Abstract
Background
Histiocytic sarcoma as a secondary malignancy following childhood leukemia is extremely uncommon with fewer than 20 cases reported worldwide. They often pose a diagnostic challenge and prognosis is dismal. There is a lack of well-established clinical treatment protocols owing to rarity of disease. Majority were managed with chemotherapy with variable outcomes.
Case presentation
Herein we report a rare case of an 8-year-old girl with secondary BRAFV600-mutant histiocytic sarcoma following T-cell acute lymphoblastic leukemia. After poor disease control with salvage chemotherapy, she was treated with MAPK-targeted therapy with dabrafenib and trametinib. She demonstrated excellent response and remained in partial remission with no signs of disease progression 3 years later.
Conclusions
There is yet to be consensus on the optimal management for this neoplasm. Description of our successful clinical experience highlights that investigation for BRAF mutations in histiocytic sarcoma is potentially advantageous. It also adds to the growing evidence that precision medicine may be a promising avenue to target this aggressive tumor and lays the foundation for future research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-025-05539-2.
Keywords: Histiocytic sarcoma, BRAF mutation, MAPK-targeted therapy
Background
Histiocytic sarcoma (HS) developing as a secondary neoplasm is extraordinarily rare [1] in childhood and presents a diagnostic challenge. Dismal prognosis is expected due to rapid progression and poor response to therapy [2]. No optimal treatment has been defined and standardized [3]. Among the reported cases of secondary HS following acute lymphoblastic leukemia (ALL) in children (Table 1), treatment modalities employed include various regimens of chemotherapy [2, 4–9], thalidomide post-stem cell transplant [10], targeted therapy with monoclonal antibodies [11] and palliative care [9, 12]. Interestingly, a recent article discovered BRAFV600E mutation in secondary HS of a child, who responded dramatically to MAPK-targeted therapy with combined BRAF inhibition (dabrafenib) and MEK inhibition (trametinib) [13]. We describe an 8-year-old girl with secondary BRAFV600-mutant HS who also demonstrated therapeutic benefit from this therapy, and provide an overview of the literature.
Table 1.
Published cases of secondary histiocytic sarcoma following acute lymphoblastic leukemia in children
| Case # | Year of publication | Author | Age/sex | Primary malignancy | Onset of secondary HS | HS location | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1. | 1996 | Soslow et al.4 | 8y/ M | preB-ALL | 10 m after ID | Paraspinous, bone, lung, liver, spleen | ETO, MEP | Died 3 m later |
| 2. | 1996 | Soslow et al.4 | 6y/ M | ALL | 20 m after ID | Bone, paravertebral, lung, liver | IFO, ETO, CBP | Alive 16 m later but developed new lesions |
| 3. | 2003 | Wongchanchailert and Laosombat5 | 8y / F | preB-ALL | 6 m after ID | Bone, extradural | CY, DNR, VCR, PRED | Died from sepsis following relapsed ALL |
| 4. | 2003 | Dalle et al.10 | 4y/ M | T-ALL | 9 m after SCT | Bone, BM, GIT, lung | Post-SCT: VIN, PRED, THA, DLI | Alive 33 m later |
| 5. | 2004 | Feldman et al.6 | 14y/ M | preB-ALL | 21 m after ID | BM, bone, spleen, kidney | VCR, CY, DNR, MTX, ETO | Not known |
| 6. | 2010 | Castro et al.7 | 5y/ M | T-ALL | 6 m after ID | Bone | Chemotherapy | Died |
