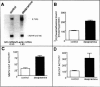
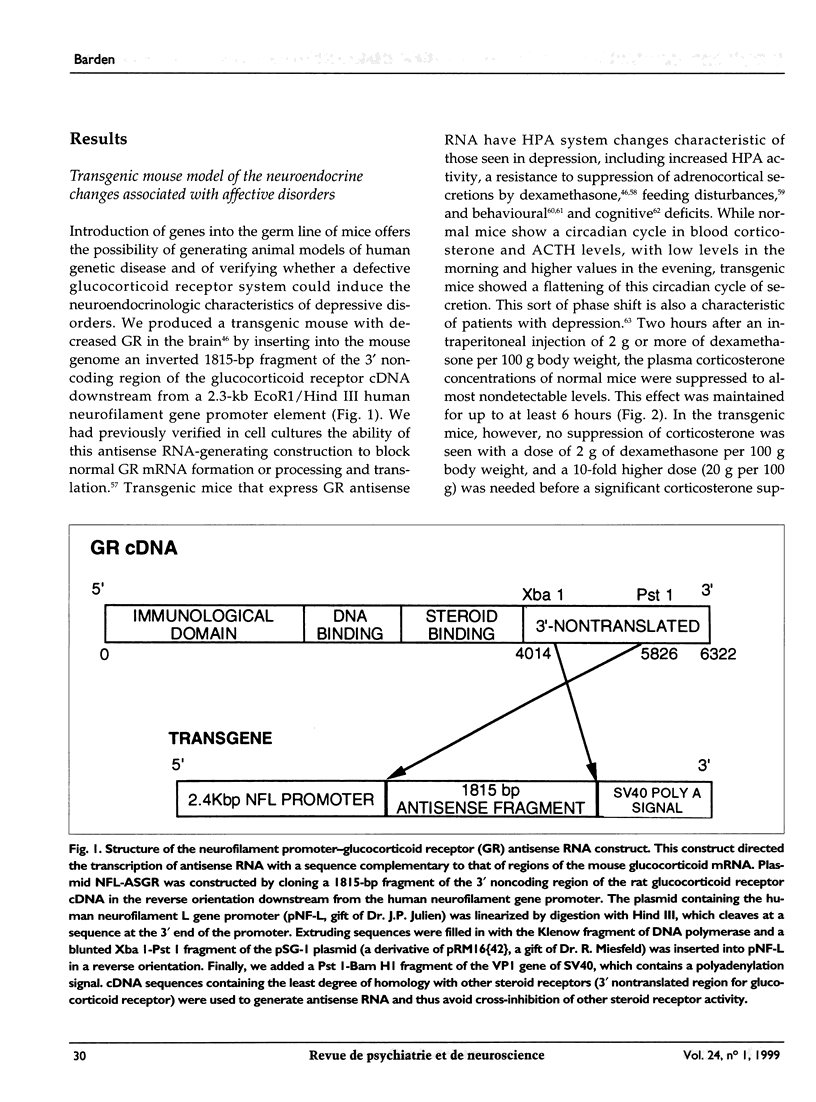
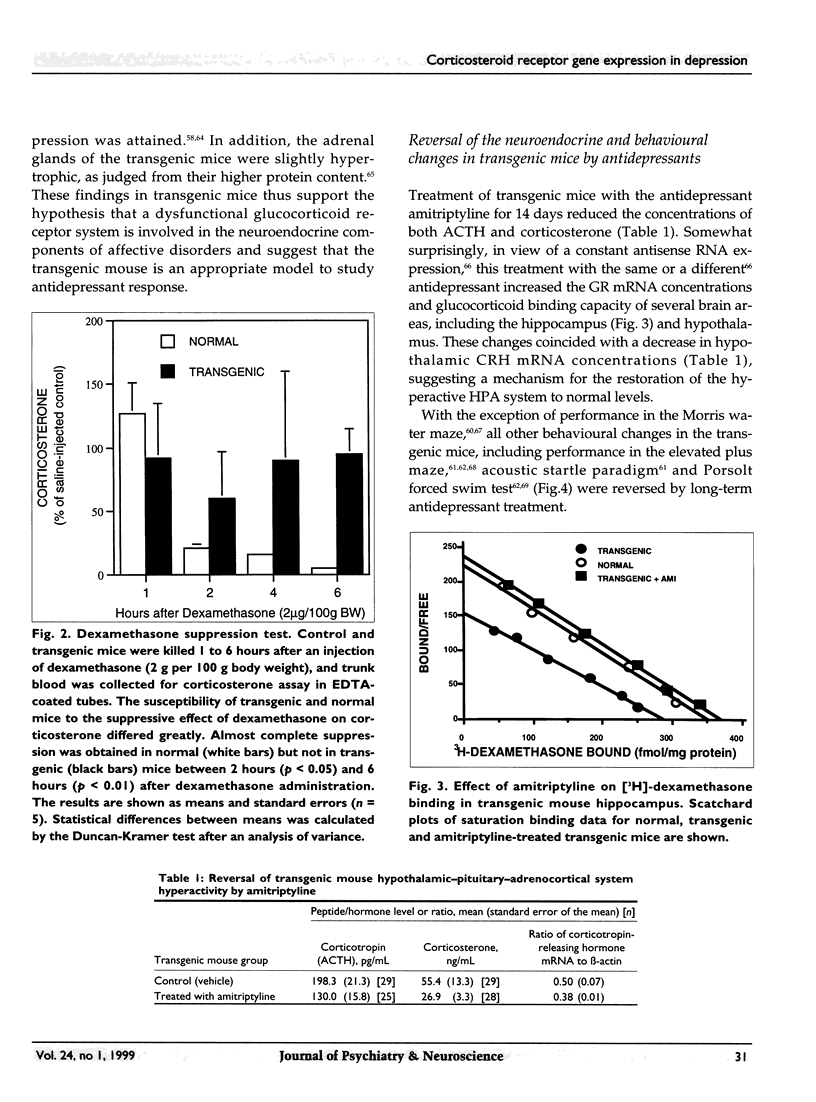
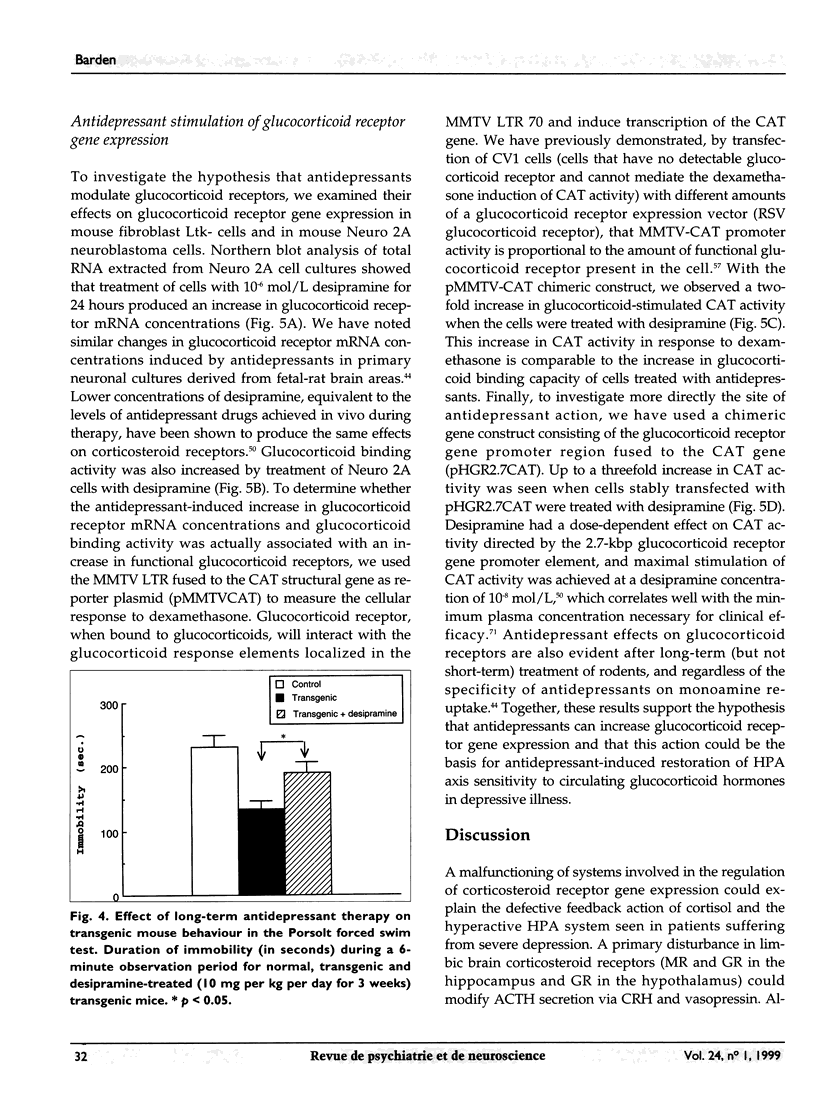
Abstract
OBJECTIVE: Major alterations of the hypothalamic-pituitary-adrenocortical (HPA) system are often seen in patients with depression, and can be reversed by successful antidepressant therapy. Persuasive evidence points to the involvement of a dysfunctional glucocorticoid receptor system in these changes. The authors developed a transgenic mouse to determine the mechanism for these changes. DESIGN: In vivo and in vitro animal experiments. ANIMALS: Transgenic mice expressing glucocorticoid receptor antisense RNA and control mice. INTERVENTIONS: In vivo: hormone assays and dexamethasone suppression tests; in vitro: cell transfection, chloramphenicol acetyl transferase assay, Northern blot analysis, binding assays of cytosolic receptor. OUTCOME MEASURES: Indicators of depressive disorder in transgenic mice, effect of antidepressant therapy on dexamethasone binding in transgenic mouse hippocampus, mouse behaviour, and glucocorticoid receptor activity. RESULTS: Transgenic mice showed no suppression of corticosterone with a dose of 2 mg per 100 g body weight dexamethasone. Treatment with amitriptyline reduced levels of corticotropin and corticosterone, increased glucocorticoid receptor mRNA concentrations and glucocorticoid binding capacity of several brain areas, and reversed behavioural changes. In vitro experiments also showed that desipramine increased glucocorticoid receptor mRNA. CONCLUSION: These transgenic mice have numerous neuroendocrine characteristics of human depression as well as altered behaviour. Many of these neuroendocrinologic and behavioural characteristics are reversed by antidepressants. The antidepressant-induced increase in glucocorticoid receptor activity may render the HPA axis more sensitive to glucocorticoid feedback. This new insight into antidepressant drug action suggests a novel approach to the development of new antidepressant drugs.
Full text
PDF




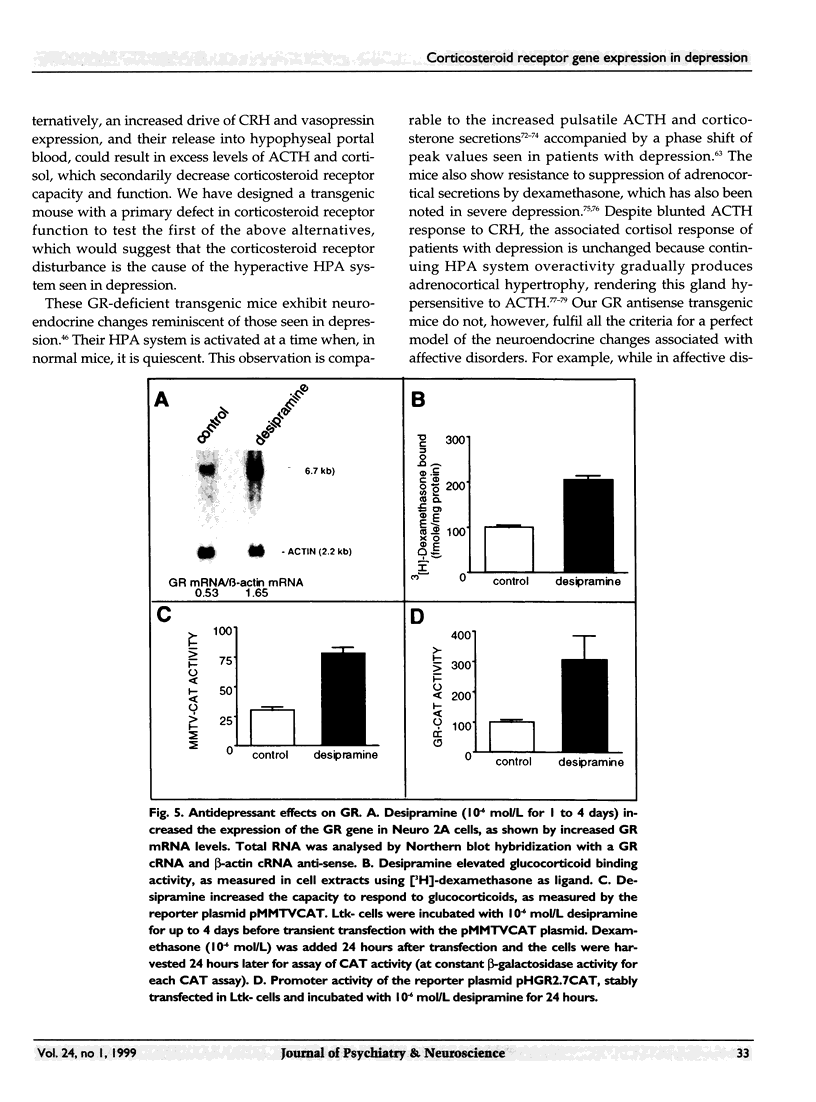
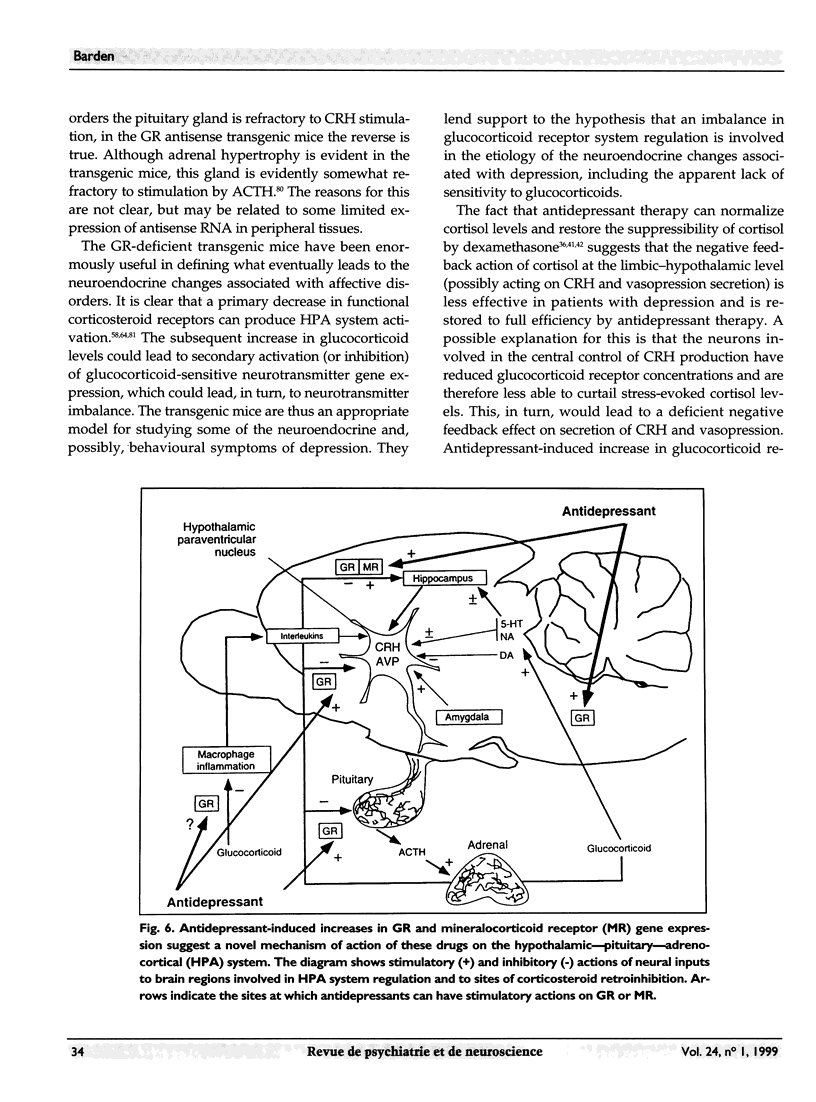





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam J. D., Marinelli D. L., Arger P., Winokur A. Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: a pilot study. Psychiatry Res. 1987 Jul;21(3):189–197. doi: 10.1016/0165-1781(87)90022-9. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Ban T. A. Monoamine uptake inhibitors. Mod Probl Pharmacopsychiatry. 1982;18:1–16. doi: 10.1159/000406233. [DOI] [PubMed] [Google Scholar]
- Barden N. Corticosteroid receptor modulation in transgenic mice. Ann N Y Acad Sci. 1994 Nov 30;746:89-98; discussion 98-100, 131-3. doi: 10.1111/j.1749-6632.1994.tb39217.x. [DOI] [PubMed] [Google Scholar]
