Abstract
Background
Systemic inflammation (SI) is a risk factor for atherosclerotic cardiovascular disease (ASCVD) and is most commonly assessed by measuring levels of high-sensitivity C-reactive protein (hsCRP).
Objective
This study aimed to determine hsCRP testing rates and SI prevalence in patients with ASCVD and in a subset of patients with chronic kidney disease (CKD).
Methods
This was a retrospective, cross-sectional analysis using US-based data from the Optum® de-identified Electronic Health Record data set (Optum® EHR; 1/1/2017–12/31/2021). hsCRP testing rates and SI prevalence (hsCRP ≥2 to <10 mg/L) were evaluated by calendar year. Demographics, comorbidities, and treatment patterns were compared between patients with ASCVD, ASCVD + CKD, and ASCVD + stage 3/4 CKD, with and without SI.
Results
1,658,476 patients with ASCVD were eligible for study inclusion. Per calendar year, 44.9 %-68.8 % and 14.9 %-18.9 % of patients had any CKD and stage 3/4 CKD, respectively. hsCRP testing was performed in 0.87 %-0.98 % (ASCVD), 0.90 %-1.17 % (ASCVD + CKD), and 0.99 %-1.41 % (ASCVD + stage 3/4 CKD) of patients. SI was present in 37.16 %-38.62 % (ASCVD), 40.61 %-44.44 % (ASCVD + CKD), and 49.44 %-53.92 % (ASCVD + stage 3/4 CKD) of tested patients. Among those with SI, patients with CKD had a greater comorbidity burden than those without.
Conclusion
While rates of hsCRP testing were low in patients with ASCVD, the prevalence of SI was high in hsCRP-tested patients with ASCVD, irrespective of CKD presence or severity. Given the low rate of testing, patients with SI may not be identified and treated.
Keywords: Atherosclerotic cardiovascular disease, Chronic kidney disease, Systemic inflammation
Graphical abstract
Glossary
- AHA
American Heart Association
- ASCVD
Atherosclerotic cardiovascular disease
- CDC
Centers for Disease Control and Prevention
- CCI
Charlson Comorbidity Index
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- CVE
Cardiovascular event
- eGFR
Estimated glomerular filtration rate
- EHR
Electronic health record
- HR
Hazard ratio
- hsCRP
High-sensitivity C-reactive protein
- ICD-10
International Classification of Diseases, Tenth Revision
- LDL-C
Low-density lipoprotein cholesterol
- MACE
Major adverse cardiovascular events
- MI
Myocardial infarction
- RWE
Real-world evidence
- SI
Systemic inflammation
1. Introduction
Heart disease is the leading cause of death in the US, accounting for over 695,000 deaths in 2021 [1]. An estimated 18.7 million adults in the US have atherosclerotic cardiovascular disease (ASCVD) [2], in which comorbidities, including chronic kidney disease (CKD), are common [3]. In fact, CKD has been reported to affect over one-third of US patients with ASCVD, with more than one-fifth having stage 3 or 4 disease [4].
Statins, which lower low-density lipoprotein cholesterol (LDL-C), are a standard treatment for ASCVD. An Expert Consensus Decision Pathway, published by the American College of Cardiology in 2022, recommends that patients with ASCVD receive high-intensity statin therapy, with LDL-C targets of <70 mg/dL and <55 mg/dL for those with high risk and very high risk ASCVD, respectively [5]. Despite the cardiovascular benefits associated with LDL-C lowering [6], many patients remain at increased risk of adverse cardiovascular events (CVEs), which may in part be due to ongoing systemic inflammation (SI) [[7], [8], [9]].
Several therapies have been or are being developed to address SI [[10], [11], [12], [13]]. The anti-IL1β monoclonal antibody canakinumab was investigated in the phase 3 CANTOS trial (NCT01327846) [10,14]. Treatment with canakinumab led to reduced SI (without an effect on LDL-C) and a lower rate of recurrent CVEs compared with placebo in patients with previous myocardial infarction (MI), highlighting the importance of addressing residual SI in secondary prevention [10]. Ziltivekimab, an anti-IL6 monoclonal antibody, was shown to reduce biomarkers of inflammation and thrombosis in patients with CKD and SI in the phase 2 RESCUE trial (NCT03926117) [13,15]. Importantly, hsCRP was reduced by 77 % to 92 % in the ziltivekimab groups vs 4 % in the placebo group [13]. Ziltivekimab is currently being investigated in patients with ASCVD, CKD, and SI in the phase 3 ZEUS trial (NCT05021835) [16]. In addition, the anti-inflammatory drug colchicine has been shown to reduce the rate of adverse CVEs in several secondary prevention trials [12]. In the phase 3 LoDoCo2 trial (ACTRN12614000093684), the risk of CVEs was significantly lower with low-dose colchicine compared with placebo in patients with chronic coronary disease [11,17]. This was further substantiated by a meta-analysis of 5 trials which demonstrated that use of colchicine was associated with reduced risk of CVEs across a broad spectrum of patients with coronary disease [12]. While these studies showed that colchicine reduced the risk of CVE, a more recent study (CLEAR SYNERGY OASIS 9) found that colchicine did not reduce the incidence of major adverse cardiovascular events (MACE) in patients with acute MI compared to placebo [18]. Further, average high-sensitivity C-reactive protein (hsCRP) levels in the colchicine group did not reach <2 mg/L [18]. This does not detract, however, from the fact that patients with SI face an increased risk of adverse CVEs [[19], [20], [21], [22], [23], [24]]. In 3 clinical trials (PROMINENT [NCT03071692] [25], REDUCE-IT [NCT01492361] [26], and STRENGTH [NCT02104817] [27]) that included participants with or at risk of ASCVD on background statin therapy, hsCRP levels were a significant predictor of CVEs and death [28].
While hsCRP is a well-established biomarker of SI that has been used for risk assessment in primary prevention [29], its use in secondary prevention is not part of standard practice [9], and the frequency with which it is utilized in this latter population is not well understood. Therefore, the aims of this study were to determine the prevalence of hsCRP testing and the prevalence of SI among hsCRP-tested patients with ASCVD with or without CKD.
2. Methods
2.1. Study design
This was a retrospective, cross-sectional study using data from January 1, 2017, to December 31, 2021, obtained from the Optum® de-identified Electronic Health Record data set (Optum® EHR). The index calendar year was defined as the first eligible calendar year with a diagnosis of ASCVD.
Patients included in this study were US adults with ≥1 inpatient medical record or ≥2 outpatient medical records for any diagnosis of ASCVD during any single calendar year between January 1, 2017, and December 31, 2021. Patients with two outpatient records in two different calendar years did not meet the inclusion criteria. ASCVD diagnoses were tracked from the initial year of diagnosis to the end of the study. These diagnoses were defined using International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes and included ischemic stroke, MI, peripheral artery disease, and other codes indicating ASCVD (eg, apraxia or dysphagia following unspecified cerebrovascular disease) or other coronary heart diseases (eg, unstable angina, angina pectoris with documented spasm). To be eligible for inclusion, patients had to be aged ≥18 years and have clinical activity in the EHR during the same calendar year as the ASCVD diagnosis.
Evidence of a CKD diagnosis in the calendar year of interest was identified using ICD-10 diagnosis codes (N18 for any CKD, N18.3X or N18.4X for stage 3 or 4 CKD), along with Current Procedural Terminology or ICD-10 procedure codes indicating dialysis and end-stage renal disease, or lab results indicative of a reduced estimated glomerular filtration rate (eGFR; ≥2 results ≤89 mL/min/1.73m2 within 90 days of each other for CKD; ≥2 results between 15 and 60 mL/min/1.73m2 within 90 days of each other for stage 3 or 4 CKD).
Patients were excluded if they had evidence of chronic inflammatory or autoimmune diseases (including lupus, rheumatoid arthritis, psoriasis, multiple sclerosis, Crohn's disease, osteoarthritis, and ulcerative colitis) or chronic infectious diseases (including hepatitis, HIV, or tuberculosis), as these conditions may increase hsCRP levels and bias the estimation of SI. Patients were also excluded if they had ≥1 pharmacy claim for treatment of autoimmune diseases, as these conditions are likely to be underreported in medical record databases. Patients with evidence of any major cancer during the studied calendar year were also excluded from the study.
In the SI analysis, hsCRP tests were excluded if results were reported as a range or laboratory values were missing. hsCRP tests with values <0 mg/L or >20 mg/L and tests conducted during a hospitalization or emergency department visit were excluded. Tests followed (within ≤7 days) by the prescription of antibiotics, antivirals, or antimycotics were excluded on the assumption that infection was the reason for hsCRP testing. Similarly, hsCRP tests during the 3 months following one of these prescriptions were excluded. Finally, hsCRP tests occurring ≤30 days after any ASCVD event (defined as any inpatient stay with any ASCVD diagnosis) were excluded. These exclusion conditions were used to ensure that hsCRP tests were not collected during the period when hsCRP levels may have been less indicative of chronic SI.