| 7. | 2010 | Castro et al.7 | 15y/ M | preB-ALL | 3 m after ID | Soft tissue, bone, lung | Chemotherapy | Died |
| 8. | 2010 | Castro et al.7 | 7y/ M | preB-ALL | 6 m after ID | Bone, kidney | Chemotherapy, SCT | Alive at last follow up |
| 9. | 2010 | Castro et al.7 | 3y/ M | T-ALL | 16 m after ID | Liver, GIT | Chemotherapy | Died |
| 10. | 2011 | Kumar et al.8 | 4y/ M | preB-ALL | 1 m into maintenance | Bone, liver, spleen | DXM, CY, MTX, IFO, ARAC, ETO, RT | Died 1 year later |
| 11. | 2013 | Karabova and Ilievova9 | 3y/ F | T-ALL | 2 m into maintenance | GIT, LN, lung | DXM, CY, CLO, ETO, palliative care | Died 7 m later |
| 12. | 2014 | Ganapule et al.12 | 4y/ M | T-ALL | 18 m into maintenance | Bone, lung | Palliative care | Not known |
| 13. | 2015 | Alten et al.2 | 6y/ M | T-ALL | 15 m after ID | BM, liver, spleen, LN | DXM, ETO, ATG, BAS | Died 6 weeks later |
| 14. | 2015 | Alten et al.2 | 10y/ M | T-ALL | 12 m after ID | BM, skin, liver, spleen | DXM, VCR, MTX, ASP, ARAC, IDA, CY, NEL, SCT | Died 3 weeks after SCT from multiorgan failure |
| 15. | 2020 | Venkataraman et el.13 | 1y/ M | T-ALL | 4 m into maintenance | Bone | CLO, DXM, MAPK | Alive 14 m later |
| 16. | 2020 | Valera et al.11 | 6y/ M | T-ALL | During maintenance | Skin, LN | CY, DOX, PRED, VCR, ALM, CLD, ARAC, SCT | Partial response, died after SCT |
| 17. | 2024 | Our patient | 8y/ F | T-ALL | 2 m into maintenance | GIT, LN | IFO, CBP, ETO, MAPK | Alive 37 m later |
ALL acute lymphoblastic leukemia, ALM alemtuzumab, ARAC cytosine arabinoside, ASP asparaginase, ATG anti-thymocyte globulin, BAS basiliximab, BM bone marrow, CBP carboplatin, CLD cladribine, CLO clofarabine, CY cyclophosphamide, DLI donor lymphocyte infusion, DNR daunorubicin, DOX doxorubicin, DXM dexamethasone, ETO etoposide, F female, GIT gastrointestinal tract, HS histiocytic sarcoma, ID initial diagnosis, IDA idarubicin, IFO ifosfamide, LN lymph node, M male, m months, MAPK mitogen-activated protein kinase targeted therapy with dabrafenib and trametinib, MEP methylprednisolone, MTX methotrexate, NEL nelarabine, preB precursor B-cell, PRED prednisolone, RT radiotherapy, SCT stem cell transplant, THA thalidomide, VCR vincristine, VIN vinblastine, y years
Case presentation
A young female presented at 7 years 5 months of age with fever, hepatosplenomegaly and white blood cell count over 800,000/mm3. Bone marrow aspiration (BMA) revealed > 95% lymphoblasts and immunophenotyping confirmed T-acute lymphoblastic leukemia. No abnormalities were detected on cytogenetics and molecular mutation studies were negative for the 30 most common chromosomal translocations in acute leukemia. Cerebrospinal fluid examination was acellular. She was treated with the AIEOP-BFM ALL 2009 chemotherapy protocol. End-of-induction BMA reassessment showed complete remission with no minimal residual disease.
Approximately two months into maintenance therapy, the patient presented with three episodes of intussusception requiring laparotomy twice. A repeat bone marrow examination ruled out disease relapse. Initial histopathological examinations of the resected bowel and mesenteric lymph nodes were mostly consistent with a peripheral T-cell lymphoma, NOS. Four courses of CHOP (cyclophosphamide, doxorubicin, prednisolone and vincristine) chemotherapy were then given.
