Abstract
Background
HNRNPC is an RNA-binding protein that is overexpressed in a variety of cancers and is well known as an m6A “reader”, but its specific function and molecular mechanism in NSCLC have not been fully understood. This study aimed to discuss molecular mechanism of HNRNPC in NSCLC.
Methods
HNRNPC expression and clinically relevant data in pan-cancer and LUAD were extracted through these websites, including UALCAN, TIMER2 and GEPIA. The target gene of HNRNPC were identified through RIP-seq, meRIP-qPCR and mRNA stability test. The differential expression of target gene in NSCLC was explored by immunohistochemistry. Lentivirus was selected to knock down HNRNPC and plasmid was selected to overexpress downstream target genes. The transfection efficiency was verified by RT-qPCR and Western Blot. In vitro colony formation assay, CCK-8, wound healing, transwell assays were performed to determine the biological functions of HNRNPC and target gene in lung adenocarcinoma cells.
Results
HNRNPC can promotes the expression of TFAP2A by recognizing the m6A modification of TFAP2A mRNA and maintaining its stability, activates the TFAP2A/CTNNB1 axis, enhances EMT, and ultimately promotes the malignant process of NSCLC and promote distant metastasis of NSCLC.
Conclusions
These results supported that HNRNPC regulate TFAP2A to promote the malignant progression and EMT of NSCLC. These findings connect m6A modification with EMT, providing a new perspective on the regulation of m6A modification in tumors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-025-03660-x.
Keywords: NSCLC, HNRNPC, TFAP2A, N6-methylation, Epithelial to mesenchymal transition
Introduction
Lung cancer is the leading cause of cancer-related death in the world [1]. The main pathological classifications are small cell lung cancer and non-small cell lung cancer (NSCLC), with NSCLC accounting for about 80–85% of cases [2]. NSCLC consists mainly of adenocarcinoma and squamous cell carcinoma, which account for more than 70% of the total risk of NSCLC [3]. And for patients with NSCLC with metastasis, treatment outcomes and prognosis remain poor [4]. Hence, further elucidation of NSCLC metastasis mechanisms and identification of metastasis-associated molecules are imperative to provide potential therapeutic targets for advanced NSCLC.
More than 160 distinct RNA chemical modifications have been identified across organisms [5]. Among these, N6-methylation (m6A) represents the most abundant internal modification in eukaryotic mRNA [6]. Emerging evidences implicate dysregulated m6A signaling in cancer pathogenesis by impacting proliferation, invasion and metastasis [7–10]. RNA-Binding Proteins (RBPs) are key homeostatic regulators, exerting post-transcriptional control over RNA abundance, processing, and function [11]. RBPs disorders are associated with various human diseases, including neurological disorders, cardiovascular diseases, and cancer [12]. Heterogeneous nuclear ribonucleoprotein C (HNRNPC) belongs to the HNRNP family of RBPs and also acts as m6A methylation “readers”, controlling multiple aspects of RNA metabolism, including alternative splicing, mRNA stabilization, and translation [13, 14]. Numerous studies have shown upregulation of HNRNPC across several tumor types, including glioblastoma, hepatocellular carcinoma, breast cancer, suggesting it plays an important role in oncogenesis and cancer progression [15–19]. Recent evidence indicates HNRNPC enhance tumorigenesis in cancer by m6A modification [20]. Further elucidation of HNRNPC molecular functions and downstream signaling in NSCLC is therefore warranted.
Signature events underlying metastasis include epithelial to mesenchymal transition (EMT), angiogenesis, initiation of an inflammatory tumor microenvironment, and apoptosis dysfunction [21, 22]. The main cause of treatment failure and death is metastasis, and EMT is involved in the metastasis of NSCLC [23]. It has been reported that the high expression of HNRNPC promotes the EMT process of tumors in a variety of tumors, and HNRNPC, as a reading protein of m6A, promotes the progression of oral squamous cell carcinoma through EMT [5, 17]. In numerous studies on NSCLC, it has also been reported that HNRNPC promotes tumor invasion and metastasis, but the mechanism of HNRNPC promoting the invasion and metastasis of NSCLC is not fully understood [13, 24]. Therefore, whether HNRNPC is involved in the regulation of EMT through m6A to promote the progression of NSCLC deserves further study.
Here, we investigated the role and mechanism of HNRNPC in NSCLC progression. Through bioinformatics analysis, we found that HNRNPC and Transcription Factor Activating Enhancer Binding Protein 2 Alpha (TFAP2A) were highly expressed in NSCLC and correlated with poor prognosis. Knocking down HNRNPC can inhibit the proliferation, migration and invasion of NSCLC cells. HNRNPC can bind and regulate TFAP2A, and its regulatory mechanism is that HNRNPC recognizes the m6A site on TFAP2A mRNA and maintains its stability, thus promoting TFAP2A expression. Overexpression of TFAP2A in stable HNRNPC knockdown cell lines can partially restore the proliferation, migration and invasion of NSCLC cells inhibited by HNRNPC knockdown. Importantly, HNRNPC/TFAP2A may promote EMT progression in NSCLC by promoting β-catenin expression, thereby contributing to the malignant progression of NSCLC.
Materials and methods
Data acquisition and differential expression analysis
The mRNA differential expression of many different types of tumor and normal samples were obtained from TIMER2.0 portal (http://timer.cistrome.org/) [25, 26]. The protein differential expression of multiple types of tumor and normal samples were obtained from UALCAN portal (https://ualcan.path.uab.edu/) [27, 28] based on TCGA data information. Overall survival analysis was performed through GEPIA (http://gepia.cancer-pku.cn/) [29] portal and Kaplan–Meier portal (https://kmplot.com/analysis/) [30].
Gene Set Enrichment Analysis (GESA)
GSEA [31] was performed to explore the potential biological mechanisms of TFAP2A in LUAD based on TCGA datasets. Gene sets enriched significantly were identified according to the criterion: a normal P-value <0.05.
Cell line and cell culture
The human LUAD cell lines (A549 and H1299) were purchased from Procell Life Science & Technology Co., Ltd (Wuhan, China). A549 cells were cultured in a complete medium composed of Ham’S F-12K, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin, and H1299 cells were cultured in a complete medium containing RPMIS −1640 supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C and 5%CO2 humidity. Then the cells were cultured, expanded and passed.
Cell transfection
For cell infection, recombinant lentivirus (Genechem, Shanghai, China) was used to introduce gene interference and overexpression in the cells, with puromycin selected as the stable cell model. Continue cultivating for 72 h, total RNA and total protein were extracted to verify the efficiency. For transfection, plasmids (Genechem, Shanghai, China) were introduced into cells using Lipofectamine3000 (Invitrogen, CA, USA) according to the manufacturer’s instructions. The sequences of the shRNAs were presented in the supplement table S1.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total cell RNA was extracted using Trizol reagent (Invitrogen, USA). Then, 1.0 μg of total RNA was synthesized into cDNA using Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) and RT-qPCR was performed throngh Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix(Yeasen, shanghai, China). All primers were designed and synthesized by Shenggong Bioengineering Co. ltd. (Shanghai, China). GAPDH was used as the internal control, and the relative mRNA expression was calculated via 2−ΔΔCT method. The nucleic acid sequences used are listed in the supplement table S2.