As this study involved retrospectively collected de-identified data, it was exempt from institutional review board review.
2.2. Variables and outcomes
Patient demographics, including age, gender, race/ethnicity, insurance type, and geographic region, were identified during the index calendar year.
Rates of hsCRP testing were estimated by calendar year and stratified by the presence and severity of CKD. Comorbidity and medication usage was assessed and compared between those that did and did not undergo hsCRP testing; these data were stratified by the presence and severity of CKD.
Comorbidities were identified during the index calendar year based on the Quan-Charlson comorbidity score, Agency for Healthcare Research and Quality comorbid conditions, evidence of prespecified comorbid conditions (including hypertension, dementia, chronic obstructive pulmonary disease, heart failure, atrial fibrillation, nonalcoholic fatty liver disease, cardiac amyloidosis, aortic stenosis, and diabetes), and the CKD indicator.
Receipt of cardiovascular disease (CVD)-related medications (mineralocorticoid receptor antagonists, lipid-lowering therapies, medications to improve glycemic control, digoxin, antiplatelet therapy, and antihypertensive medications), or medications that increase cardiovascular risk (nonsteroidal anti-inflammatory drugs) were identified from medical records (therefore excluding over-the-counter medications), medications administered, and procedures performed during the index calendar year.
SI was defined as an hsCRP level ≥2 mg/L and <10 mg/L; this definition is consistent with that used in recent trials and real-world evidence (RWE) studies [10,13,16,19,[30], [31], [32], [33], [34], [35]]. To align with the American Heart Association (AHA) definition of SI (hsCRP ≥3 mg/L [36]), hsCRP levels ≥2 mg/L and <3 mg/L and ≥3 mg/L and ≤10 mg/L were also evaluated in sensitivity analyses. The prevalence of SI was evaluated by calendar year and patients were stratified into 3 groups: patients with ASCVD, patients with ASCVD and any stage of CKD, and patients with ASCVD and stage 3 or 4 CKD. Comorbidities and concomitant medications used were assessed and stratified by CKD severity.
2.3. Statistical methods
Numbers and percentages were provided for dichotomous and polychotomous variables, while means, medians, and standard deviations were provided for continuous variables. Bivariate comparisons of patient characteristics, comorbidities, and medication usage were conducted between tested and untested cohorts. Appropriate statistical tests, including t-tests, Wilcoxon rank-sum tests, and chi-square tests, were used based on the distribution of the measurements. All analyses were conducted using SAS 9.4 (SAS Institute, Inc.). P values <0.05 were considered statistically significant. Due to the small sample size, comparisons between patients with and without SI were analyzed descriptively.
3. Results
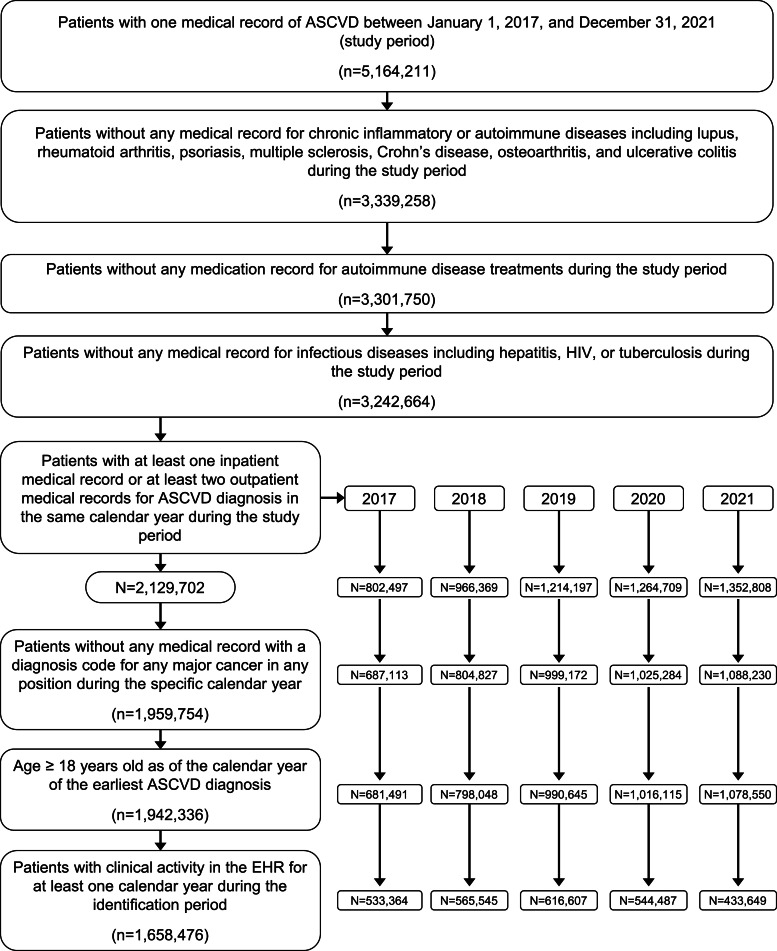
A total of 1,658,476 patients with ASCVD were eligible for inclusion in the study (Fig. 1). Of the patients with ASCVD, 20,873 (1.3 %) received hsCRP testing and 1,626,255 (98.1 %) did not (Table 1; 11,348 patients were excluded based on pre-specified exclusion criteria). The proportion of patients with ASCVD identified per calendar year with any CKD ranged from 44.9 % to 68.8 % and the proportion of patients with ASCVD with stage 3 or 4 CKD ranged from 14.9 % to 18.9 % (Table S1). Details regarding the eGFR laboratory results used to diagnose CKD can be found in Table S2.
Fig. 1.
hsCRP testing analysis patient identification and attrition.
ASCVD, atherosclerotic cardiovascular disease; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; EHR, electronic health record.
Table 1.
hsCRP testing analysis patient baseline characteristics, comorbidity level and medication use.
| ASCVD |
ASCVD + CKD |
ASCVD + CKD stage 3 and 4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| hsCRP tested n = 20,873 | hsCRP untested n = 1,626,255 | P value | hsCRP tested n = 14,576 | hsCRP untested n = 998,568 | P value | hsCRP tested n = 5949 | hsCRP untested n = 456,655 | P value | |
| Demographics | |||||||||
| Age, years, mean (SD) | 62.82 (12.36) |
66.42 (12.85) |
<0.001 | 64.04 (12.46) |
67.46 (12.71) |
<0.001 | 67.87 (12.15) |
70.94 (11.90) |
<0.001 |
| Age group, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| 18–44 | 1504 (7.21) |
90,644 (5.57) |
946 (6.49) |
48,506 (4.86) |
238 (4.00) |
13,090 (2.87) |
|||
| 45–64 | 9843 (47.16) |
588,943 (36.21) |
6305 (43.26) |
336,814 (33.73) |
1919 (32.26) |
112,101 (24.55) |
|||
| 65+ | 9526 (45.64) |
946,668 (58.21) |
7325 (50.25) |
613,248 (61.41) |
3792 (63.74) |
331,464 (72.59) |
|||
| Gender, n % | <0.001 | <0.001 | <0.001 | ||||||
| Female | 7880 (37.75) |
668,353 (41.10) |
5686 (39.01) |
425,974 (42.66) |
2698 (45.35) |
218,220 (47.79) |
|||
| Male | 12,956 (62.07) |
955,253 (58.74) |
8860 (60.78) |
571,135 (57.20) |
3238 (54.43) |
237,740 (52.06) |
|||
| Unknown/Missing | 37 (0.18) |
2649 (0.16) |
30 (0.21) |
1459 (0.15) |
13 (0.22) |
695 (0.15) |
|||
| Region, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| Northeast | 5347 (25.62) |
291,534 (17.93) |
3457 (23.72) |
163,694 (16.39) |
1220 (20.51) |
73,326 (16.06) |
|||
| Midwest | 10,282 (49.26) |
788,856 (48.51) |
7446 (51.08) |
501,117 (50.18) |
3088 (51.91) |
230,122 (50.39) |
|||
| South | 3138 (15.03) |
358,891 (22.07) |
2268 (15.56) |
215,738 (21.60) |
1061 (17.83) |
97,391 (21.33) |
|||
| West | 1352 (6.48) |
134,133 (8.25) |
892 (6.12) |
85,708 (8.58) |
370 (6.22) |
41,335 (9.05) |
|||
| Other/Unknown | 754 (3.61) |
52,841 (3.25) |
513 (3.52) |
32,311 (3.24) |
210 (3.53) |
14,481 (3.17) |
|||
| Insurance, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| Commercial | 9578 (45.89) |
551,691 (33.92) |
5754 (39.48) |
294,585 (29.50) |
1549 (26.04) |