- Barden N., Stec I. S., Montkowski A., Holsboer F., Reul J. M. Endocrine profile and neuroendocrine challenge tests in transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuroendocrinology. 1997 Sep;66(3):212–220. doi: 10.1159/000127240. [DOI] [PubMed] [Google Scholar]
- Beaulieu S., Di Paolo T., Côté J., Barden N. Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinology. 1987 Jan;45(1):37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- Beaulieu S., Pelletier G., Vaudry H., Barden N. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 1989 Mar;49(3):255–261. doi: 10.1159/000125125. [DOI] [PubMed] [Google Scholar]
- Benkelfat C., Ellenbogen M. A., Dean P., Palmour R. M., Young S. N. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994 Sep;51(9):687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Biegon A., Rainbow T. C., McEwen B. S. Corticosterone modulation of neurotransmitter receptors in rat hippocampus: a quantitative autoradiographic study. Brain Res. 1985 Apr 22;332(2):309–314. doi: 10.1016/0006-8993(85)90599-2. [DOI] [PubMed] [Google Scholar]
- Bradbury M. J., Akana S. F., Cascio C. S., Levin N., Jacobson L., Dallman M. F. Regulation of basal ACTH secretion by corticosterone is mediated by both type I (MR) and type II (GR) receptors in rat brain. J Steroid Biochem Mol Biol. 1991;40(1-3):133–142. doi: 10.1016/0960-0760(91)90176-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady L. S., Whitfield H. J., Jr, Fox R. J., Gold P. W., Herkenham M. Long-term antidepressant administration alters corticotropin-releasing hormone, tyrosine hydroxylase, and mineralocorticoid receptor gene expression in rat brain. Therapeutic implications. J Clin Invest. 1991 Mar;87(3):831–837. doi: 10.1172/JCI115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J., Martin F. I., Davies B. Resistance to suppression by dexamethasone of plasma 11-O.H.C.S. levels in severe depressive illness. Br Med J. 1968 Aug 3;3(5613):285–287. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982 Mar;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- Carroll, Curtis G. C., Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch Gen Psychiatry. 1976 Sep;33(9):1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- Cascio C. S., Shinsako J., Dallman M. F. The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 1987 Oct 13;423(1-2):173–178. doi: 10.1016/0006-8993(87)90837-7. [DOI] [PubMed] [Google Scholar]