In view of mixed response to chemotherapy and following further consultation, additional immunohistochemical staining was performed on the previous gastrointestinal tissue and revealed that the neoplastic cells were positive for CD45+, CD4+, CD14+, CD68+, CD163 + and S100+, with weak and patchy expression of CD33 (Fig. 1). Negative staining was demonstrated for Langerhans cells, follicular dendritic cells, myeloid, B and T cells, epithelial, melanocytic and precursor markers (CD1a, CD23, MPO, CD117, CD56, CD30, CD20, CD79a, CD3, CD5, CD7, AE1/AE3, HMB45, TdT, CD34, CD99). Mutation in exon 15 of BRAF V600 (V600E, V600K and V600R) was detected, and the diagnosis was revised to histiocytic sarcoma. There is no family history of malignancy, and she does not exhibit any neurocutaneous or physical abnormalities to suggest an underlying cancer predisposition syndrome.
Fig. 1.
Immunohistochemical studies on gastrointestinal tissue
The neoplastic cells showed histiocytic differentiation and stained strongly for (A) CD163 and (B) CD68 (original magnification 400x)
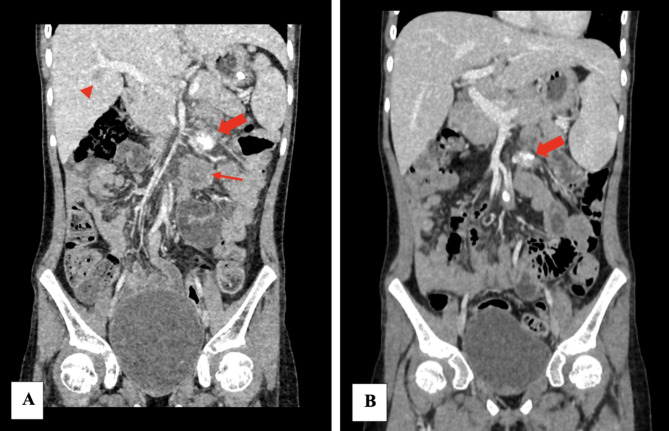
Salvage chemotherapy with ifosfamide, carboplatin and etoposide (ICE) was commenced in an effort to treat the histiocytic sarcoma. Despite five courses, reassessment CT scans of the abdomen and pelvis revealed that the disease was still progressing (Fig. 2A). She presented again with intussusception warranting a third laparotomy. In view of the presence of BRAFV600 mutation, MAPK-targeted therapy with dabrafenib and trametinib was instituted, with dabrafenib at 5.25 mg/kg/day divided into 2 doses and trametinib at 0.025 mg/kg/dose daily. She developed fever of unknown origin at the beginning of treatment. Trametinib dose was reduced temporarily but subsequently increased back to optimal dose once fever settled. She achieved a partial response and remained well more than 3 years later with no signs of disease progression (Fig. 2B). At time of writing (3 years 4 months of targeted therapy), she did not experience further pyrexia or any other side effects including dermatological, metabolic, hematological, neurological, gastrointestinal and musculoskeletal side effects.
Fig. 2.
Coronal images of the child’s abdominal and pelvic CT scan
A CT image 4 months prior to MAPK-targeted therapy revealed an enlarged calcified left mesenteric node (thick arrow), large left paraaortic node (thin arrow) and hypodense liver lesion (arrowhead). B CT image after 34 months of MAPK-targeted therapy showed a smaller left mesenteric node (thick arrow), smaller subcentimeter paraaortic nodes and resolution of liver lesion, indicating stable disease and no signs of disease progression
Discussion and conclusions
Second malignant neoplasms are well-recognized long-term health problems in individuals diagnosed with and treated for ALL at infancy, childhood and adolescence [11]. Histiocytosis, particularly HS, is very rarely described following ALL [11] with less than 20 cases reported in children to date [2, 4–13]. The overall survival of those with secondary HS was significantly lower at 11.8 months compared with 70 months for those with de novo HS as reported in a study of 23 adults and children [3]. Among the published cases of pediatric secondary HS as summarized in Table 1, there is an 82% male predominance and the mean age of affected children is 6.6 years. It most frequently presents during maintenance therapy for ALL but can occur as early as 3 months from initial diagnosis of ALL and up to 18 months after commencement of the maintenance chemotherapy. Commonly affected areas are extranodal in nature especially the bone (65% of cases), and to a lesser extent the lung, liver and spleen.