97,505 (21.35) |
|||
| Medicare | 4464 (21.39) |
450,184 (27.68) |
3509 (24.07) |
289,240 (28.97) |
1860 (31.27) |
153,972 (33.72) |
|||
| Medicaid | 750 (3.59) |
73,262 (4.50) |
627 (4.30) |
49,733 (4.98) |
272 (4.57) |
20,965 (4.59) |
|||
| Uninsured | 359 (1.72) |
29,671 (1.82) |
233 (1.60) |
19,246 (1.93) |
67 (1.13) |
6798 (1.49) |
|||
| Race | <0.001 | <0.001 | 0.028 | ||||||
| White | 17,404 (83.38) |
1,334,651 (82.07) |
11,953 (82.00) |
809,769 (81.09) |
4736 (79.61) |
364,727 (79.87) |
|||
| Black/African American | 1917 (9.18) |
161,346 (9.92) |
1623 (11.13) |
116,077 (11.62) |
827 (13.90) |
59,766 (13.09) |
|||
| Asian | 448 (2.15) |
25,132 (1.55) |
274 (1.88) |
15,588 (1.56) |
98 (1.65) |
6844 (1.50) |
|||
| Other/unknown | 1104 (5.29) |
105,126 (6.46) |
726 (4.98) |
57,134 (5.72) |
288 (4.84) |
25,318 (5.54) |
|||
| Ethnicity | <0.001 | <0.001 | 0.024 | ||||||
| Hispanic, Latino, or Spanish | 755 (3.62) |
66,337 (4.08) |
590 (4.05) |
44,664 (4.47) |
275 (4.62) |
19,461 (4.26) |
|||
| Not Hispanic, Latino, or Spanish | 17,235 (82.57) |
1,372,093 (84.37) |
12,260 (84.11) |
860,376 (86.16) |
5086 (85.49) |
395,919 (86.70) |
|||
| Unknown | 2883 (13.81) |
187,825 (11.55) |
1726 (11.84) |
93,528 (9.37) |
588 (9.88) |
41,275 (9.04) |
|||
| Comorbidities, n (%) | |||||||||
| Hypertension | 14,696 (70.41) |
1,203,039 (73.98) |
<0.001 | 11,291 (77.46) |
815,485 (81.67) |
<0.001 | 5178 (87.04) |
396,272 (86.78) |
0.552 |
| Dementia | 1066 (5.11) |
108,279 (6.66) |
<0.001 | 925 (6.35) |
86,300 (8.64) |
<0.001 | 548 (9.21) |
52,729 (11.55) |
<0.001 |
| COPD | 2421 (11.60) |
240,905 (14.81) |
<0.001 | 2115 (14.51) |
185,762 (18.60) |
<0.001 | 1191 (20.02) |
102,130 (22.36) |
<0.001 |
| Heart failure | 2464 (11.80) |
244,932 (15.06) |
<0.001 | 2292 (15.72) |
203,371 (20.37) |
<0.001 | 1527 (25.67) |
131,210 (28.73) |
<0.001 |
| Atrial fibrillation | 2629 (12.60) |
257,733 (15.85) |
<0.001 | 2261 (15.51) |
193,648 (19.39) |
<0.001 | 1307 (21.97) |
112,651 (24.67) |
<0.001 |
| Type 2 diabetes | 5808 (27.83) |
501,556 (30.84) |
<0.001 | 4937 (33.87) |
373,341 (37.39) |
<0.001 | 2727 (45.84) |
200,925 (44.00) |
0.004 |
| Fatty liver disease | 511 (2.45) |
28,147 (1.73) |
<0.001 | 408 (2.80) |
21,839 (2.19) |
<0.001 | 145 (2.44) |
8902 (1.95) |
0.007 |
| Cardiac amyloidosis | 22 (0.11) |
1782 (0.11) |
0.856 | 20 (0.14) |
1534 (0.15) |
0.615 | 11 (0.18) |
966 (0.21) |
0.657 |
| Aortic stenosis | 1402 (6.72) |
116,363 (7.16) |
0.015 | 1128 (7.74) |
84,159 (8.43) |
0.003 | 593 (9.97) |
45,324 (9.93) |
0.913 |
| Medications, n (%) | |||||||||
| NSAIDs | 10,246 (49.09) |
757,228 (46.56) |
<0.001 | 8414 (57.73) |
571,903 (57.27) |
0.273 | 3526 (59.27) |
252,409 (55.27) |
<0.001 |
| MRA | 871 (4.17) |
63,750 (3.92) |
0.062 | 761 (5.22) |
51,995 (5.21) |
0.940 | 475 (7.98) |
31,757 (6.95) |
0.002 |
| Antidiabetics | 5528 (26.48) |
439,132 (27.00) |
0.093 | 4802 (32.94) |
352,670 (35.32) |
<0.001 | 2684 (45.12) |
190,643 (41.75) |
<0.001 |
| Digoxin | 285 (1.37) |
27,130 (1.67) |
<0.001 | 260 (1.78) |
23,231 (2.33) |
<0.001 | 178 (2.99) |
15,052 (3.30) |
0.192 |
| Lipid-lowering therapy | 14,290 (68.46) |
941,749 (57.91) |
<0.001 | 10,389 (71.27) |
646,836 (64.78) |
<0.001 | 4256 (71.54) |
295,298 (64.67) |
<0.001 |
| Anti-platelet | 9712 (46.53) |
719,400 (44.24) |
<0.001 | 7911 (54.27) |
535,486 (53.63) |
0.119 | 3393 (57.03) |
243,377 (53.30) |
<0.001 |
| Antihypertensives | 15,353 (73.55) |
1,166,394 (71.21) |
<0.001 | 11,744 (80.57) |
810,180 (81.13) |
0.084 | 5143 (86.45) |
383,677 (84.02) |
<0.001 |
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary artery disease; hsCRP, high-sensitivity C-reactive protein; MRA, mineralocorticoid receptor antagonist; NSAIDs, nonsteroidal anti-inflammatory drugs.
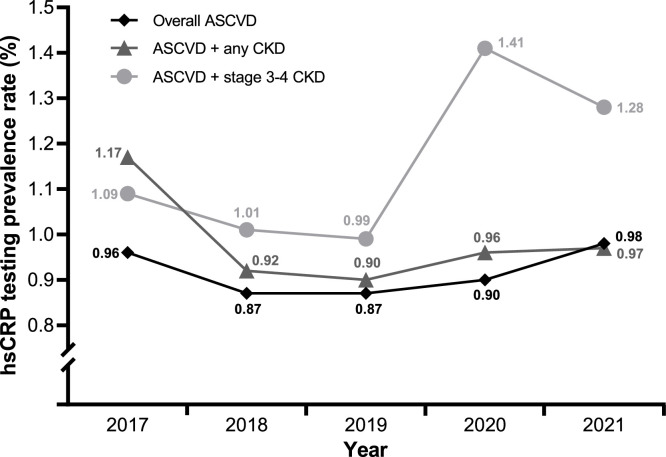
Calendar year rates of hsCRP testing in patients with ASCVD ranged from 0.87 % to 0.98 % (Fig. 2). The distribution of hsCRP test results in patients with ASCVD can be found in Fig. S1. hsCRP testing rates were similar among those with ASCVD, ASCVD and any CKD (0.90 % to 1.17 %), and ASCVD and stage 3 or 4 CKD (0.99 % to 1.41 %; Fig. 2).
Fig. 2.
Prevalence of any hsCRP test in each patient group (ASCVD, n = 1,658,476; ASCVD + CKD, n = 1,021,146; ASCVD + stage 3 or 4 CKD, n = 466,212) by calendar year.a
aDifferences in testing rates across the study period may seem large due to the scale of the y-axis. However, most of these changes are minimal. For example, the change in hsCRP testing rates before and after the beginning of the COVID-19 pandemic is only approximately 0.5 %.
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; hsCRP, high-sensitivity C-reactive protein.
Patients with ASCVD who underwent hsCRP testing were younger and generally had fewer comorbidities compared with untested patients, regardless of CKD severity (Table 1). hsCRP-tested patients with ASCVD with or without stage 3 or 4 CKD were significantly more likely to receive nonsteroidal anti-inflammatory drugs, lipid-lowering therapies, antiplatelet therapies, or antihypertensives compared with untested patients (P < 0.05; Table 1). A slightly higher percentage of patients underwent hsCRP testing during the pre-COVID-19 calendar years (2017 to 2019) compared to calendar years 2020 to 2021 for all three patient groups (1.42 % vs 1.17 % for patients with ASCVD, 1.53 % vs 1.19 % for patients with ASCVD and CKD, and 1.23 % vs 1.01 % for patients with ASCVD and stage 3 or 4 CKD; Table S3).
Approximately 3000 patients with ASCVD who underwent hsCRP testing were identified as eligible for analysis of SI in each calendar year (Fig. 3). Patient baseline characteristics, comorbidities, and medication use are stratified by calendar year and the presence of SI in Table 2 (patients with ASCVD), Table 3 (patients with ASCVD and CKD), and Table 4 (patients with ASCVD and stage 3 or 4 CKD).
