glioblastoma cells are sensitive to drugs that inhibit eIF2alpha dephosphorylation and its phosphomimetic S51D variant. Front Oncol. 2022;12:959133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Udugama M, Hii L, Garvie A, et al. Mutations inhibiting KDM4B drive ALT activation in ATRX-mutated glioblastomas. Nat Commun. 2021;12(1):2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martin C, Zhang Y.. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. [DOI] [PubMed] [Google Scholar]
- 53. Valle-Garcia D, Qadeer ZA, McHugh DS, et al. ATRX binds to atypical chromatin domains at the 3’ exons of zinc finger genes to preserve H3K9me3 enrichment. Epigenetics. 2016;11(6):398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yadav RK, Jablonowski CM, Fernandez AG, et al. Histone H3G34R mutation causes replication stress, homologous recombination defects and genomic instability in S. pombe. Elife. 2017;6:e27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lucas C-HG, Mueller S, Reddy A, et al. Diffuse hemispheric glioma, H3 G34-mutant: Genomic landscape of a new tumor entity and prospects for targeted therapy. Neuro-Oncology. 2021;23(11):1974–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turkalo TK, Maffia A, Schabort JJ, et al. A non-genetic switch triggers alternative telomere lengthening and cellular immortalization in ATRX deficient cells. Nat Commun. 2023;14(1):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Juhasz S, Elbakry A, Mathes A, Lobrich M.. ATRX promotes DNA repair synthesis and sister chromatid exchange during homologous recombination. Mol Cell. 2018;71(1):11–24.e7. [DOI] [PubMed] [Google Scholar]
- 58. Stundon JL, Ijaz H, Gaonkar KS, et al. ALT in pediatric high-grade gliomas can occur without ATRX mutation and is enriched in patients with pathogenic germline MMR Variants. Neuro Oncol. 2023;25(7):1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu I, Alencastro Veiga Cruzeiro G, Bjerke L, et al. GABAergic neuronal lineage development determines clinically actionable targets in diffuse hemispheric glioma, H3G34-mutant. Cancer Cell. 2024;42(9):1528–1548.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mackay A, Burford A, Molinari V, et al. Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY Phase II Randomized Trial. Cancer Cell. 2018;33(5):829–842.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valiakhmetova A, Gorelyshev S, Konovalov A, et al. Treatment of pediatric glioblastoma with combination olaparib and temozolomide demonstrates 2-year durable response. Oncologist. 2020;25(2):e198–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schafer ES, Rau RE, Berg SL, et al. Phase 1/2 trial of talazoparib in combination with temozolomide in children and adolescents with refractory/recurrent solid tumors including Ewing sarcoma: A Children’s Oncology Group Phase 1 Consortium study (ADVL1411). Pediatr Blood Cancer. 2020;67(2):e28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Z, Xu M, Sakandar A, et al. Successful treatment of a patient with brain metastasis from ovarian cancer with BRCA wild type using niraparib: A case report and review of the literature. Front Oncol. 2022;12:873198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Q, Zhang F, Gao H, Xu Y.. Successful treatment of a patient with brain metastases from endometrial cancer using Niraparib: A case report. Ann Palliat Med. 2021;10(1):818–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
WES data of pHGG patient-derived cell models will be available upon request to the corresponding authors. Upon publication data will be deposited on the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). Bulk RNA seq data of iPSCs will be available upon request to the corresponding authors.