The diagnosis of HS can be extremely challenging owing to its rarity and paucity of clinical and genetic information on pediatric cases of secondary HS [11]. The unclear distinction between neoplastic and non-neoplastic proliferation of histiocytes, such as reactive histiocytosis [3] and histologic overlap with diverse mimics adds to the challenge. Patient #13, a 6-year-old boy, was initially diagnosed with hemophagocytic lymphohistiocytosis but died 10 days later despite highly intensive immunosuppressive treatment [2]. Histological evaluation of an abdominal lymph node (CD163+, CD68+, CD3-) removed during laparotomy ultimately revealed a diagnosis of HS [2]. The malignant cells of HS are typically CD163+, CD68+, lysosome + and CD1a- [14]. Prior to the additional immunohistochemical staining which eventually demonstrated CD163+, CD68 + and CD1a- in our patient, she was initially diagnosed and treated for peripheral T-cell lymphoma, stressing the importance of an awareness of HS and thorough clinical, morphological and immunohistochemical examinations.
Unsurprisingly, treatment modalities for secondary HS following ALL in children, are notably variable for this aggressive tumour, with differing outcomes. Castro et al. described four children with ALL preceding HS who were treated with chemotherapy (regimen not specified), three of whom succumbed to disease (#6, #7, #9), while one who also underwent stem cell transplantation was alive at last follow up (#8) [7]. Soslow et al. described an 8-year-old boy who received etoposide and methylprednisolone but died after 3 months (#1), and a 6-year-old boy who was treated with ifosfamide, etoposide and carboplatin but showed evidence of disease progression (#2) [4]. A 10-year-old boy with secondary HS received ALL relapse treatment (ALL-REZ BFM) and allogeneic stem cell transplantation despite absence of evidence of ALL relapse, due to lack of standardized treatment for HS and insufficient minimal residual disease clearance (#14) [2].
Additionally, even thalidomide which is generally only recommended as a last resort when all therapies fail was used in secondary HS following hematopoietic stem cell transplantation with favourable results (#4) [10]. Targeted therapy with monoclonal antibodies alemtuzumab was offered in a child with CD52 positivity in tumour cells but with only partial response, complicated with opportunistic infections, and he subsequently passed away (#16) [11]. A 4-year-old boy’s family opted for palliative care considering the dismal prognosis of the secondary HS he developed 1.5 years after maintenance therapy for T-cell ALL (#12) [12].
In adults, BRAFV600 mutations are commonly found in melanoma and thyroid cancers, and to a lesser degree other tumour types [15]. Mutation in BRAF at codon 600 causes constitutive activation of the MAPK pathway [16]. Successful inhibition of this pathway with BRAF/ MEK inhibitors results in clinically meaningful benefits [16]. This precision medicine approach has shown promising results in BRAFV600-mutated melanoma, non-small-cell lung carcinoma and thyroid cancer and is the standard-of-care option [17–19]. The NCI-MATCH trial involving more than 16 different tumour types with BRAF mutations including one patient with HS, reported that dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) therapy resulted in responses in 38% of patients and showed a high rate of disease control, suggesting that BRAF/MEK inhibition may be a viable treatment strategy across the majority of BRAFV600-mutated cancers [15].
Langerhans cell histiocytosis, another histiocytic neoplasm, primarily affecting children, is well known to harbour BRAF mutations in more than 50% of cases [20]. Dabrafenib monotherapy or in combination with trametinib showed preliminary evidence of clinical efficacy in BRAF-mutant pediatric Langerhans cell histiocytosis, with a safety profile comparable to that observed in solid tumours in adults [20]. The first reported child with T-cell ALL developing secondary BRAFV600E-mutant HS, also treated with MAPK-targeted therapy had demonstrated therapeutic benefit and he remained in remission for 14 months (#15) [13]. Similarly, our patient with secondary BRAFV600-mutant HS responded dramatically to MAPK-targeted therapy and remains in partial remission with good quality of life and no evidence of disease progression for 3 years (#17).