Fig. 3.
Patient identification and attrition in the systemic inflammation analysis.
(A) Identification and attrition of patients with ASCVD. (B) Identification and attrition of patients with ASCVD and CKD. (C) Identification and attrition of patients with ASCVD and stage 3 or 4 CKD.
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; hsCRP, high-sensitivity C-reactive protein.
Table 2.
Systemic inflammation analysis ASCVD patient baseline characteristics, comorbidity level, and medication use.
| 2017 |
2018 |
2019 |
2020 |
2021 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SI n = 1933 |
SI n = 1143 | No SI n = 1927 |
SI n = 1184 |
No SI n = 2168 |
SI n = 1364 |
No SI n = 1834 |
SI n = 1135 |
No SI n = 1765 |
SI n = 1088 |
|
| Demographics | ||||||||||
| Age, years, mean (SD) | 63.29 (11.46) | 64.46 (11.25) | 62.63 (11.06) | 62.90 (11.47) | 63.00 (11.15) | 63.15 (11.42) | 63.77 (10.85) | 63.39 (11.33) | 63.38 (11.63) | 63.42 (11.40) |
| 18–44, n (%) | 99 (5.12) |
48 (4.20) |
100 (5.19) | 68 (5.74) |
111 (5.12) |
82 (6.01) |
79 (4.31) |
59 (5.20) | 103 (5.84) | 50 (4.60) |
| 45–64, n (%) | 921 (47.65) | 523 (45.76) | 996 (51.69) | 578 (48.82) | 1071 (49.40) | 658 (48.24) | 870 (47.44) | 539 (47.49) | 817 (46.29) | 534 (49.08) |
| 65+, n (%) | 913 (47.23) | 572 (50.04) | 831 (43.12) | 538 (45.44) | 986 (45.48) | 624 (45.75) | 885 (48.26) | 537 (47.31) | 845 (47.88) | 504 (46.32) |
| Gender, n (%) | ||||||||||
| Female | 532 (27.52) | 456 (39.90) | 545 (28.28) | 469 (39.61) | 613 (28.27) | 548 (40.18) | 532 (29.01) | 451 (39.74) | 534 (30.25) | 466 (42.83) |
| Male | 1398 (72.32) | 687 (60.10) | 1377 (71.46) | 712 (60.14) | 1551 (71.54) | 813 (59.60) | 1301 (70.94) | 681 (60.00) | 1228 (69.58) | 621 (57.08) |
| Unknown/Missing | 3 (0.16) | 0 (0) | 5 (0.26) | 3 (0.25) | 4 (0.18) | 3 (0.22) | 1 (0.05) | 3 (0.26) | 3 (0.17) | 1 (0.09) |
| Region, n (%) | ||||||||||
| Northeast | 453 (23.44) | 224 (19.60) | 480 (24.91) | 245 (20.69) | 659 (30.40) | 382 (28.01) | 698 (38.06) | 411 (36.21) | 845 (47.88) | 451 (41.45) |
| Midwest | 961 (49.72) | 605 (52.93) | 1027 (53.30) | 650 (54.90) | 1062 (48.99) | 672 (49.27) | 759 (41.38) | 493 (43.44) | 606 (34.33) | 401 (36.86) |
| South | 268 (13.86) | 150 (13.12) | 220 (11.42) | 175 (14.78) | 253 (11.67) | 185 (13.56) | 193 (10.52) | 134 (11.81) | 170 (9.63) | 143 (13.14) |
| West | 183 (9.47) |
119 (10.41) | 115 (5.97) | 69 (5.83) |
122 (5.63) |
71 (5.21) | 123 (6.71) | 60 (5.29) | 92 (5.21) | 49 (4.50) |
| Other/Unknown | 68 (3.52) |
45 (3.94) |
85 (4.41) | 45 (3.80) |
72 (3.32) |
54 (3.96) | 61 (3.33) | 37 (3.26) | 52 (2.95) | 44 (4.04) |
| Insurance, n (%) | ||||||||||
| Commercial | 1037 (53.65) | 521 (45.58) | 1070 (55.53) | 602 (50.84) | 1159 (53.46) | 663 (48.61) | 943 (51.42) | 522 (45.99) | 926 (52.46) | 552 (50.74) |
| Medicare | 357 (18.47) | 276 (24.15) | 296 (15.36) | 220 (18.58) | 419 (19.33) | 283 (20.75) | 380 (20.72) | 229 (20.18) | 377 (21.36) | 226 (20.77) |
| Medicaid | 25 (1.29) |
27 (2.36) |
34 (1.76) | 26 (2.20) |
28 (1.29) |
28 (2.05) | 24 (1.31) | 30 (2.64) | 16 (0.91) | 14 (1.29) |
| Uninsured | 33 (1.71) |
18 (1.57) |
59 (3.06) |
14 (1.18) |
50 (2.31) |
11 (0.81) |
26 (1.42) |
11 (0.97) |
8 (0.45) |
8 (0.74) |
| Comorbidities, n (%) | ||||||||||
| Hypertension | 1174 (60.73) | 817 (71.48) | 1139 (59.11) | 857 (72.38) | 1355 (62.50) | 968 (70.97) | 1179 (64.29) | 821 (72.33) | 1143 (64.76) | 788 (72.43) |
| Dementia | 65 (3.36) |
33 (2.89) |
71 (3.68) | 48 (4.05) |
70 (3.23) |
55 (4.03) | 62 (3.38) | 31 (2.73) | 52 (2.95) | 37 (3.40) |
| COPD | 105 (5.43) |
116 (10.15) | 109 (5.66) | 107 (9.04) | 102 (4.70) |
157 (11.51) | 120 (6.54) | 111 (9.78) | 117 (6.63) | 102 (9.38) |
| Heart failure | 124 (6.41) |
115 (10.06) | 100 (5.19) | 111 (9.38) | 123 (5.67) |
139 (10.19) | 85 (4.63) | 107 (9.43) | 113 (6.40) | 104 (9.56) |
| Atrial fibrillation | 183 (9.47) |
146 (12.77) | 150 (7.78) | 137 (11.57) | 199 (9.18) |
176 (12.90) | 176 (9.60) | 141 (12.42) | 173 (9.80) | 124 (11.40) |
| Type 2 diabetes | 379 (19.61) | 329 (28.78) | 322 (16.71) | 307 (25.93) | 414 (19.10) | 394 (28.89) | 356 (19.41) | 334 (29.43) | 336 (19.04) | 315 (28.95) |
| Fatty liver disease | 22 (1.14) |
28 (2.45) |
29 (1.50) | 44 (3.72) |
36 (1.66) |
48 (3.52) | 38 (2.07) | 37 (3.26) | 39 (2.21) | 30 (2.76) |
| Cardiac amyloidosis | 1 (0.05) | 1 (0.09) | 2 (0.10) | 0 (0) | 0 (0) | 1 (0.07) | 1 (0.05) | 1 (0.09) | 1 (0.06) | 1 (0.09) |
| Aortic stenosis | 103 (5.33) |
74 (6.47) |
121 (6.28) | 72 (6.08) |
149 (6.87) |
80 (5.87) | 118 (6.43) | 76 (6.70) | 117 (6.63) | 65 (5.97) |
| Medications, n (%) | ||||||||||
| NSAIDs | 604 (31.25) | 452 (39.55) | 598 (31.03) | 431 (36.40) | 639 (29.47) | 463 (33.94) | 479 (26.12) | 373 (32.86) | 487 (27.52) | 361 (33.18) |
| MRA | 56 (2.90) |
54 (4.72) |
51 (2.65) | 29 (2.45) |
59 (2.72) |
50 (3.67) | 56 (3.05) | 47 (4.14) | 62 (3.51) | 65 (5.97) |
| Antidiabetics | 310 (16.04) |
268 (23.45) | 295 (15.31) | 258 (21.79) | 346 (15.96) | 309 (22.65) | 308 (16.79) | 259 (22.82) | 316 (17.90) | 285 (26.19) |
| Digoxin | 16 (0.83) |
14 (1.22) |
12 (0.62) | 9 (0.76) |
10 (0.46) |
13 (0.95) | 6 (0.33) |
9 (0.79) |
6 (0.34) |
10 (0.92) |
| Lipid-lowering therapy | 1351 (69.89) | 799 (69.9) | 1346 (69.85) | 794 (67.06) | 1496 (69.00) | 942 (69.06) | 1299 (70.83) | 809 (71.28) | 1342 (76.03) | 819 (75.28) |
| Anti-platelet | 660 (34.14) | 448 (39.20) | 658 (34.15) | 441 (37.25) | 686 (31.64) | 466 (34.16) | 571 (31.13) | 389 (34.27) | 538 (30.48) | 400 (36.76) |
| Antihypertensives | 1251 (64.72) | 853 (74.63) | 1221 (63.36) | 855 (72.21) | 1384 (63.84) | 967 (70.89) | 1204 (65.65) | 820 (72.25) | 1189 (67.37) | 808 (74.26) |
ASCVD, atherosclerotic cardiovascular disease; COPD, chronic obstructive pulmonary artery disease; MRA, mineralocorticoid receptor antagonist; NSAIDs, nonsteroidal anti-inflammatory drugs; SI, systemic inflammation.