Western blot
Protein from cell lysate was isolated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was incubated with primary specific antibody (GAPDH: 10494-1-AP, Proteintech, Wuhan, China; HNRNPC: MAB2486, Abnova, China; TFAP2A: 13019-3-AP, Proteintech, Wuhan, China; β-catenin: 51067-2-AP, Proteintech, Wuhan, China; E-Cadherin: 20874-1-AP, Proteintech, Wuhan, China; N-Cadherin: 22018-1-AP, Proteintech, Wuhan, China; Vimentin: 10366-1-AP, Proteintech, Wuhan, China) at 4 ℃ overnight, followed by a second HRP conjugated antibody shaker at room temperature for 1 h. ECL reagent (MA0186-Apr-041, Meilunbio, China) was used for protein detection.
CCK8 proliferation assays
Several growing cells were selected, thoroughly mixed following digestion, and 2 × 103 cells were inoculated into each well of 96-well plate for 96 h. The number of living cells was measured every 24 h by CCK8 kit (Meilunbio, Dalian, China).
Plate cloning experiments
Cells in the logarithmic growth stage were selected, digested with pancreatic enzymes and thoroughly mixed into single-cell suspension state. 1 × 103 cells were selected, inoculated into 6-well plates, fixed with paraformaldehyde for 10–14 days, stained with crystal violet, and the number of cell clones was counted.
Transwell experiments
The log-grown cells were starved and cultured one day in advance for transwell invasion and migration experiment. The transwell cells (NEST, Wuxi, China) were placed into 24-well plates for 4 h, followed by incubation with Matrigel (CORNING, USA) in a 37 ℃ incubator. 4 × 104 cells were suspended in medium without FBS and inoculated in the upper chamber, and 600ul of culture base containing 20%FBS was added to the lower chamber. After 48 h of cultivation, the cells were fixed with paraformaldehyde and stained with crystal violet. The cells residing on the lower surface of the filter are counted for statistical analysis. In a similar manner, no transwell chamber equipped with Matrigel was employed for cell migration experiments.
Wound-healing assays
One day prior to the experiment, logarithmic growth cells were inoculated in a six-well plate. The cell layer was carefully scraped with a 200 μL pipette tip to create a wound, and the wound was photographed 0, 24 or 48 h later for statistical analysis of the wound area.
RNA immunoprecipitation (RIP)-PCR
A549 cells and H1299 cells cultured in 10 cm dishes were harvested, and RIP assays were carried out using HNRNPC (MAB2486, Abnova, China) antibodies and a RNA Immunoprecipitation kit (BersinBio Best5101). The RIP assay was performed based on the instructions of the RIP kit. RNA was extracted from the immunoprecipitate and reverse transcribed into cDNA for further RT-PCR detection. Primer sequences are listed in supplement table S2.
Methylated RNA immunoprecipitation assays (MeRIP)
Total RNA was isolated from A549 cells. Following the standard operating protocol of the EpiQuik™ CUT&RUN m6A RNA enrichment Kit (Epigentek, NY, USA), the RNA samples were fragmented and incubated with anti-M6A antibodies (#A-1802, Epigentek) at room temperature for 90 min. Non-immune IgG served as a negative control. The m6A sites on target gene mRNA were predicted using the Whistle database (http://180.208.58.19/whistle/index.html) [32]. The concentration of m6a-RNA was quantified by RT-qPCR. The primer sequence information used in this study is summarized in the supplement table S3.
RNA stability test
To assess the stability of RNA, lentivirus-infected A549 cells were treated with actinomycin D (ActD, 5 mg/mL) for 0, 2 and 4 h. Subsequently, total RNA was isolated and analyzed by real-time fluorescent quantitative PCR. The corresponding level of TFAP2A was normalized to GAPDH.
Prediction of CTNNB1 promoter transcription factor binding sites
Transcription factor binding sites of CTNNB1 promoter were predicted via UCSC portal (https://genome.ucsc.edu/) [33]. The specific binding sequence of TFAP2A was predicted by JASPAR database [34].
Immunohistochemistry and its evaluation
This study was approved by the ethics committee of The Affiliated Hospital of Guizhou Medical University (Document ID: 2021-342) and implemented in accordance with the Declaration of Helsinki, 109 LUAD specimens (56 cancerous and 53 para-cancerous) of LUAD-TMA were collected from the Affiliated Hospital of Guizhou Medical University, China. The process involved collecting pathological sections, which were dewaxed, antigen repaired, treated with hydrogen peroxide, and sealed. These sections were then incubated with secondary antibodies labeled with specific primary antibodies (HNRNPC:11760-1-AP, Proteintech, Wuhan, China,TFAP2A:67076-1-Ig, Proteintech, Wuhan, China) and horseradish peroxidase (HRP). Staining, restaining and microscopy were then performed. The level of protein expression was assessed using the IHC score (H), calculated as follows: H = ∑ (pi*i), where “pi” indicates the percentage of positive cells and “I” indicates the strength of staining.
All of the clinical cases information in this study is listed in the supplement table S4.
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed using student’s t-test or one-way ANOVA. Statistical analysis and chart production were performed using GraphPad Prism (version 9, GraphPad Software, Inc.). P < 0.05 was considered statistically significant (*P < 0.05; *P < 0.01; ***P < 0.001; ****P < 0.0001; NS: not significant).
Results
High expression of HNRNPC in non-small cell lung cancer is associated with poor prognosis
HNRNPC serves as a recognized m6A methylation reading protein [35]. Through the analysis of TIMER2.0 online database, we found that HNRNPC mRNA is highly expressed in cancer tissues including NSCLC (Fig. 1A). And the analysis performed though UALCAN portal showed that HNRNPC protein expression was highly expressed in a variety of tumors including NSCLC (Fig. 1B). Overall survival analysis by GEPIA and Kaplan–Meier showed that high expression of HNRNPC was associated with poor prognosis of LUAD (Fig. 1C, D). And disease free survival analysis by GEPIA showed that high expression of HNRNPC was associated with poor prognosis of LUSC (Supplementary Fig. 1).
Fig. 1.