In a study of histiocytic sarcoma in adults in Japan, only 6.1% (2 out of 33 patients) harboured BRAFV600E mutation [21]. However moving forward, it may become essential to investigate for BRAF mutation in childhood histiocytic sarcoma. Insights into such genetic alterations have significant treatment implications as highly effective therapies with BRAF/ MEK inhibitors are now available. We are unable to conclude the origin of BRAFV600 mutation in our patient as it was not tested for in the initial ALL specimens. Given the rarity of secondary HS, compounded by the low overall rate of BRAF mutation in most tumour types [15], the feasibility of conducting disease-specific studies is limited. This highlights the value of our report as there is no consensus on optimal treatment yet, laying the foundation for future work on such malignancies.
HS as a secondary neoplasm following childhood leukemia is an exceptional and aggressive tumour which lacks uniform, well-established treatment protocols. For many rare cancers, it is challenging to develop clinical trials that recruit enough patients to show benefit from certain therapies, more so for strategies that target tumours with unique genetic mutations. We described a second child with BRAFV600-mutant secondary HS who showed therapeutic benefit from MAPK-targeted therapy, underlining the importance of investigation for BRAF mutations in HS and increasing the confidence in precision medicine to approach this highly malignant tumour. Further research is needed as we are still far from defining the standard treatment and the long-term effects of these newer avenues remain largely unknown.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Universiti Malaysia Sarawak (UNIMAS) for supporting the publication fees of this article.
Abbreviations
- HS
Histiocytic sarcoma
- ALL
Acute lymphoblastic leukemia
- BMA
Bone marrow aspiration
Author contributions
TSL conceptualized and drafted the manuscript. BHLS managed the patient, provided patient’s clinical data and expertise on the subject. YTT is involved in the acquisition and reporting of the patient’s CT images. DY contributed the histology slides and interpretation. All authors reviewed and approved the final version.
Funding
Open Access funding provided by Universiti Malaysia Sarawak.
Data availability
Data is provided within the manuscript and supplementary information files.
Declarations
Ethics approval and consent to participate
NA.
Consent for publication
Written informed consent for publication of the patient’s clinical details and investigation results was obtained from the patient’s parents.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khoury JD, Solary E, Abla O, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–19. 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alten J, Klapper W, Leuschner I, Eckert C, Beier R, Vallo E, Krause M, Claviez A, Vieth S, Bleckmann K, Möricke A. Secondary Histiocytic sarcoma May cause apparent persistence or recurrence of minimal residual disease in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62(9):1656–60. [DOI] [PubMed] [Google Scholar]
- 3.Broadwater DR, Conant JL, Czuchlewski DR, Hall JM, Wei S, Siegal GP, Peker D. Clinicopathologic features and clinical outcome differences in de Novo versus secondary Histiocytic sarcomas: a multi-institutional experience and review of the literature. Clin Lymphoma Myeloma Leuk. 2018;18(10):e427–35. [DOI] [PubMed] [Google Scholar]
- 4.Soslow RA, Davis RE, Warnke RA, Cleary ML, Kamel OW. True Histiocytic lymphoma following therapy for lymphoblastic neoplasms. Blood. 1996 June;87(12):5207–12. [PubMed]
- 5.Wongchanchailert M, Laosombat V. True Histiocytic lymphoma following acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40(1):51–3. [DOI] [PubMed] [Google Scholar]