Table 3.
Systemic inflammation analysis ASCVD and CKD patient baseline characteristics, comorbidity level, and medication use.
| 2017 |
2018 |
2019 |
2020 |
2021 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SI n = 686 |
SI n = 520 |
No SI n = 825 |
SI n = 660 |
No SI n = 1130 |
SI n = 832 |
No SI n = 1072 |
SI n = 733 |
No SI n = 1010 |
SI n = 693 |
|
| Demographics | ||||||||||
| Age, years, mean (SD) | 65.72 (11.23) | 66.19 (11.89) | 64.67 (10.96) | 64.47 (11.34) | 64.55 (11.09) | 64.79 (11.19) | 65.30 (10.92) | 64.81 (10.96) | 64.83 (11.39) | 64.70 (11.31) |
| 18–44, n (%) | 22 (3.21) |
24 (4.62) |
30 (3.64) | 33 (5.00) | 42 (3.72) | 37 (4.45) | 41 (3.82) | 28 (3.82) | 48 (4.75) | 24 (3.46) |
| 45–64, n (%) | 276 (40.23) | 193 (37.12) | 378 (45.82) | 291 (44.09) | 508 (44.96) | 368 (44.23) | 438 (40.86) | 320 (43.66) | 423 (41.88) | 315 (45.45) |
| 65+, n (%) | 388 (56.56) | 303 (58.27) | 417 (50.55) | 336 (50.91) | 580 (51.33) | 427 (51.32) | 593 (55.32) | 385 (52.52) | 539 (53.37) | 354 (51.08) |
| Gender, n (%) | ||||||||||
| Female | 193 (28.13) | 211 (40.58) | 248 (30.06) | 266 (40.30) | 316 (27.96) | 346 (41.59) | 307 (28.64) | 294 (40.11) | 322 (31.88) | 303 (43.72) |
| Male | 492 (71.72) | 309 (59.42) | 574 (69.58) | 393 (59.55) | 811 (71.77) | 483 (58.05) | 764 (71.27) | 436 (59.48) | 687 (68.02) | 389 (56.13) |
| Unknown/Missing | 1 (0.15) | 0 (0) | 3 (0.36) | 1 (0.15) | 3 (0.27) | 3 (0.36) | 1 (0.09) | 3 (0.41) | 1 (0.10) | 1 (0.14) |
| Region, n (%) | ||||||||||
| Northeast | 158 (23.03) | 92 (17.69) | 194 (23.52) | 124 (18.79) | 326 (28.85) | 218 (26.20) | 406 (37.87) | 250 (34.11) | 477 (47.23) | 289 (41.70) |
| Midwest | 354 (51.60) | 275 (52.88) | 446 (54.06) | 388 (58.79) | 551 (48.76) | 424 (50.96) | 446 (41.60) | 334 (45.57) | 361 (35.74) | 268 (38.67) |
| South | 89 (12.97) |
64 (12.31) | 93 (11.27) | 94 (14.24) | 124 (10.97) | 109 (13.10) | 115 (10.73) | 87 (11.87) | 104 (10.30) | 91 (13.13) |
| West | 62 (9.04) |
72 (13.85) | 57 (6.91) | 29 (4.39) | 83 (7.35) | 47 (5.65) | 73 (6.81) | 37 (5.05) | 40 (3.96) | 19 (2.74) |
| Other/Unknown | 23 (3.35) |
17 (3.27) |
35 (4.24) | 25 (3.79) | 46 (4.07) | 34 (4.09) | 32 (2.99) | 25 (3.41) | 28 (2.77) | 26 (3.75) |
| Insurance, n (%) | ||||||||||
| Commercial | 310 (45.19) | 197 (37.88) | 407 (49.33) | 296 (44.85) | 539 (47.70) | 352 (42.31) | 493 (45.99) | 301 (41.06) | 480 (47.52) | 327 (47.19) |
| Medicare | 161 (23.47) | 138 (26.54) | 162 (19.64) | 145 (21.97) | 247 (21.86) | 194 (23.32) | 238 (22.20) | 162 (22.10) | 226 (22.38) | 156 (22.51) |
| Medicaid | 13 (1.90) |
12 (2.31) |
18 (2.18) | 17 (2.58) | 19 (1.68) | 18 (2.16) | 16 (1.49) | 22 (3.00) |
8 (0.79) |
8 (1.15) |
| Uninsured | 4 (0.58) |
8 (1.54) |
18 (2.18) | 8 (1.21) |
17 (1.50) | 6 (0.72) |
14 (1.31) | 7 (0.95) |
5 (0.50) |
4 (0.58) |
| Comorbidities, n (%) | ||||||||||
| Hypertension | 511 (74.49) | 411 (79.04) | 580 (70.30) | 517 (78.33) | 814 (72.04) | 629 (75.60) | 760 (70.90) | 576 (78.58) | 722 (71.49) | 551 (79.51) |
| Dementia | 31 (4.52) |
19 (3.65) |
38 (4.61) | 33 (5.00) | 46 (4.07) | 42 (5.05) | 51 (4.76) | 29 (3.96) | 37 (3.66) | 33 (4.76) |
| COPD | 58 (8.45) |
80 (15.38) | 64 (7.76) | 73 (11.06) | 73 (6.46) | 120 (14.42) | 96 (8.96) | 86 (11.73) | 84 (8.32) | 75 (10.82) |
| Heart failure | 77 (11.22) |
92 (17.69) | 79 (9.58) | 88 (13.33) | 100 (8.85) | 117 (14.06) | 71 (6.62) | 94 (12.82) | 86 (8.51) | 93 (13.42) |
| Atrial fibrillation | 104 (15.16) | 91 (17.50) | 93 (11.27) | 100 (15.15) | 146 (12.92) | 129 (15.50) | 144 (13.43) | 109 (14.87) | 119 (11.78) | 98 (14.14) |
| Type 2 diabetes | 201 (29.30) | 200 (38.46) | 197 (23.88) | 213 (32.27) | 292 (25.84) | 285 (34.25) | 251 (23.41) | 264 (36.02) | 237 (23.47) | 240 (34.63) |
| Fatty liver disease | 11 (1.60) |
14 (2.69) |
17 (2.06) | 30 (4.55) | 27 (2.39) | 37 (4.45) | 26 (2.43) | 28 (3.82) | 26 (2.57) | 22 (3.17) |
| Cardiac amyloidosis | 1 (0.15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.12) | 1 (0.09) | 1 (0.14) | 1 (0.10) | 1 (0.14) |
| Aortic stenosis | 52 (7.58) |
44 (8.46) |
65 (7.88) | 48 (7.27) | 90 (7.96) | 48 (5.77) | 82 (7.65) | 58 (7.91) | 79 (7.82) | 52 (7.50) |
| Medications, n (%) | ||||||||||
| NSAIDs | 328 (47.81) | 262 (50.38) | 363 (44.00) | 289 (43.79) | 412 (36.46) | 328 (39.42) | 347 (32.37) | 272 (37.11) | 346 (34.26) | 259 (37.37) |
| MRA | 34 (4.96) |
41 (7.88) |
38 (4.61) | 20 (3.03) | 47 (4.16) | 43 (5.17) | 44 (4.10) | 40 (5.46) | 46 (4.55) | 58 (8.37) |
| Antidiabetics | 163 (23.76) | 170 (32.69) | 182 (22.06) | 171 (25.91) | 242 (21.42) | 233 (28.00) | 223 (20.80) | 211 (28.79) | 222 (21.98) | 224 (32.32) |
| Digoxin | 10 (1.46) |
10 (1.92) |
9 (1.09) |
7 (1.06) |
8 (0.71) |
11 (1.32) | 3 (0.28) |
7 (0.95) |
4 (0.40) |
8 (1.15) |
| Lipid-lowering therapy | 520 (75.80) | 385 (74.04) | 612 (74.18) | 448 (67.88) | 820 (72.57) | 601 (72.24) | 778 (72.57) | 530 (72.31) | 800 (79.21) | 534 (77.06) |
| Anti-platelet | 334 (48.69) | 264 (50.77) | 378 (45.82) | 290 (43.94) | 456 (40.35) | 332 (39.90) | 411 (38.34) | 285 (38.88) | 376 (37.23) | 288 (41.56) |
| Antihypertensives | 532 (77.55) | 434 (83.46) | 613 (74.30) | 516 (78.18) | 832 (73.63) | 643 (77.28) | 778 (72.57) | 562 (76.67) | 742 (73.47) | 559 (80.66) |
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary artery disease; MRA, mineralocorticoid receptor antagonist; NSAIDs, nonsteroidal anti-inflammatory drugs; SI, systemic inflammation.