- Chalmers D. T., López J. F., Vázquez D. M., Akil H., Watson S. J. Regulation of hippocampal 5-HT1A receptor gene expression by dexamethasone. Neuropsychopharmacology. 1994 May;10(3):215–222. doi: 10.1038/npp.1994.24. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cintra A., Zoli M., Rosén L., Agnati L. F., Okret S., Wikström A. C., Gustaffsson J. A., Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994 Oct;62(3):843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Levin N., Cascio C. S., Akana S. F., Jacobson L., Kuhn R. W. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology. 1989 Jun;124(6):2844–2850. doi: 10.1210/endo-124-6-2844. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., Kovács G. L., Szabó G., Telegdy G., Bohus B., Versteeg D. H. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 1982 May 13;239(2):659–663. doi: 10.1016/0006-8993(82)90546-7. [DOI] [PubMed] [Google Scholar]
- Dunn A. J., Berridge C. W. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990 May-Aug;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J. W. Corticosteroid receptors and the central nervous system. J Steroid Biochem Mol Biol. 1994 Jun;49(4-6):381–384. doi: 10.1016/0960-0760(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Chrousos G., Kellner C., Post R., Roy A., Augerinos P., Schulte H., Oldfield E., Loriaux D. L. Psychiatric implications of basic and clinical studies with corticotropin-releasing factor. Am J Psychiatry. 1984 May;141(5):619–627. doi: 10.1176/ajp.141.5.619. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Loriaux D. L., Roy A., Kling M. A., Calabrese J. R., Kellner C. H., Nieman L. K., Post R. M., Pickar D., Gallucci W. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986 May 22;314(21):1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt U., Schweiger U., Fahrenberg J., Lauer C. J., Holsboer F., Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995 Apr;268(4 Pt 2):R865–R873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- Greden J. F., Gardner R., King D., Grunhaus L., Carroll B. J., Kronfol Z. Dexamethasone suppression tests in antidepressant treatment of melancholia. The process of normalization and test-retest reproducibility. Arch Gen Psychiatry. 1983 May;40(5):493–500. doi: 10.1001/archpsyc.1983.01790050019002. [DOI] [PubMed] [Google Scholar]
- Heuser I. J., Gotthardt U., Schweiger U., Schmider J., Lammers C. H., Dettling M., Holsboer F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994 Mar-Apr;15(2):227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Heuser I., Yassouridis A., Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994 Jul-Aug;28(4):341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E., Stohler R., Hatzinger M. Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res. 1991 Aug;38(2):163–171. doi: 10.1016/0165-1781(91)90041-m. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Gerken A., Stalla G. K., Müller O. A. ACTH, cortisol, and corticosterone output after ovine corticotropin-releasing factor challenge during depression and after recovery. Biol Psychiatry. 1985 Mar;20(3):276–286. doi: 10.1016/0006-3223(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Gerken A., von Bardeleben U., Grimm W., Beyer H., Müller O. A., Stalla G. K. Human corticotropin-releasing hormone in depression--correlation with thyrotropin secretion following thyrotropin-releasing hormone. Biol Psychiatry. 1986 Jun;21(7):601–611. doi: 10.1016/0006-3223(86)90121-6. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Liebl R., Hofschuster E. Repeated dexamethasone suppression test during depressive illness. Normalisation of test result compared with clinical improvement. J Affect Disord. 1982 Jun;4(2):93–101. doi: 10.1016/0165-0327(82)90039-8. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Spengler D., Heuser I. The role of corticotropin-releasing hormone in the pathogenesis of Cushing's disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog Brain Res. 1992;93:385–417. doi: 10.1016/s0079-6123(08)64586-0. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Von Bardeleben U., Gerken A., Stalla G. K., Müller O. A. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984 Oct 25;311(17):1127–1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991 May;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jasper M. S., Engeland W. C. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology. 1994 Feb;59(2):97–109. doi: 10.1159/000126645. [DOI] [PubMed] [Google Scholar]
- Kitayama I., Janson A. M., Cintra A., Fuxe K., Agnati L. F., Ogren S. O., Härfstrand A., Eneroth P., Gustafsson J. A. Effects of chronic imipramine treatment on glucocorticoid receptor immunoreactivity in various regions of the rat brain. Evidence for selective increases of glucocorticoid receptor immunoreactivity in the locus coeruleus and in 5-hydroxytryptamine nerve cell groups of the rostral ventromedial medulla. J Neural Transm. 1988;73(3):191–203. doi: 10.1007/BF01250136. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Mikuni M., Ogawa T., Takahashi K. Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor-mediated wet-dog shake behaviors. Psychopharmacology (Berl) 1992;108(1-2):27–32. doi: 10.1007/BF02245281. [DOI] [PubMed] [Google Scholar]