- 6.Feldman AL, Minniti C, Santi M, Downing JR, Raffeld M, Jaffe ES. Histiocytic sarcoma after acute lymphoblastic leukaemia: a common clonal origin. Lancet Oncol. 2004;5(4):248–50. [DOI] [PubMed] [Google Scholar]
- 7.Castro EC, Blazquez C, Boyd J, Correa H, De Chadarevian JP, Felgar RE, Graf N, Levy N, Lowe EJ, Manning JT Jr, Proytcheva MA. Clinicopathologic features of Histiocytic lesions following ALL, with a review of the literature. Pediatr Dev Pathol. 2010;13(3):225–37. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Khan SP, Joshi DD, Shaw GR, Ketterling RP, Feldman AL. Pediatric Histiocytic sarcoma clonally related to precursor B-cell acute lymphoblastic leukemia with homozygous deletion of CDKN2A encoding p16INK4A. Pediatr Blood Cancer. 2011;56(2):307–10. [DOI] [PubMed] [Google Scholar]
- 9.Karabová Z, Ilievová L. Palliative care in the home: a case study of secondary Histiocytic sarcoma in a 3-year-old child. J Health Sci. 2013;3(3):267–70. [Google Scholar]
- 10.Dalle JH, Leblond P, Decouvelaere A, Yakoub-Agha I, Preudhomme C, Nelken B, Mazingue F. Efficacy of thalidomide in a child with Histiocytic sarcoma following allogeneic bone marrow tranplantation for T-ALL. Leukemia. 2003;17(10):2056–7. [DOI] [PubMed] [Google Scholar]
- 11.Valera ET, Brassesco MS, Reis MB, Maggioni G, Guerino-Cunha RL, Grecco CE Jr, Kato JE, Tone M. Short-term response to alemtuzumab in CD52-positive secondary Histiocytic sarcoma in a child: is it time to consider new targets? Pediatr Hematol Oncol. 2020;38(1):89–96. [DOI] [PubMed] [Google Scholar]
- 12.Ganapule AP, Gupta M, Kokil G, Viswabandya A. Histiocytic sarcoma with acute lymphoblastic leukemia a rare association: case report and literature review. Indian J Hematol Blood Transfus. 2014;30:305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkataraman V, Massoth LR, Sullivan RJ, Friedmann AM. Secondary Histiocytic sarcoma with BRAFV600E mutation after T-cell acute lymphoblastic leukemia in a very young child with dramatic response to Dabrafenib and Trametinib. Pediatr Blood Cancer. 2020;67(5):e28200. [DOI] [PubMed] [Google Scholar]
- 14.Heath JL, Burgett SE, Gaca AM, Jaffe R, Wechsler DS. Successful treatment of pediatric Histiocytic sarcoma using abbreviated high-risk leukemia chemotherapy. Pediatr Blood Cancer. 2014;61(10):1874–6. [DOI] [PubMed] [Google Scholar]
- 15.Salama AK, Li S, Macrae ER, Park JI, Mitchell EP, Zwiebel JA, Chen HX, Gray RJ, McShane LM, Rubinstein LV, Patton D. Dabrafenib and Trametinib in patients with tumors with BRAFV600E mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol. 2020;38(33):3895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. [DOI] [PubMed] [Google Scholar]
- 17.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V. Improved overall survival in melanoma with combined Dabrafenib and Trametinib. N Engl J Med. 2015;372(1):30–9. [DOI] [PubMed] [Google Scholar]
- 18.Planchard D, Besse B, Groen HJ, Souquet PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, Rigas JR. Dabrafenib plus Trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbiah V, Kreitman RJ, Wainberg ZA et al. Dabrafenib plus Trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Annals of oncology. 2022;33(4):406–15. [DOI] [PMC free article] [PubMed]
- 20.Whitlock JA, Geoerger B, Dunkel IJ, Roughton M, Choi J, Osterloh L, Russo M, Hargrave D. Dabrafenib, alone or in combination with Trametinib, in BRAF V600–mutated pediatric Langerhans cell histiocytosis. Blood Adv. 2023;7(15):3806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimono J, Miyoshi H, Arakawa F, et al. Prognostic factors for Histiocytic and dendritic cell neoplasms. Oncotarget. 2017;8(58):98723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript and supplementary information files.