Table 4.
Systemic inflammation analysis ASCVD and stage 3/4 CKD patient baseline characteristics, comorbidity level, and medication use.
| 2017 |
2018 |
2019 |
2020 |
2021 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SI n = 177 |
SI n = 186 |
No SI n = 153 |
SI n = 179 |
No SI n = 187 |
SI n = 205 |
No SI n = 181 |
SI n = 177 |
No SI n = 172 |
SI n = 176 |
|
| Demographics | ||||||||||
| Age, years, mean (SD) | 71.28 (10.54) | 70.49 (10.36) | 70.52 (9.63) | 69.24 (10.28) | 72.02 (9.41) | 69.76 (10.61) | 71.50 (9.35) | 70.07 (10.64) | 70.35 (10.86) | 70.28 (10.75) |
| 18–44, n (%) | 3 (1.69) | 4 (2.15) | 0 (0) | 2 (1.12) | 1 (0.53) | 4 (1.95) | 2 (1.10) | 1 (0.56) | 3 (1.74) | 3 (1.70) |
| 45–64, n (%) | 39 (22.03) |
40 (21.51) | 45 (29.41) | 50 (27.93) | 36 (19.25) | 53 (25.85) | 41 (22.65) | 50 (28.25) | 47 (27.33) | 46 (26.14) |
| 65+, n (%) | 135 (76.27) |
142 (76.34) | 108 (70.59) | 127 (70.95) | 150 (80.21) | 148 (72.20) | 138 (76.24) | 126 (71.19) | 122 (70.93) | 127 (72.16) |
| Gender, n (%) | ||||||||||
| Female | 56 (31.64) |
86 (46.24) | 52 (33.99) | 76 (42.46) | 64 (34.22) |
98 (47.80) | 62 (34.25) | 79 (44.63) | 57 (33.14) | 77 (43.75) |
| Male | 121 (68.36) |
100 (53.76) | 100 (65.36) | 103 (57.54) | 122 (65.24) | 106 (51.71) | 119 (65.75) | 98 (55.37) | 115 (66.86) | 99 (56.25) |
| Unknown/Missing | 0 (0) | 0 (0) | 1 (0.65) | 0 (0) | 1 (0.53) | 1 (0.49) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Region, n (%) | ||||||||||
| Northeast | 26 (14.69) |
27 (14.52) | 27 (17.65) | 34 (18.99) | 45 (24.06) | 48 (23.41) | 64 (35.36) | 48 (27.12) | 82 (47.67) | 74 (42.05) |
| Midwest | 95 (53.67) |
95 (51.08) | 82 (53.59) | 105 (58.66) | 91 (48.66) | 106 (51.71) | 78 (43.09) | 86 (48.59) | 53 (30.81) | 67 (38.07) |
| South | 25 (14.12) |
27 (14.52) | 22 (14.38) | 18 (10.06) | 22 (11.76) | 24 (11.71) | 19 (10.50) | 23 (12.99) | 27 (15.70) | 21 (11.93) |
| West | 24 (13.56) |
33 (17.74) | 17 (11.11) | 11 (6.15) |
25 (13.37) | 17 (8.29) |
13 (7.18) | 13 (7.34) | 6 (3.49) |
8 (4.55) |
| Other/Unknown | 7 (3.95) | 4 (2.15) | 5 (3.27) | 11 (6.15) | 4 (2.14) | 10 (4.88) | 7 (3.87) | 7 (3.95) | 4 (2.33) | 6 (3.41) |
| Insurance, n (%) | ||||||||||
| Commercial | 46 (25.99) |
43 (23.12) | 63 (41.18) | 54 (30.17) | 50 (26.74) | 52 (25.37) | 55 (30.39) | 50 (28.25) | 62 (36.05) | 54 (30.68) |
| Medicare | 60 (33.90) |
67 (36.02) | 43 (28.10) | 58 (32.40) | 67 (35.83) | 74 (36.10) | 60 (33.15) | 59 (33.33) | 49 (28.49) | 56 (31.82) |
| Medicaid | 4 (2.26) | 4 (2.15) | 1 (0.65) | 4 (2.23) | 1 (0.53) | 4 (1.95) | 1 (0.55) | 3 (1.69) | 1 (0.58) | 3 (1.70) |
| Uninsured | 2 (1.13) | 2 (1.08) | 1 (0.65) | 1 (0.56) | 3 (1.60) | 1 (0.49) | 0 (0) | 1 (0.56) | 0 (0) | 2 (1.14) |
| Comorbidities, n (%) | ||||||||||
| Hypertension | 151 (85.31) |
168 (90.32) | 125 (81.70) | 160 (89.39) | 165 (88.24) | 185 (90.24) | 159 (87.85) | 158 (89.27) | 145 (84.30) | 160 (90.91) |
| Dementia | 11 (6.21) |
9 (4.84) |
12 (7.84) | 11 (6.15) |
10 (5.35) |
16 (7.80) |
11 (6.08) | 12 (6.78) | 9 (5.23) |
12 (6.82) |
| COPD | 20 (11.30) |
31 (16.67) | 22 (14.38) | 22 (12.29) | 20 (10.70) | 36 (17.56) | 25 (13.81) | 27 (15.25) | 23 (13.37) | 29 (16.48) |
| Heart failure | 39 (22.03) |
51 (27.42) | 27 (17.65) | 45 (25.14) | 28 (14.97) | 49 (23.90) | 21 (11.60) | 40 (22.60) | 29 (16.86) | 45 (25.57) |
| Atrial fibrillation | 36 (20.34) |
44 (23.66) | 26 (16.99) | 45 (25.14) | 35 (18.72) | 47 (22.93) | 35 (19.34) | 31 (17.51) | 29 (16.86) | 38 (21.59) |
| Type 2 diabetes | 77 (43.50) |
105 (56.45) | 57 (37.25) | 71 (39.66) | 77 (41.18) | 92 (44.88) | 73 (40.33) | 84 (47.46) | 69 (40.12) | 82 (46.59) |
| Fatty liver disease | 2 (1.13) | 5 (2.69) | 2 (1.31) | 4 (2.23) | 5 (2.67) | 10 (4.88) | 5 (2.76) | 3 (1.69) | 7 (4.07) | 0 (0) |
| Cardiac amyloidosis | 1 (0.56) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.49) | 1 (0.55) | 1 (0.56) | 0 (0) | 1 (0.57) |
| Aortic stenosis | 17 (9.60) |
21 (11.29) | 10 (6.54) | 20 (11.17) | 24 (12.83) | 12 (5.85) |
19 (10.50) | 19 (10.73) | 21 (12.21) | 23 (13.07) |
| Medications, n (%) | ||||||||||
| NSAIDs | 73 (41.24) |
75 (40.32) | 72 (47.06) | 90 (50.28) | 69 (36.90) | 90 (43.90) | 55 (30.39) | 69 (38.98) | 73 (42.44) | 74 (42.05) |
| MRA | 18 (10.17) |
25 (13.44) | 8 (5.23) |
5 (2.79) |
13 (6.95) |
18 (8.78) |
13 (7.18) | 18 (10.17) | 14 (8.14) | 21 (11.93) |
| Antidiabetics | 63 (35.59) |
86 (46.24) | 53 (34.64) | 69 (38.55) | 71 (37.97) | 78 (38.05) | 65 (35.91) | 69 (38.98) | 61 (35.47) | 70 (39.77) |
| Digoxin | 4 (2.26) | 7 (3.76) | 3 (1.96) | 4 (2.23) | 5 (2.67) | 4 (1.95) | 0 (0) | 4 (2.26) | 1 (0.58) | 4 (2.27) |
| Lipid-lowering therapy | 137 (77.40) |
147 (79.03) | 118 (77.12) | 128 (71.51) | 141 (75.40) | 147 (71.71) | 130 (71.82) | 137 (77.40) | 145 (84.30) | 136 (77.27) |
| Anti-platelet | 82 (46.33) |
87 (46.77) | 67 (43.79) | 92 (51.40) | 73 (39.04) | 85 (41.46) | 69 (38.12) | 81 (45.76) | 79 (45.93) | 86 (48.86) |
| Antihypertensives | 148 (83.62) |
161 (86.56) | 120 (78.43) | 158 (88.27) | 166 (88.77) | 181 (88.29) | 150 (82.87) | 150 (84.75) | 150 (87.21) | 151 (85.80) |
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary artery disease; MRA, mineralocorticoid receptor antagonist; NSAIDs, nonsteroidal anti-inflammatory drugs; SI, systemic inflammation.