HNRNPC expression is up-regulated in a variety of tumors, and its up-regulation LUAD is associated with poor prognosis. A Analysis based on TIMER2.0 website showed that HNRNPC mRNA is highly expressed in many types of cancer tissues. B Analysis based on UALCAN portal showed that HNRNPC protein is highly expressed in many types of cancer tissues. C, D The effect of HNRNPC expression on overall survival of lung adenocarcinoma patients was analyzed based on Kaplan–Meier and GEPIA databases
Knocking down HNRNPC can inhibit non-small cell lung cancer NSCLC cell proliferation, migration and invasion in vitro
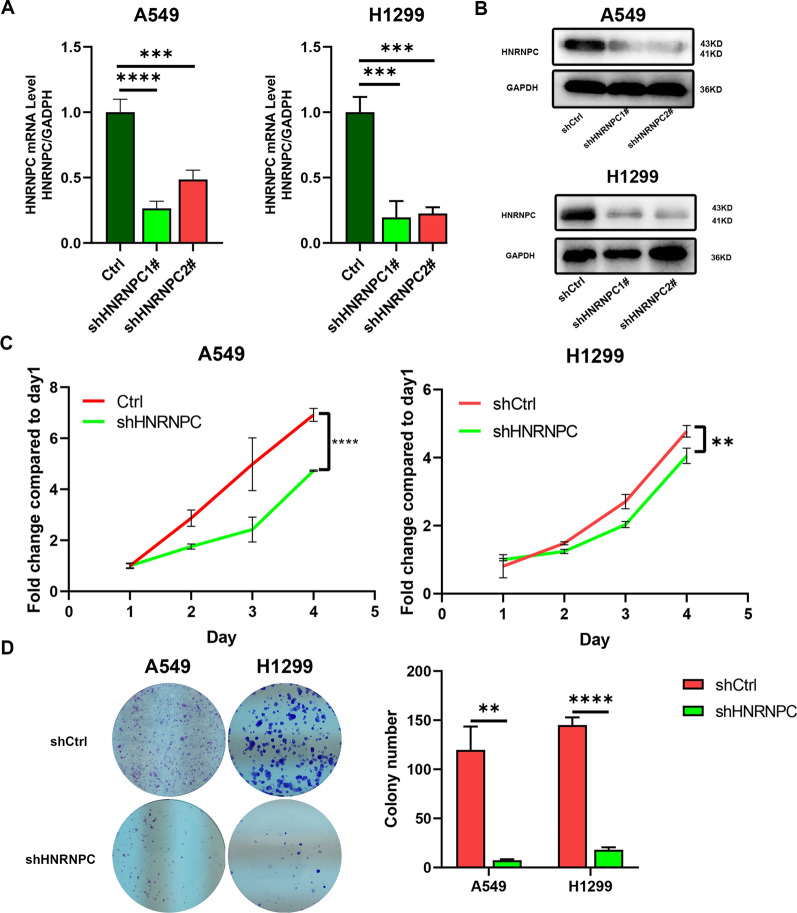
To investigate the function of HNRNPC in NSCLC cells, we established knockdown models of HNRNPC in A549 cells and H1299 cells by using lentiviruses. The silencing effect demonstrated that HNRNPC was effectively knocked down in both mRNA and protein (Fig. 2A, B). Among them, shHNRNPC1# exhibited a better knocking effect, thus we chose shHNRNPC1# for subsequent experiments.
Fig. 2.
The role of HNRNPC in non-small cell lung cancer cells. A The inhibitory effect of HNRNPC in non-small cell lung cancer cell lines (A549, H1299) was determined by RT-qPCR. B Western Blot analysis demonstrating the silencing effect of HNRNPC in non-small cell lung cancer cell lines (A549, H1299). C Proliferation capacity was evaluated by CCK8 in HNRNPC knockdown cells. D Proliferation capacity was evaluated by colony formation assay in HNRNPC knockdown cells. The error bar represents the standard deviation derived from at least three independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
The CCK8 test results showed that the proliferation capacity of A549 and H1299 cells in the shHNRNPC group was reduced compared to the shCtrl group (Fig. 2C), while fewer cell clones were formed in the clonogenesis test (Fig. 2D). Scratches showed that A549 and H1299 cells migrated less in shHNRNPC group than in shCtrl group (Fig. 3A). In transwell migration experiments, the number of A549 and H1299 cells in shHNRNPC group was lower than that in shCtrl group (Fig. 3B). In the Transwell invasion assay, lentivirus-transfected A549 and H1299 cells had fewer invasive cells (Fig. 3C).
Fig. 3.
Effects of HNRNPC knockdown on migration and invasion of non-small cell lung cancer cells. A Wound healing experiments (representative holes) evaluated the cell migration capacity of HNRNPC knockdown cell lines. B, C The Transwell migration and invasion assay measured the migration and invasion abilities of HNRNPC knockdown cell lines. The error bar represents the standard deviation derived from at least three independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
HNRNPC binds to TFAP2A, which is highly expressed in non-small cell lung cancer and associated with poor prognosis
Our previous RIP-seq was designed to further explore the HNRNPC-bound target RNAs (GSE249094, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE249094). The results combined with differential expression genes dataset with HNRNPC knockdown in H1299 cells (GSE199483) and the differential expression and prognostic genes based on TCGA-LUAD, we screened a total of 23 target mRNAs (Fig. 4A, left) that were directly bound to HNRNPC and expressed significantly changed after HNRNPC knockdown. Then we intersected the 23 genes obtained by the intersection of the Venn diagram with a dataset of m6A modification in head and neck squamous cell cancer cells (GSE185885) to obtain 15 genes. TFAP2A was included (Fig. 4A, right), which was found to be closely related to HNRNPC through the GEPIA database (Fig. 4B). The m6A sites on TFAP2A were predicted using the Whistle database (Fig. 4C): There are two m6A sites on TFAP2A mRNA, and these two m6A sites contain rich biological processes and molecular functions, indicating that TFAP2A mRNA can undergo m6A methylation and play important functions.
Fig. 4.
Identification of HNRNPC targets in non-small cell lung cancer. A, left The Venn diagram overlap contains 23 genes. A, right The intersection contains 15 genes with the intersection taken again that can be modified by m6A, including TFAP2A. B The correlation between TFAP2A and HNRNPC was analyzed using the GEPIA database. C The Whistle database predicted the m6A site on TFAP2A mRNA. D Analysis based on TIMER2.0 website showed that TFAP2A mRNA is highly expressed in many types of cancer tissues. E Analysis based on UALCAN portal showed that TFAP2A protein is highly expressed in many types of cancer tissues. F The effect of TFAP2A expression on overall survival of lung adenocarcinoma patients was analyzed based on GEPIA databases. G RNA Immunoprecipitation PCR of HNRNPC and TFAP2A
Through the analysis of TIMER2.0 online database, we found that TFAP2A mRNA is highly expressed in cancer tissues including NSCLC (Fig. 4D). And the analyses based on UALCAN portal showed that TFAP2A protein was highly expressed in a variety of tumors including NSCLC (Fig. 4E). Overall survival analysis by GEPIA showed that high expression of TFAP2A was associated with poor prognosis of NSCLC (Fig. 4F). Moreover, RIP-PCR was conducted and confirmed that TFAP2A transcripts were directly bound by HNRNPC (Fig. 4G).
Meanwhile, to further verify the expression of TFAP2A, immunohistochemical staining was performed on 56 cancerous and 53 para-cancerous of NSCLC cancer tissues, and the results confirmed that TFAP2A expression in NSCLC tissues was significantly higher than that in adjacent tissues (Fig. 5A).
Fig. 5.