- Kvetnanský R., Fukuhara K., Pacák K., Cizza G., Goldstein D. S., Kopin I. J. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993 Sep;133(3):1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Kelsoe J. R., Remick R. A., Sadovnick A. D., Rapaport M. H., Lin M., Pazur B. A., Roe A. M., Saito T., Papolos D. F. Linkage studies suggest a possible locus for bipolar disorder near the velo-cardio-facial syndrome region on chromosome 22. Am J Med Genet. 1997 Apr 18;74(2):121–128. doi: 10.1002/(sici)1096-8628(19970418)74:2<121::aid-ajmg2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Linkowski P., Kerkhofs M., Van Onderbergen A., Hubain P., Copinschi G., L'Hermite-Balériaux M., Leclercq R., Brasseur M., Mendlewicz J., Van Cauter E. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry. 1994 Aug;51(8):616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- Linkowski P., Mendlewicz J., Leclercq R., Brasseur M., Hubain P., Golstein J., Copinschi G., Van Cauter E. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985 Sep;61(3):429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- Lisansky J., Peake G. T., Strassman R. J., Qualls C., Meikle A. W., Risch S. C., Fava G. A., Zownir-Brazis M., Hochla P., Britton D. Augmented pituitary corticotropin response to a threshold dosage of human corticotropin-releasing hormone in depressives pretreated with metyrapone. Arch Gen Psychiatry. 1989 Jul;46(7):641–649. doi: 10.1001/archpsyc.1989.01810070067011. [DOI] [PubMed] [Google Scholar]
- Lister R. G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Maes M., Meltzer H. Y., D'Hondt P., Cosyns P., Blockx P. Effects of serotonin precursors on the negative feedback effects of glucocorticoids on hypothalamic-pituitary-adrenal axis function in depression. Psychoneuroendocrinology. 1995;20(2):149–167. doi: 10.1016/0306-4530(94)00049-g. [DOI] [PubMed] [Google Scholar]
- Mendelson S. D., McEwen B. S. Autoradiographic analyses of the effects of restraint-induced stress on 5-HT1A, 5-HT1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology. 1991 Nov;54(5):454–461. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Montkowski A., Barden N., Wotjak C., Stec I., Ganster J., Meaney M., Engelmann M., Reul J. M., Landgraf R., Holsboer F. Long-term antidepressant treatment reduces behavioural deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995 Nov;7(11):841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mortola J. F., Liu J. H., Gillin J. C., Rasmussen D. D., Yen S. S. Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab. 1987 Nov;65(5):962–968. doi: 10.1210/jcem-65-5-962. [DOI] [PubMed] [Google Scholar]
- Murphy B. E., Filipini D., Ghadirian A. M. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J Psychiatry Neurosci. 1993 Nov;18(5):209–213. [PMC free article] [PubMed] [Google Scholar]