Among those with SI, a higher Charlson Comorbidity Index (CCI) was noted in those with CKD compared to those without CKD. Annual range of mean CCI was 0.75 to 0.78 in patients with ASCVD, 0.99 to 1.21 in those with ASCVD and CKD, and 1.71 to 1.89 in those with ASCVD and stage 3 or 4 CKD. The most common medications used by patients with SI were lipid-lowering therapy (70.5 % [ASCVD], 72.7 % [ASCVD and CKD], and 75.4 % [ASCVD and stage 3 or 4 CKD]) and antihypertensive therapy (72.8 % [ASCVD], 79.3 % [ASCVD and CKD], and 86.7 % [ASCVD and stage 3 or 4 CKD]).
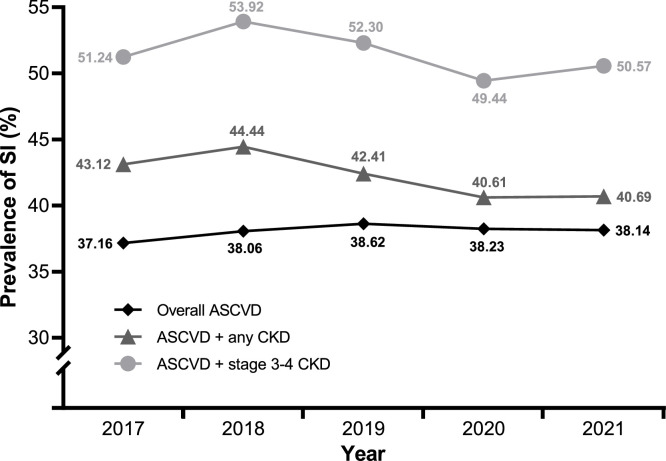
Among patients who underwent hsCRP testing, SI (hsCRP levels ≥2 mg/L and ≤10 mg/L) was present in a mean of 38.0 %, 42.3 %, and 51.5 % of patients with ASCVD, ASCVD with CKD, and ASCVD with stage 3 or 4 CKD, respectively (Table 2, Table 3, Table 4). The prevalence of SI remained largely unchanged over the study period in all three groups (Fig. 4).
Fig. 4.
Prevalence of patients with systemic inflammation (hsCRP ≥2 mg/L and ≤10 mg/L) over the study period (2017–2021).
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; SI, systemic inflammation.
Patients with hsCRP levels ≥2 mg/L and <3 mg/L and levels ≥3 mg/L and ≤10 mg/L were also further evaluated. Patient baseline characteristics, comorbidities, and medication use were stratified by calendar year and hsCRP level in Table S4 (patients with ASCVD), Table S5 (patients with ASCVD and CKD), and Table S6 (patients with ASCVD and stage 3 or 4 CKD). Among patients who underwent hsCRP testing, 12.3 % of patients with ASCVD, 13.4 % of patients with ASCVD and CKD, and 14.5 % of patients with ASCVD and stage 3 or 4 CKD had hsCRP levels ≥2 mg/L and <3 mg/L averaged across the study period (Fig. S2) and 25.7 %, 28.8 %, and 37.0 % of patients with ASCVD, ASCVD and CKD, and ASCVD and stage 3 or 4 CKD, respectively, had hsCRP levels of ≥3 mg/L and ≤10 mg/L averaged across the study period (Fig. S3).
4. Discussion
In this study, we identified a persistent, low rate of hsCRP testing in a large cohort of US patients with ASCVD. Relative to the overall ASCVD population, there was no appreciable increase in the hsCRP testing rate among patients with ASCVD and any CKD or ASCVD and stage 3 or 4 CKD. Among patients who underwent hsCRP testing, SI was prevalent in patients with ASCVD, with a prevalence that increased with CKD severity. These findings have important implications for anti-inflammatory therapies that are dependent on hsCRP testing to determine treatment eligibility.
Currently, there is little guidance on the role of hsCRP testing in patients with ASCVD. While AHA/CDC guidelines recommend consideration of hsCRP testing in primary prevention [36], such testing is not recommended in secondary prevention. In our study, only about 1 % of patients with ASCVD had hsCRP testing performed.
The lack of strong clinical evidence to support the need for hsCRP testing and address SI in secondary prevention likely contributes to low hsCRP testing rates. For example, hsCRP testing was not required in the LoDoCo, LoDoCo2, and COLCOT trials [11,37,38]. In the context of primary prevention, existing RWE is mixed, and the implications of these findings are unclear [[39], [40], [41], [42], [43], [44]]. However, findings from some RWE studies support the use of hsCRP testing in secondary prevention. One real-world study in patients with MI demonstrated that higher hsCRP levels correlated with increased risk of MACE and death [19]. Another prospective study in patients with established CVD showed that hsCRP was associated with increased risk of incident heart failure [23]. Similarly, a meta-analysis of 8 studies found that high hsCRP was predictive of MACE in patients with peripheral artery disease [45].
In our study, regional differences in rates of hsCRP testing were observed. Patients located in the Northeast were more likely than patients in other regions to undergo hsCRP testing. It is possible that, because clinical trials investigating treatments for SI have most often been conducted in the Northeast [10,14,16,34,35], clinicians in this region may have been more likely to order hsCRP testing to evaluate SI. In addition, patients who were covered by commercial insurance plans were more likely to undergo hsCRP testing than those covered by other insurance plans or who were uninsured. In general, commercial plans are likely to have more flexible test reimbursement policies compared with Medicare and Medicaid [46]. However, commercial plans are largely private, and coverage is assessed on a case-by-case basis; therefore, this hypothesis cannot be tested.
Regardless of hsCRP testing status, patients who identified as White accounted for the highest percentage of patients in the ASCVD, ASCVD and any stage CKD, and ASCVD and stage 3 or 4 CKD groups. Patients who identified as Black/African American made up a higher percentage of patients with ASCVD and stage 3 or 4 CKD versus patients with ASCVD or ASCVD and any stage CKD. This may indicate the presence of health disparities or differences in testing behavior between racial groups.
While many patients are at risk for SI, there is a potential unmet need to identify patients that may benefit from treatments to address SI and associated residual cardiovascular risk. Further, a lack of treatment options specifically targeting SI may explain the low rate of hsCRP testing in clinical practice. That being said, results from recent clinical trials evaluating treatments for SI may lead to increased future use of hsCRP testing to identify at-risk patients with ASCVD with or without CKD [11,13,16,35,37,38]. Additionally, data from the RESCUE trial highlight the ability of an IL6 inhibitor to reduce SI, potentially addressing unmet needs [13].
Among patients with ASCVD undergoing hsCRP testing, SI was common, particularly among those with stage 3 or 4 CKD. Regardless of CKD stage, patients with SI displayed increased rates of comorbidities such as hypertension, heart failure, and atrial fibrillation. These findings show that there may be many at-risk patients who are likely to benefit from assessment of SI and subsequent treatment. Due to low rates of hsCRP testing, however, these patients may not be properly identified.
4.1. Limitations
As this was a retrospective observational study, it may be limited by issues related to confounding, misclassification, selection, generalizability, and random error. Therefore, results should be interpreted with caution. Further, data collection reflected routine clinical practice rather than mandatory assessments at prespecified time points, potentially impacting the amount of data available and its interpretation. No imputation was performed in this study because it was unclear whether a lack of data should be attributed to misclassification, a true lack of disease, or incompleteness due to censoring or lack of observation time.
While our study found that SI was common in patients with ASCVD undergoing hsCRP testing, it is possible that hsCRP testing may be conducted more frequently in patients suspected to have higher levels of SI, leading to an overestimate of the prevalence of SI. Finally, some of the hsCRP results were extracted from clinician notes using natural language processing; therefore, the data collected was subject to human error and data processing errors. For example, clinicians may not have had access to final hsCRP results or may not have accurately transcribed these test results in their notes.
5. Conclusion
The prevalence of hsCRP testing in ASCVD patients is low, regardless of the presence or severity of CKD. However, among those who receive hsCRP testing, the prevalence of patients with SI is high, and this prevalence increases with CKD severity. Increasing the rate of hsCRP testing is likely to help identify patients with SI and who are at risk for CVEs.
Data sharing statement
The Optum® de-identified Electronic Health Record data set (Optum® EHR) was commercially licensed from the data vendor. The data contained in the Optum® EHR contains proprietary elements owned by Optum and, therefore, cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third party clients assumes certain data security and privacy protocols are in place and that the third-party client has executed the standard license agreement which includes restrictive covenants governing the use of the data.