The regulation of TFAP2A by HNRNPC. A The expression and score of TFAP2A in cancer tissues and adjacent tissues of NSCLC patients were detected by immunohistochemical method. B qRT-PCR was used to detect HNRNPC and TFAP2A mRNA expression in non-small cell lung cancer cell lines (A549, H1299). C Western Blot analysis of TFAP2A protein expression after HNRNPC silencing in non-small cell lung cancer cell lines (A549, H1299). D In H1299 cell line, TFAP2A m6A modification was significantly reduced after HNRNPC knockdown. E RNA stability analysis revealed that the degradation rate of TFAP2A mRNA was significantly increased in HNRNPC knockdown cell lines compared to shCtrl. The error bar represents the standard deviation derived from at least three independent tests; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
HNRNPC regulates TFAP2A by maintaining the stability of TFAP2A mRNA in an m6A-dependent manner
To investigate whether HNRNPC can regulate TFAP2A expression in NSCLC, we established stable knockdown models of HNRNPC in A549 and H1299 cells for grouping treatment. RT-qPCR and Western blot results showed that HNRNPC knockdown significantly inhibited TFAP2A mRNA levels and protein expression (Fig. 5B, C). Meanwhile, in order to test whether TFAP2A would affect the expression of HNRNPC, we designed a rescue test in which TFAP2A was simultaneously overexpressed in cells with stable HNRNPC knockdown (Fig. 6A). By RT-qPCR and Western Blot, TFAP2A overexpression promoted its own expression, but did not affect the expression of HNRNPC (Fig. 6B, C).
Fig. 6.
Overexpression of TFAP2A can partially recover NSCLC cell proliferation caused by HNRNPC knockdown. A Overexpression of TFAP2A in lentiviral knockdown HNRNPC NSCLC cell lines (A549, H1299). B The lvevl of HNRNPC and TFAP2A protein after HNRNPC knockdown and TFAP2A overexpression in NSCLC cell lines (A549, H1299) were detected by Western Blot. C The lvevl of HNRNPC and TFAP2A mRNA after HNRNPC knockdown and TFAP2A overexpression in NSCLC cell lines (A549, H1299) were detected by RT-qPCR. D, E Proliferation capacity was evaluated by CCK8 and colony formation assay in cells that knocked down HNRNPC and TFAP2A overexpression. The error bar represents the standard deviation obtained from at least three independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
Studies on breast cancer have shown that HNRNPC enhances TFAP2A expression by means of VIRMA’s recognizing m6A modifications in TFAP2A mRNA [36]. Subsequently, we detected the m6A methylation modification variation of TFAP2A mRNA in H1299 cells in the case of HNRNPC changed using MeRIP-qPCR, and found that compared to sh-Ctrl, the m6A methylation level of TFAP2A mRNA was significantly reduced following HNRNPC knockdown (Fig. 5D).
RIP-seq and MeRIP-qPCR analyses have confirmed that HNRNPC can bind to TFAP2A and promote its expression by recognizing TFAP2A m6A modifications. Studies in esophageal squamous cell carcinoma have found that interaction with HNRNPC enhances the stability of ZEB1 and ZEB2 mRNA, thereby promoting the development of esophageal squamous cell carcinoma [37]. To further explore how HNRNPC promotes TFAP2A mRNA expression, we treated H1299 cells with actinomycin D, a well-known mRNA stability inhibitor, to evaluate TFAP2A mRNA stability. The results showed that the degradation rate of TFAP2A mRNA increased after HNRNPC knockdown (Fig. 5E). These results confirm that HNRNPC regulates TFAP2A by maintaining the stability of TFAP2A mRNA in an m6A-dependent manner.
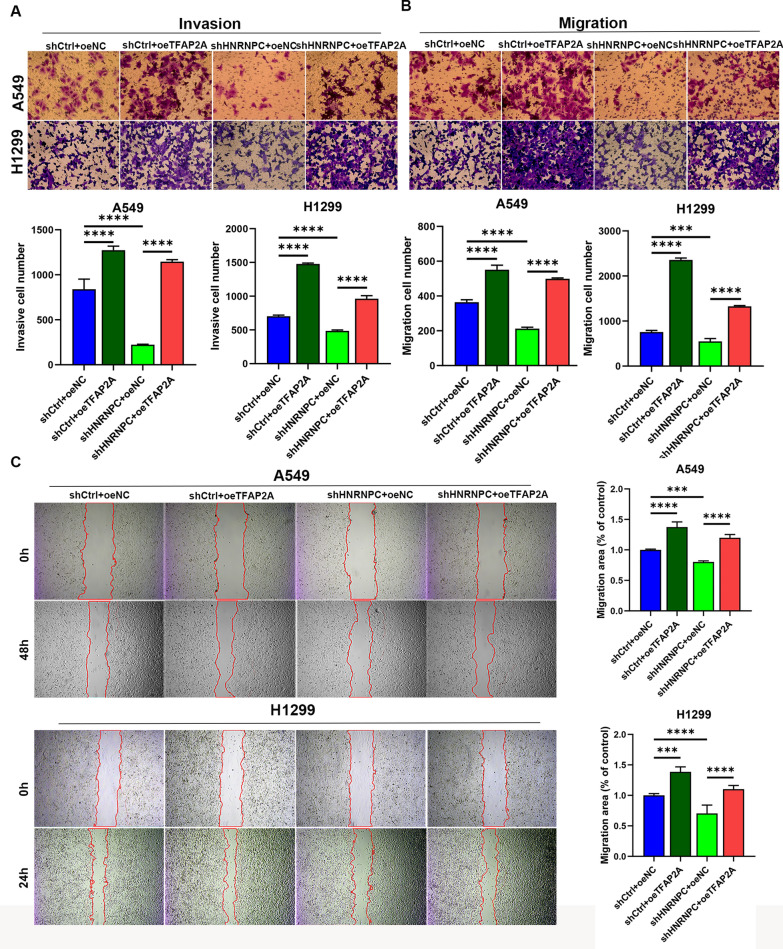
Overexpression of TFAP2A can partially recover the decreased proliferation, invasion and migration of NSCLC cells due to HNRNPC knockdown
In order to further study the role of HNRNPC via TFAP2A, we first knocked down HNRNPC (shHNRNPC) in A549 and H1299 cells, and then overexpressed TFAP2A (HNRNPC−/TFAP2A+) in these cells (Fig. 6A). CCK-8 and colony formation experiments showed that TFAP2A overexpression increased the proliferation capacity of A549 and H1299 cells with HNRNPC knockdown (Fig. 6D, E). In the transwell invasion study, that TFAP2A overexpression increased the invasion capacity of A549 and H1299 cells with HNRNPC knockdown (Fig. 7A). The transwell migration and scratch tests showed that TFAP2A overexpression increased the migration capacity of A549 and H1299 cells with HNRNPC knockdown (Fig. 7B, C).
Fig. 7.