- Murphy B. E. Steroids and depression. J Steroid Biochem Mol Biol. 1991 May;38(5):537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Krishnan K. R., Reed D., Leder R., Beam C., Dunnick N. R. Adrenal gland enlargement in major depression. A computed tomographic study. Arch Gen Psychiatry. 1992 May;49(5):384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Owens M. J., Bissette G., Andorn A. C., Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988 Jun;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Widerlöv E., Bissette G., Walléus H., Karlsson I., Eklund K., Kilts C. D., Loosen P. T., Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984 Dec 14;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Mikuni M., Kuroda Y., Muneoka K., Mori K. J., Takahashi K. Effects of the altered serotonergic signalling by neonatal treatment with 5,7-dihydroxytryptamine, ritanserin or clomipramine on the adrenocortical stress response and the glucocorticoid receptor binding in the hippocampus in adult rats. J Neural Transm Gen Sect. 1994;96(2):113–123. doi: 10.1007/BF01277933. [DOI] [PubMed] [Google Scholar]
- Owens M. J., Nemeroff C. B. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991 Dec;43(4):425–473. [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Peiffer A., Veilleux S., Barden N. Antidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brain. Psychoneuroendocrinology. 1991;16(6):505–515. doi: 10.1016/0306-4530(91)90034-q. [DOI] [PubMed] [Google Scholar]
- Pepin M. C., Barden N. Decreased glucocorticoid receptor activity following glucocorticoid receptor antisense RNA gene fragment transfection. Mol Cell Biol. 1991 Mar;11(3):1647–1653. doi: 10.1128/mcb.11.3.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin M. C., Beaulieu S., Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Res Mol Brain Res. 1989 Jul;6(1):77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Pepin M. C., Govindan M. V., Barden N. Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol Pharmacol. 1992 Jun;41(6):1016–1022. [PubMed] [Google Scholar]
- Pepin M. C., Pothier F., Barden N. Antidepressant drug action in a transgenic mouse model of the endocrine changes seen in depression. Mol Pharmacol. 1992 Dec;42(6):991–995. [PubMed] [Google Scholar]
- Pepin M. C., Pothier F., Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992 Feb 20;355(6362):725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- Porsolt R. D., Bertin A., Jalfre M. "Behavioural despair" in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978 Oct 1;51(3):291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Raadsheer F. C., Hoogendijk W. J., Stam F. C., Tilders F. J., Swaab D. F. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994 Oct;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Ratka A., Sutanto W., Bloemers M., de Kloet E. R. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989 Aug;50(2):117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- Regestein Q. R., Rose L. I., Williams G. H. Psychopathology in Cushing's syndrome. Arch Intern Med. 1972 Jul;130(1):114–117. [PubMed] [Google Scholar]