CRediT authorship contribution statement
Lei Lv: Writing – review & editing, Visualization, Supervision, Methodology, Conceptualization. Jigar Rajpura: Writing – review & editing, Visualization, Methodology, Conceptualization. Meixia Liu: Writing – review & editing, Visualization, Supervision, Methodology, Conceptualization. Matt Strum: Writing – review & editing, Visualization, Methodology, Conceptualization. Benjamin Chastek: Writing – review & editing, Visualization, Supervision, Methodology, Conceptualization. Jonathan Johnson: Writing – review & editing, Visualization, Methodology, Formal analysis, Conceptualization. Ty J. Gluckman: Writing – review & editing, Visualization, Methodology, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Lei Lv reports a relationship with Novo Nordisk Inc that includes: employment and equity or stocks. Jigar Rajpura reports a relationship with Novo Nordisk Inc that includes: employment and equity or stocks. Matt Strum reports a relationship with Novo Nordisk Inc that includes: employment and equity or stocks. Meixia Liu reports a relationship with Optum that includes: employment and equity or stocks. Benjamin Chastek reports a relationship with Optum that includes: employment and equity or stocks. Jonathan Johnson reports a relationship with Optum that includes: employment and equity or stocks. Ty J. Gluckman reports a relationship with OptumRx that includes: consulting or advisory. Funding for this study was provided to Optum by Novo Nordisk Inc. Medical writing and editing assistance was financially supported by Novo Nordisk Inc. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Adele Musicant, PhD, and Kelsey Pinckard Schaefers, PhD, of Precision AQ (Bethesda, Maryland) for providing writing and editing assistance in accordance with Good Publication Practice (GPP 2022) guidelines. This assistance was financially supported by Novo Nordisk Inc.
Funding sources
Funding for this study was provided to Optum by Novo Nordisk Inc. Novo Nordisk Inc. took the lead on study design, data interpretation, study report development, and the decision to publish study findings. Novo Nordisk Inc. did not collect or analyze any data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2025.100950.
Appendix. Supplementary materials
References
- 1.Centers for Disease Control and Prevention. Heart disease facts. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed July 9, 2024).
- 2.Alanaeme C.J., Bittner V., Brown T.M., et al. Estimated number and percentage of US adults with atherosclerotic cardiovascular disease recommended add-on lipid-lowering therapy by the 2018 AHA/ACC multi-society cholesterol guideline. Am Heart J Plus. 2022;21:100201. doi: 10.1016/j.ahjo.2022.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Y., Li D., Cui H., et al. Epidemiology of multimorbidity associated with atherosclerotic cardiovascular disease in the United States, 1999-2018. BMC Public Health. 2024;24:267. doi: 10.1186/s12889-023-17619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanna M., Sloan L., Faurby M., et al. Abstract 11398: Prevalence and characteristics of systemic inflammation in adults with atherosclerotic cardiovascular disease and chronic kidney disease: results from the National Health and Nutrition Examination Survey. Circulation. 2022;146:A11398. doi: 10.1161/circ.146.suppl_1.11398. [DOI] [Google Scholar]
- 5.Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., et al. 2022 ACC Expert Consensus Decision Pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80:1366–1418. doi: 10.1016/j.jacc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Ridker P.M., Danielson E., Fonseca F.A., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 7.Sampson U.K., Fazio S., Linton M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong N.D., Zhao Y., Quek R.G.W., et al. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: the Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol. 2017;11:1223–1233. doi: 10.1016/j.jacl.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawler P.R., Bhatt D.L., Godoy L.C., et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42:113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 10.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 11.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 12.Fiolet A.T.L., Opstal T.S.J., Mosterd A., et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42:2765–2775. doi: 10.1093/eurheartj/ehab115. [DOI] [PubMed] [Google Scholar]
- 13.Ridker P.M., Devalaraja M., Baeres F.M.M., et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060–2069. doi: 10.1016/S0140-6736(21)00520-1. [DOI] [PubMed] [Google Scholar]
- 14.Cardiovascular Risk Reduction Study (Reduction in Recurrent Major CV Disease Events) (CANTOS). ClinicalTrials.gov identifier: NCT01327846. https://www.clinicaltrials.gov/study/NCT01327846 (accessed September 9, 2024).
- 15.Trial to evaluate reduction in inflammation in patients with advanced chronic renal disease utilizing antibody mediated IL-6 inhibition (RESCUE). ClinicalTrials.gov identifier: NCT03926117. https://clinicaltrials.gov/study/NCT03926117 (accessed July 9, 2024).
- 16.ZEUS - A research study to look at how Ziltivekimab works compared to placebo in people with cardiovascular disease, chronic kidney disease and inflammation (ZEUS). ClinicalTrials.gov identifier: NCT05021835. https://clinicaltrials.gov/study/NCT05021835 (accessed July 9, 2024).
- 17.The LoDoCo2 Trial: a randomised controlled trial on the effect of low dose colchicine for secondary prevention of cardiovascular disease in patients with established stable coronary artery disease. ANZCTR identifier: ACTRN12614000093684. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363771 (accessed September 9, 2024).
- 18.Jolly S.S., d'Entremont M.A., Lee S.F., et al. Colchicine in acute myocardial infarction. N Engl J Med. 2024:1–10. doi: 10.1056/NEJMoa2405922. [DOI] [PubMed] [Google Scholar]
- 19.Carrero J.J., Andersson Franko M., Obergfell A., Gabrielsen A., Jernberg T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: a healthcare-based study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., He G., Huo X., et al. Long-term cumulative high-sensitivity C-reactive protein and mortality among patients with acute heart failure. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.123.029386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A., Liu J., Li C., et al. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z.H., Zhong W.F., Lv Y.B., et al. Associations of plasma high-sensitivity C-reactive protein concentrations with all-cause and cause-specific mortality among middle-aged and elderly individuals. Immun Age. 2019;16:28. doi: 10.1186/s12979-019-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger P.M., Koudstaal S., Mosterd A., et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. 2023;82:414–426. doi: 10.1016/j.jacc.2023.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Burger P.M., Pradhan A.D., Dorresteijn J.A.N., et al. C-reactive protein and risk of cardiovascular events and mortality in patients with various cardiovascular disease locations. Am J Cardiol. 2023;197:13–23. doi: 10.1016/j.amjcard.2023.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts with diabeTes (PROMINENT) ClinicalTrials.gov identifier: NCT03071692. https://clinicaltrials.gov/study/NCT03071692 (accessed July 9, 2024).
- 26.A study of AMR101 to evaluate its ability to reduce cardiovascular events in high-risk patients with hypertriglyceridemia and on statin (REDUCE-IT) ClinicalTrials.gov identifier: NCT01492361. https://clinicaltrials.gov/study/NCT01492361 (accessed July 9, 2024).
- 27.Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia (STRENGTH). ClinicalTrials.gov identifier: NCT02104817. https://clinicaltrials.gov/study/NCT02104817 (accessed July 9, 2024).
- 28.Ridker P.M., Bhatt D.L., Pradhan A.D., et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401:1293–1301. doi: 10.1016/S0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 29.Ridker P.M. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. 2016;67:712–723. doi: 10.1016/j.jacc.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Peikert A., Kaier K., Merz J., et al. Residual inflammatory risk in coronary heart disease: incidence of elevated high-sensitive CRP in a real-world cohort. Clin Res Cardiol. 2020;109:315–323. doi: 10.1007/s00392-019-01511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aday A.W., Ridker P.M. Antiinflammatory therapy in clinical care: the CANTOS trial and beyond. Front Cardiovasc Med. 2018;5:62. doi: 10.3389/fcvm.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., Yue Q., Fang F., et al. Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc Diabetol. 2023;22:96. doi: 10.1186/s12933-023-01826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A research study looking into how ziltivekimab works compared to placebo in participants with heart failure and inflammation (ATHENA) ClinicalTrials.gov identifier: NCT06200207 https://clinicaltrials.gov/study/NCT06200207 (accessed September 9, 2024).
- 34.Wang T.Y., Kaltenbach L.A., Cannon C.P., et al. Effect of medication co-payment vouchers on P2Y12 inhibitor use and major adverse cardiovascular events among patients with myocardial infarction: the ARTEMIS randomized clinical trial. JAMA. 2019;321:44–55. doi: 10.1001/jama.2018.19791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A research study to look at how Ziltivekimab works compared to placebo in people with heart failure and inflammation (HERMES). ClinicalTrials.gov identifier: NCT05636176. https://www.clinicaltrials.gov/study/NCT05636176 (accessed September 9, 2024).
- 36.Pearson T.A., Mensah G.A., Alexander R.W., et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 37.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Tardif J.C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 39.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 40.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 41.The Emerging Risk Factors Collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeboah J., Young R., McClelland R.L., et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaha M.J., Budoff M.J., DeFilippis A.P., et al. Association between hsCRP≥2, coronary artery calcium, and cardiovascular events – implications for the JUPITER population: multi-ethnic study of atherosclerosis (MESA) Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blankstein R., Budoff M.J., Shaw L.J., et al. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:364–374. doi: 10.1016/j.jacc.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 45.Singh T.P., Morris D.R., Smith S., Moxon J.V., Golledge J. Systematic review and meta-analysis of the association between C-reactive protein and major cardiovascular events in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2017;54:220–233. doi: 10.1016/j.ejvs.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 46.U.S. Centers for Medicare & Medicaid Services. Billing and coding: C-reactive protein high sensitivity testing (hsCRP). https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=56643 (accessed November 11, 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.