Overexpression of TFAP2A can partially recover NSCLC cell migration and invasion caused by HNRNPC knockdown. A, B Transwell invasion and migration test evaluated the invasion and migration ability of HNRNPC knockdown and TFAP2A overexpressing cell lines. C Wound healing assay (representative hole) assessed the cell migration capacity of HNRNPC knockdown and TFAP2A overexpressing cell lines. The error bar represents the standard deviation obtained from at least three independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
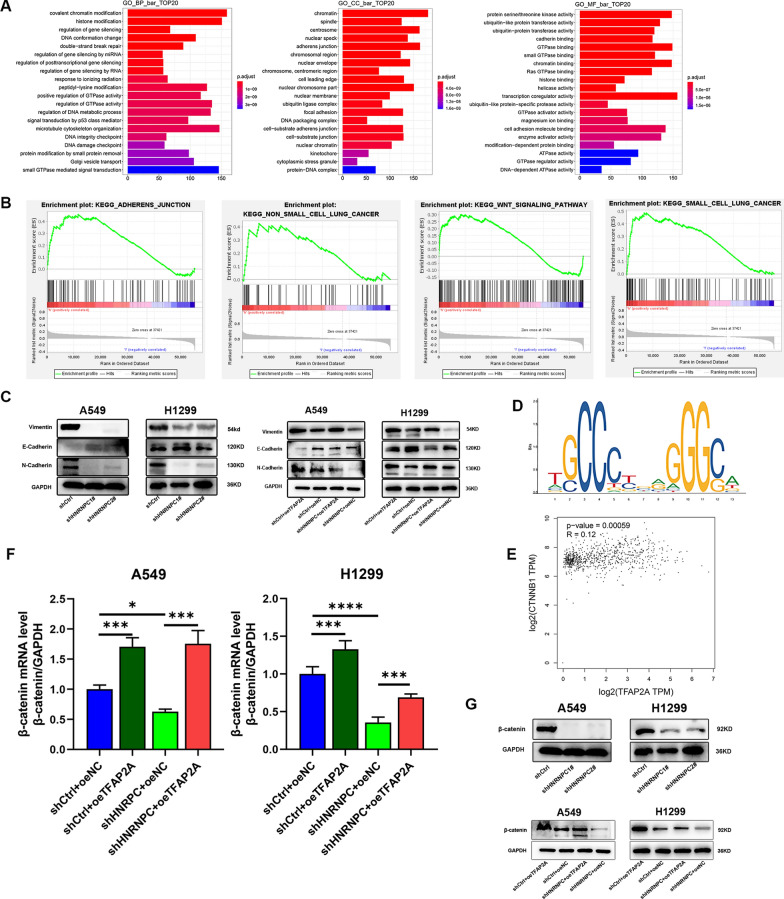
HNRNPC/TFAP2A is involved in the regulation of EMT in NSCLC
Previous studies have shown that HNRNPC accelerates the progression of EMT in oral squamous cell carcinoma [5, 38]. By performing GO enrichment analysis on HNRNPC from the RIP-seq, we found that the top 20 functions enriched in HNRNPC include adhesive bonding (Fig. 8A). Through GSEA gene enrichment analysis, we discovered that the functional enrichment of TFAP2A is mainly detected in adherens junction, WNT signaling pathway, non-small cell lung cancer, and small cell lung cancer (Fig. 8B). Dimitrova et al. [39] identified that TFAP2A can bind to Zeb2, the core transcription network of EMT, and its motif is also a conserved component of mouse and human EMT networks, and its target gene is upregulated during EMT. It is proved that TFAP2A is involved in the occurrence of EMT process and possibly functions at the initial stage of EMT.
Fig. 8.
HNRNPC/TFAP2A is involved in the regulation of EMT in NSCLC. A GO enrichment analysis of HNRNPC based on RIP-seq. B GSEA analysis of TFAP2A based on TCGA-LUAD dataset. C Expression of E-Cadherin, N-Cadherin and Vimentin protein after HNRNPC silencing and TFAP2A overexpression in non-small cell lung cancer cell lines (A549, H1299) were detected by Western Blot. D The highest scoring binding site on CTNNB1 promoters by Jasper database. E Correlation analysis between β-catenin and TFAP2A in GEPIA database. F β-catenin mRNA was detected in non-small cell lung cancer cell lines (A549, H1299) when HNRNPC knockdown and TFAP2A overexpression. G Protein expression of β-catenin was detected in non-small cell lung cancer cell lines (A549, H1299) when HNRNPC knockdown and TFAP2A overexpression. The error bars represent standard differences from at least three independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
Therefore, the hypothesis that HNRNPC may affect EMT process in NSCLC by regulating TFAP2A was proposed. To verify this hypothesis, we identified several EMT markers. In stable knockdown HNRNPC cells, the protein expression levels of N-Cadherin and Vimentin were decreased, while the protein expression level of E-Cadherin was up-regulated, and such changes could be reversed by overexpression of TFAP2A (Fig. 8C). These findings indicated that HNRNPC/TFAP2A contributes to EMT progression via modulating the expression of EMT associated genes.
HNRNPC/TFAP2A may promote NSCLC by control β-catenin expression of EMT process
It is well known that β-catenin mediates the classical pathway in the WNT signaling pathway, which is rich in cell proliferation, differentiation, migration and other aspects, and it can form a complex with E-Cadherin [40]. As demonstrated in a recent study on oral squamous cell carcinoma, TFAP2A directly binds to β-catenin and regulates its transcriptional activity [41]. Predicting CTNNB1 promoter regions of transcription factors binding through the online database UCSC (https://genome.ucsc.edu/) and JASPAR (https://jaspar.genereg.net), while TFAP2A was included (Minimum Score: 500). The TFAP2A sequences binding on the CTNNB1 promoter region was predicted (Fig. 8D). Correlation analysis using the online database GEPIA revealed a positive correlation between CTNNB1 and TFAP2A (Fig. 8E). In stable knockdown HNRNPC cells, the mRNA level and protein expression level of β-catenin were decreased, and such changes could be reversed by overexpression of TFAP2A (Fig. 8F, G).
Discussion
As an RNA-binding protein, HNRNPC plays an important role in the occurrence and development of many tumors. In a study on breast cancer, LvW et al. found that the progression of breast cancer could be promoted through the circBACH2/has-miR-944/HNRNPC axis [42]. In the study of glioblastoma, HNRNPC could combine with the oncogene DDX11-AS1 to enhance Wnt/β-catenin and AKT pathways as well as EMT, thereby promoting the proliferation and migration of glioma cells [17]. With the further study of HNRNPC, HNRNPC can promote the occurrence of EMT in OSCC tumors, thus facilitating tumor progression [5].
Although significant advancements have been made in the diagnosis and treatment of non-small cell lung cancer, it remains the leading cause of cancer-related death and it considered one of the most aggressive malignancies worldwide [43, 44]. Invasion and metastasis are the main causes of high mortality in advanced NSCLC patients [45, 46]. However, the process of invasion and metastasis is complex and partially understood [47]. Although it is known that m6A regulators-mediated gene methylation plays a key role in NSCLC development, the underlying molecular mechanisms of m6A regulator promotes tumor progression are still not fully understood [48]. Previous studies have confirmed that HNRNPC, an important m6A reader, promotes the invasion and metastasis of NSCLC [13]. However, whether HNRNPC regulates gene expression in the manner of N6-methylation and thus participates in the progression of NSCLC deserves further study.
In this study, HNRNPC was highly expressed in various tumors, including NSCLC, and its high expression was associated with poor prognosis. The proliferation, migration and invasion of NSCLC cells were inhibited in the case of knocking down HNRNPC. LiuD et al. indicated that down-regulation of HNRNPC could inhibit liver cancer metastasis [12], which was consistent with our findings. These data emphasize the potential role of HNRNPC in promoting tumor malignancy progression. Blocking HNRNPC might thus represent a promising therapeutic target for NSCLC.