- Reul J. M., Labeur M. S., Grigoriadis D. E., De Souza E. B., Holsboer F. Hypothalamic-pituitary-adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology. 1994 Nov;60(5):509–519. doi: 10.1159/000126788. [DOI] [PubMed] [Google Scholar]
- Reul J. M., Stec I., Söder M., Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology. 1993 Jul;133(1):312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Richard D., Chapdelaine S., Deshaies Y., Pépin M. C., Barden N. Energy balance and lipid metabolism in transgenic mice bearing an antisense GCR gene construct. Am J Physiol. 1993 Jul;265(1 Pt 2):R146–R150. doi: 10.1152/ajpregu.1993.265.1.R146. [DOI] [PubMed] [Google Scholar]
- Rochford J., Beaulieu S., Rousse I., Glowa J. R., Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharmacology (Berl) 1997 Jul;132(2):145–152. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- Rousse I., Beaulieu S., Rowe W., Meaney M. J., Barden N., Rochford J. Spatial memory in transgenic mice with impaired glucocorticoid receptor function. Neuroreport. 1997 Mar 3;8(4):841–845. doi: 10.1097/00001756-199703030-00007. [DOI] [PubMed] [Google Scholar]
- Sachar E. J., Hellman L., Roffwarg H. P., Halpern F. S., Fukushima D. K., Gallagher T. F. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry. 1973 Jan;28(1):19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- Seckl J. R., Fink G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology. 1992 Jun;55(6):621–626. doi: 10.1159/000126180. [DOI] [PubMed] [Google Scholar]
- Shimoda K., Yamada N., Ohi K., Tsujimoto T., Takahashi K., Takahashi S. Chronic administration of tricyclic antidepressants suppresses hypothalamo-pituitary-adrenocortical activity in male rats. Psychoneuroendocrinology. 1988;13(5):431–440. doi: 10.1016/0306-4530(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Silva C. M., Powell-Oliver F. E., Jewell C. M., Sar M., Allgood V. E., Cidlowski J. A. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994 Jul;59(7):436–442. doi: 10.1016/0039-128x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Singh V. B., Corley K. C., Phan T. H., Boadle-Biber M. C. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res. 1990 May 14;516(1):66–76. doi: 10.1016/0006-8993(90)90898-l. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stec I., Barden N., Reul J. M., Holsboer F. Dexamethasone nonsuppression in transgenic mice expressing antisense RNA to the glucocorticoid receptor. J Psychiatr Res. 1994 Jan-Feb;28(1):1–5. doi: 10.1016/0022-3956(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Suda T., Tozawa F., Yamada M., Ushiyama T., Tomori N., Sumitomo T., Nakagami Y., Demura H., Shizume K. Insulin-induced hypoglycemia increases corticotropin-releasing factor messenger ribonucleic acid levels in rat hypothalamus. Endocrinology. 1988 Sep;123(3):1371–1375. doi: 10.1210/endo-123-3-1371. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A., Alonso G., Ixart G., Malaval F., Assenmacher I. Diurnal-stimulated and stress-induced ACTH release in rats is mediated by ventral noradrenergic bundle. Am J Physiol. 1985 Aug;249(2 Pt 1):E219–E226. doi: 10.1152/ajpendo.1985.249.2.E219. [DOI] [PubMed] [Google Scholar]
- Szigethy E., Conwell Y., Forbes N. T., Cox C., Caine E. D. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry. 1994 Sep 15;36(6):374–380. doi: 10.1016/0006-3223(94)91212-2. [DOI] [PubMed] [Google Scholar]
- Torpy D. J., Grice J. E., Hockings G. I., Walters M. M., Crosbie G. V., Jackson R. V. Alprazolam attenuates vasopressin-stimulated adrenocorticotropin and cortisol release: evidence for synergy between vasopressin and corticotropin-releasing hormone in humans. J Clin Endocrinol Metab. 1994 Jul;79(1):140–144. doi: 10.1210/jcem.79.1.8027217. [DOI] [PubMed] [Google Scholar]
- Whalley L. J., Borthwick N., Copolov D., Dick H., Christie J. E., Fink G. Glucocorticoid receptors and depression. Br Med J (Clin Res Ed) 1986 Mar 29;292(6524):859–861. doi: 10.1136/bmj.292.6524.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J. L., Seckl J. R. Central 6-hydroxydopamine lesions decrease mineralocorticoid, but not glucocorticoid receptor gene expression in the rat hippocampus. Neurosci Lett. 1992 Aug 17;142(2):159–162. doi: 10.1016/0304-3940(92)90363-c. [DOI] [PubMed] [Google Scholar]
- Young A. H., MacDonald L. M., St John H., Dick H., Goodwin G. M. The effects of corticosterone on 5-HT receptor function in rodents. Neuropharmacology. 1992 May;31(5):433–438. doi: 10.1016/0028-3908(92)90080-9. [DOI] [PubMed] [Google Scholar]
- Young E. A., Haskett R. F., Grunhaus L., Pande A., Weinberg V. M., Watson S. J., Akil H. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994 Sep;51(9):701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- Young E. A., Haskett R. F., Murphy-Weinberg V., Watson S. J., Akil H. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991 Aug;48(8):693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U., Stalla G. K., Müller O. A., Holsboer F. Blunting of ACTH response to human CRH in depressed patients is avoided by metyrapone pretreatment. Biol Psychiatry. 1988 Nov;24(7):782–786. doi: 10.1016/0006-3223(88)90254-5. [DOI] [PubMed] [Google Scholar]