Subsequently, we identified the target gene TFAP2A of HNRNPC through RIP-seq, meRIP-qPCR and other experiments, while its high expression in NSCLC cancer tissues was confirmed by immunohistochemistry. In a recent study on immune escape in breast cancer, HNRNPC can promote TFAP2A mRNA expression by recognizing m6A modification [36]. Similarly, our study revealed that HNRNPC enhances TFAP2A expression by recognizing m6A modification in TFAP2A mRNA and regulating its expression. In the study of head and neck squamous cell carcinoma, HNRNPC can maintain the stability of ANLN210 mRNA and promote its expression by recognizing m6A modifications [49]. The next work is to investigate how HNRNPC promotes TFAP2A expression. The degradation rate of TFAP2A mRNA increased after HNRNPC knockdown of NSCLC cells treated with actinomycin D. These findings and reports indicate that HNRNPC enhances the expression of TFAP2A by recognizing the m6A of TFAP2A mRNA and maintaining its stability, thereby further promoting the proliferation, migration and invasion of NSCLC.
In addition, in previous studies, TFAP2A has been shown to promote metastasis in NSCLC [50]. The function of TFAP2A as a target gene of HNRNPC m6A modification in NSCLC needs to be further explored. Through rescue experiments, this study discovered that TFAP2A overexpression could partially restore the proliferation, migration and invasion ability of NSCLC cells inhibited by HNRNPC knockdown. The aforementioned results and reports serve to illustrate that HNRNPC might further promote the proliferation, migration and invasion of NSCLC by regulating TFAP2A expression.
In the malignant process, the mesenchymal properties induced by EMT enable cancer cells to complete many steps of the invasion-metastasis cascade [51]. EMT enhances cellular mobility, which is deemed to the first step of the metastasis cascade. Studies in many tumors have found that HNRNPC affects tumor migration through EMT [17, 38]. However, whether HNRNPC promotes the metastasis of NSCLC through EMT is unclear. In our study, E-Cadherin was increased, Vimentin and N-Cadherin were decreased when HNRNPC was knocked down. In breast cancer researches, TFAP2A has been found to regulate the early stage of EMT and promote its occurrence. TFAP2A can bind to Zeb2, the core transcription network of EMT, and participate in the occurrence of EMT [39]. Bioinformatics analysis in this study revealed that both HNRNPC and TFAP2A were enriched in adhesive junctions. Overexpression of TFAP2A can partially reverse the regulation of HNRNPC knockdown on the expression of EMT-related marker proteins. Therefore, it revealed that HNRNPC/TFAP2A promotes the metastasis of NSCLC via EMT.
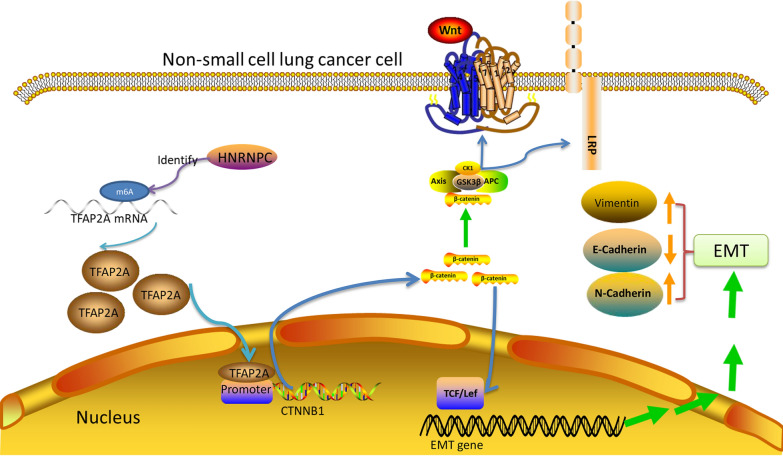
TFAP2A belongs to the Activator Protein 2 family of transcription factors, the best characterized member of the family [52]. A study in oral cancer by 2023 YangK et al. found that TFAP2A directly binds to the promoter region of β-catenin, thereby, regulating its transcriptional activity [41]. The expression decreaed trend of β-catenin was consistent with HNRNPC and TFAP2A by HNRNPC silenced, while the expression of β-catenin was increased after TFAP2A overexpressed. So we consider that HNRNPC/TFAP2A may promote NSCLC by control β-catenin expression of EMT process. In summary, these findings and reports suggest that HNRNPC can promotes the expression of TFAP2A by recognizing the m6A modification of TFAP2A mRNA and maintaining its stability, activates the TFAP2A/β-catenin axis, enhances EMT, and ultimately promotes the malignant process of NSCLC (Fig. 9).
Fig. 9.
Schematic diagram of the proposed mechanism. In NSCLC, HNRNPC increases TFAP2A expression by maintaining its mRNA stability in an m6A-dependent manne. Accumulated TFAP2A enters the nucleus and binds to β-catenin promoter and transcriptionally regulates the expression of β-catenin. Subsequently, β-catenin enters the nucleus to promote the occurrence of EMT by enhancing the transcription of EMT-related genes
Conclusions
In summary, we revealed a new HNRNPC/TFAP2A/CTNNB1 signaling axis, which promotes tumor proliferation, migration and invasion by enhancing protein expression and enhancing EMT development. This study not only expands our understanding of tumor progression mediated by m6A from a different perspective but also elucidates the regulatory mechanism of the HNRNPC/TFAP2A/CTNNB1 axis in NSCLC EMT. However, the selection of non-small cell lung cancer cells has limited the clinical relevance of these two cell types, which has not been extensively discussed in existing literature. In future studies, we aim to further address these shortcomings. And our research into the correlation among HNRNPC, TFAP2A and CTNNB1 is insufficient, and in vivo validation is lacking. Further investigation is necessary to explore the relationship between these factors and validate their interplay in tumorigenesis and progression.
Supplementary Information
Additional file 1: Table S1. Correlation between TFAP2A levels in NSCLC patients and their clinicopathologic characteristics.
Additional file 2: Table S2. Primer sequences for shRNA and used in the study.
Additional file 3: Table S3. Primer sequences for real-time PCR used in the study.
Additional file 4: Table S4. m6A primer sequences for real-time PCR used in the study.
Acknowledgements
We would like to thank TCGA, GEO, GEPIA, TIMER2, Kaplan-Meier Plotter, UALCAN, GSEA, JASPAR, and UCSC databases for the availability of the data. The authors thank the reviewers for their helpful comments.
Abbreviations
- HNRNPC
Heterogeneous nuclear ribonucleoprotein C
- NSCLC
Non-small cell lung cancer
- LUAD
Lung adenocarcinoma
- m6A
N6-methylation
- RBPs
RNA-Binding Proteins
- EMT
Epithelial to mesenchymal transition
- TFAP2A
Transcription Factor Activating Enhancer Binding Protein 2 Alpha
Author contributions
Minghua Liao: Study design, Experiment execution, Acquisition and analysis of data, Writing—original draft. Chunyu Li: Funding acquisition, Study design, Experiment execution, Acquisition and analysis of data, Writing—review & editing. Rui Yang: Supervision, Review & editing. Ke Wu: Acquisition and analysis of data, Visualization. Jun Li: Methodology, Writing—review & editing. Jiayi Zhang: Visualization, Data sorting. Qian Zhu: Visualization, Data sorting. Yingchang Shi: Visualization, Investigation. Xianming Zhang: Funding acquisition, Project administration. All authors read and approved the final manuscript.
Funding
This work was supported by the Cultivate project 2021 for National Natural Science Foundation of China, Guizhou Medical University (20NSP038); the Cultivate project 2021 for National Natural Science Foundation of China, Affiliated Hospital of Guizhou Medical University (gyfynsfc-2021-33); Science and Technology Fund Project of Guizhou Health Committee, China (gzwjkj2020-1-052, gzwkj2021-090, gzwkj2021-096, 2025GZWJKJXM1082); Science and Technology Support Program of Science and Technology Department of Guizhou Province (NO:Qian Ke He Zhi Cheng[2021]Yi Ban 061); Basic research project of Science and Technology Department of Guizhou Province, Basic of Guizhou—ZK[2022] General 450. Guizhou Provincial Basic Research Program(Natural Science) (Qian Ke He Ji Chu-zk[2025] Mian Shang 444).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Minghua Liao and Chunyu Li equally contributed to the manuscript.
References
- 1.Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198(2020):897–907. 10.1007/s00408-020-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suster DI, Mino-Kenudson M. Molecular pathology of primary non-small cell lung cancer. Arch Med Res. 2020;51:784–98. 10.1016/j.arcmed.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Chen S, Wang X, Liu X. Immune-related pulmonary toxicities of checkpoint inhibitors in non-small cell lung cancer: diagnosis, mechanism, and treatment strategies. Front Immunol. 2023;14:1138483. 10.3389/fimmu.2023.1138483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardarabadi P, Kojabad AA, Jafari D, Liu C-H. Liquid biopsy-based biosensors for MRD detection and treatment monitoring in non-small cell lung cancer (NSCLC). Biosensors. 2021;11:394. 10.3390/bios11100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang G-Z, Wu Q-Q, Zheng Z-N, Shao T-R, Chen Y-C, Zeng W-S, Lv X-Z. M6A-related bioinformatics analysis reveals that HNRNPC facilitates progression of OSCC via EMT. Aging. 2020;12:11667–84. 10.18632/aging.103333. [DOI] [PMC free article] [PubMed]
- 6.Quan W, Li J, Liu L, Zhang Q, Qin Y, Pei X, Chen J. Influence of N6-methyladenosine modification gene HNRNPC on cell phenotype in Parkinson’s disease. Parkinson’s Disease. 2021;2021:1–10. 10.1155/2021/9919129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, Li J, An P, Lu L, Luo N, Du J, Shan H, Liu H, Wang H. m6A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Li M, Rao X, Zhang W, Li X, Wang L, Huang G. KIAA1429 regulates the migration and invasion of hepatocellular carcinoma by altering m6A modification of ID2 mRNA. OTT. 2019;12:3421–8. 10.2147/OTT.S180954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Eckert MA, Harada BT, Liu S-M, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, Huang J-T, Chen S-M, Xu Z-G, Leng X-H, Yu X-C, Cao J, Zhang Z, Liu J, Lengyel E, He C. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–83. 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m 6 A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–45. 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krismer K, Bird MA, Varmeh S, Handly ED, Gattinger A, Bernwinkler T, Anderson DA, Heinzel A, Joughin BA, Kong YW, Cannell IG, Yaffe MB. Transite: a computational motif-based analysis platform that identifies RNA-binding proteins modulating changes in gene expression. Cell Rep. 2020;32:108064. 10.1016/j.celrep.2020.108064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Luo X, Xie M, Zhang T, Chen X, Zhang B, Sun M, Wang Y, Feng Y, Ji X, Li Y, Liu B, Huang W, Xia L. HNRNPC downregulation inhibits IL-6/STAT3-mediated HCC metastasis by decreasing HIF1A expression. Cancer Sci. 2022;113:3347–61. 10.1111/cas.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Z, Yang Y, Ma Q, Wang H, Zhao S, Qi Y, Li Y. HNRNPC, a predictor of prognosis and immunotherapy response based on bioinformatics analysis, is related to proliferation and invasion of NSCLC cells. Respir Res. 2022;23:362. 10.1186/s12931-022-02227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Bao C, Zhang X, Lin X, Fu Y. Knockdown of LINC00662 represses AK4 and attenuates radioresistance of oral squamous cell carcinoma. Cancer Cell Int. 2020;20:244. 10.1186/s12935-020-01286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Zhao W, Liu Y, Tan X, Li X, Zou Q, Xiao Z, Xu H, Wang Y, Yang X. Function of HNRNPC in breast cancer cells by controlling the dsRNA‐induced interferon response. EMBO J. 2018;37:e99017. 10.15252/embj.201899017. [DOI] [PMC free article] [PubMed]
- 16.He Q, Yang C, Xiang Z, Huang G, Wu H, Chen T, Dou R, Song J, Han L, Song T, Wang S, Xiong B. LINC00924-induced fatty acid metabolic reprogramming facilitates gastric cancer peritoneal metastasis via hnRNPC-regulated alternative splicing of Mnk2. Cell Death Dis. 2022;13:987. 10.1038/s41419-022-05436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang Z, Lv Q, Zhang Y, Chen X, Guo R, Liu S, Peng X. Long non-coding RNA DDX11-AS1 promotes the proliferation and migration of glioma cells by combining with HNRNPC. Molecular Therapy Nucleic Acids. 2022;28:601–12. 10.1016/j.omtn.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Cai D, Zhao Z, Zhong G-C, Gong J. Suppression of heterogeneous nuclear ribonucleoprotein C Inhibit hepatocellular carcinoma proliferation, migration, and invasion via Ras/MAPK signaling pathway. Front Oncol. 2021;11:659676. 10.3389/fonc.2021.659676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Huai Q, Zhang G, Guo L, Song P, Xue X, Tan F, Xue Q, Gao S, He J. Elevated heterogeneous nuclear ribonucleoprotein C expression correlates with poor prognosis in patients with surgically resected lung adenocarcinoma. Front Oncol. 2021;10:598437. 10.3389/fonc.2020.598437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–4. 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie W, Jiang Q, Wu X, Wang L, Gao B, Sun Z, Zhang X, Bu L, Lin Y, Huang Q, Li J, Guo J. IKBKE phosphorylates and stabilizes Snail to promote breast cancer invasion and metastasis. Cell Death Differ. 2022;29:1528–40. 10.1038/s41418-022-00940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundamaraju R, Lu W, Paul MK, Jha NK, Gupta PK, Ojha S, Chattopadhyay I, Rao PV, Ghavami S. Autophagy and EMT in cancer and metastasis: who controls whom? Biochimica et Biophysica Acta (BBA) Molecular Basis of Disease. 2022;1868:166431. 10.1016/j.bbadis.2022.166431. [DOI] [PubMed]
- 23.Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed Pharmacother. 2021;138:111450. 10.1016/j.biopha.2021.111450. [DOI] [PubMed] [Google Scholar]
- 24.Park YM, Hwang SJ, Masuda K, Choi K-M, Jeong M-R, Nam D-H, Gorospe M, Kim HH. Heterogeneous nuclear ribonucleoprotein C1/C2 controls the metastatic potential of glioblastoma by regulating PDCD4. Mol Cell Biol. 2012;32:4237–44. 10.1128/MCB.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Can Res. 2017;77:e108–10. 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Severson E, Pignon J-C, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Chandrashekar DS, Varambally S, Creighton CJ. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun. 2019;10:5679. 10.1038/s41467-019-13528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58. 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102. 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao T, Pan W, Sun X, Shen H. Increased expression of TET3 predicts unfavorable prognosis in patients with ovarian cancer—a bioinformatics integrative analysis. J Ovarian Res. 2019;12:101. 10.1186/s13048-019-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Wei Z, Zhang Q, Wu X, Rong R, Lu Z, Su J, de Magalhães JP, Rigden DJ, Meng J. WHISTLE: a high-accuracy map of the human N6-methyladenosine (m6A) epitranscriptome predicted using a machine learning approach. Nucleic Acids Res. 2019;47:e41–e41. 10.1093/nar/gkz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speir ML, Bhaduri A, Markov NS, Moreno P, Nowakowski TJ, Papatheodorou I, Pollen AA, Raney BJ, Seninge L, Kent WJ, Haeussler M. UCSC cell browser: visualize your single-cell data. Bioinformatics. 2021;37:4578–80. 10.1093/bioinformatics/btab503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Mondragon JA, Riudavets-Puig R, Rauluseviciute I, Berhanu Lemma R, Turchi L, Blanc-Mathieu R, Lucas J, Boddie P, Khan A, Manosalva Pérez N, Fornes O, Leung TY, Aguirre A, Hammal F, Schmelter D, Baranasic D, Ballester B, Sandelin A, Lenhard B, Vandepoele K, Wasserman WW, Parcy F, Mathelier A. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50(2022):D165–73. 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Ren H, Zhang X, Chang L, Wang Z, Wu H, Zhang J, Ren J, Zhou L. Research advances of N6-methyladenosine in diagnosis and therapy of pancreatic cancer. Clin Lab Anal. 2022;36:e24611. 10.1002/jcla.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian B, Yan S, Li J, Bai Z, Li J. HNRNPC promotes collagen fiber alignment and immune evasion in breast cancer via activation of the VIRMA-mediated TFAP2A/DDR1 axis. Mol Med. 2023;29:103. 10.1186/s10020-023-00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen W, Pan T, Wang H, Zhang Y, Li C. LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs. Biochem Biophys Res Commun. 2019;511:566–72. 10.1016/j.bbrc.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Wang J, Liu X, Xu Y, Zhai R, Zhang J, Wang M, Wang M, Liu L. lncRNA CYTOR promotes aberrant glycolysis and mitochondrial respiration via HNRNPC-mediated ZEB1 stabilization in oral squamous cell carcinoma. Cell Death Dis. 2022;13:703. 10.1038/s41419-022-05157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitrova Y, Gruber AJ, Mittal N, Ghosh S, Dimitriades B, Mathow D, Grandy WA, Christofori G, Zavolan M. TFAP2A is a component of the ZEB1/2 network that regulates TGFB1-induced epithelial to mesenchymal transition. Biol Direct. 2017;12:8. 10.1186/s13062-017-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P. Role of cadherins in cancer—a review. IJMS. 2020;21:7624. 10.3390/ijms21207624. [DOI] [PMC free article] [PubMed]
- 41.Yang K, Zhao J, Liu S, Man S. RELA promotes the progression of oral squamous cell carcinoma via TFAP2A-Wnt/β-catenin signaling. Mol Carcinog. 2023;62:641–51. 10.1002/mc.23512. [DOI] [PubMed] [Google Scholar]
- 42.Lv W, Tan Y, Xiong M, Zhao C, Wang Y, Wu M, Wu Y, Zhang Q. Analysis and validation of m6A regulatory network: a novel circBACH2/has-miR-944/HNRNPC axis in breast cancer progression. J Transl Med. 2021;19:527. 10.1186/s12967-021-03196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mokhlesi A, Talkhabi M. Comprehensive transcriptomic analysis identifies novel regulators of lung adenocarcinoma. J Cell Commun Signal. 2020;14:453–65. 10.1007/s12079-020-00565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. [DOI] [PubMed]
- 45.Yuanhua L, Pudong Q, Wei Z, Yuan W, Delin L, Yan Z, Geyu L, Bo S. TFAP2A induced KRT16 as an oncogene in lung adenocarcinoma via EMT. Int J Biol Sci. 2019;15:1419–28. 10.7150/ijbs.34076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Gao X, Ma M, Zhao C, Zhang Y, Guo S. CircRNA_101237 promotes NSCLC progression via the miRNA-490-3p/MAPK1 axis. Sci Rep. 2020;10:9024. 10.1038/s41598-020-65920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao H. MiR-7-5p suppresses tumor metastasis of non-small cell lung cancer by targeting NOVA2. Cell Mol Biol Lett. 2019;24:60. 10.1186/s11658-019-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Z, Cai Q, Zhang P, He B, Peng X, Tu G, Peng W, Wang L, Yu F, Wang X. N6-methyladenosine RNA methylation regulator-related alternative splicing (AS) gene signature predicts non-small cell lung cancer prognosis. Front Mol Biosci. 2021;8:657087. 10.3389/fmolb.2021.657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin S, Li M, Chang H, Wang R, Zhang Z, Zhang J, He Y, Ma H. The m6A demethylase ALKBH5 promotes tumor progression by inhibiting RIG-I expression and interferon alpha production through the IKKε/TBK1/IRF3 pathway in head and neck squamous cell carcinoma. Mol Cancer. 2022;21:97. 10.1186/s12943-022-01572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong Y, Feng Y, Zhao J, Lei J, Qiao T, Zhou Y, Lu Q, Jiang T, Jia L, Han Y. TFAP2A potentiates lung adenocarcinoma metastasis by a novel miR-16 family/TFAP2A/PSG9/TGF-β signaling pathway. Cell Death Dis. 2021;12:352. 10.1038/s41419-021-03606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361–73. 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallberg AR, Vorrink SU, Hudachek DR, Cramer-Morales K, Milhem MM, Cornell RA, Domann FE. Aberrant CpG methylation of the TFAP2A gene constitutes a mechanism for loss of TFAP2A expression in human metastatic melanoma. Epigenetics. 2014;9:1641–7. 10.4161/15592294.2014.988062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Correlation between TFAP2A levels in NSCLC patients and their clinicopathologic characteristics.
Additional file 2: Table S2. Primer sequences for shRNA and used in the study.
Additional file 3: Table S3. Primer sequences for real-time PCR used in the study.
Additional file 4: Table S4. m6A primer sequences for real-time PCR used in the study.
Data Availability Statement
No datasets were generated or analysed during the current study.