Abstract
Background
To comprehensively evaluate the risk factors for recurrent gestational diabetes mellitus (GDM) in women with a history of GDM during re-pregnancy.
Methods
Articles about risk factors for recurrent GDM were searched in China National Knowledge Infrastructure, Wanfang Data, VIP Database for Chinese Technical Periodicals, PubMed, EMBASE, the Cochrane Library, and Web of Science from the date of establishment to January 2023. Meta-analysis of risk factors for recurrent GDM was performed using STATA/SE 15.1 software.
Results
A total of 19 studies were included in the meta-analysis, comprising 15 case-control studies and 4 cohort studies, involving 11,385 patients. Among them, 2,462 patients experienced recurrent GDM, while 2,909 did not. The analysis of case-control studies revealed a GDM recurrence rate of 48%. Meta-analysis identified several significant risk factors for GDM recurrence: advanced maternal age at subsequent pregnancy [ES = 3.02, 95% CI (1.24,2.79), P = 0.003], increased BMI prior to the subsequent pregnancy [ES = 2.23, 95% CI (1.04,1.72), P = 0.026], elevated 1-hour plasma glucose levels in oral glucose tolerance test (OGTT) during previous pregnancy [ES = 2.79, 95% CI (1.11,1.78), P = 0.005], increased 2-hour OGTT glucose levels in previous pregnancy [ES = 2.75, 95% CI (1.11,1.91), P = 0.006], and previous delivery of macrosomia [ES = 3.48, 95% CI (1.38,3.18), P = 0.001]. All these factors showed statistically significant differences between the recurrence and non-recurrence groups. Pregnant women with a history of GDM can reduce the risk of recurrence by adopting a reasonable pregnancy plan, such as avoiding advanced maternal age, managing body weight, controlling blood glucose levels during pregnancy, and losing weight before conception.
Conclusion
Advanced maternal age, elevated BMI before subsequent pregnancy, increased OGTT levels during the previous pregnancy, and the delivery of macrosomia are significant risk factors for recurrent GDM.
Keywords: Gestational diabetes mellitus, Recurrence, Risk factors, Meta-analysis
Background
Gestational diabetes mellitus (GDM) refers to the abnormal glucose metabolism of women only in pregnancy, excluding the disorder of glucose metabolism that existed before pregnancy. The GDM recurrence is defined as the recurrence of GDM during re-pregnancy in a woman who has been diagnosed with GDM during a previous pregnancy and whose blood sugar has returned to normal after delivery. GDM not only increases the incidence of adverse events such as hypertensive disorder in pregnancy, macrosomia, caesarean section, and postpartum hemorrhage, but also increases the long-term risk of type 2 diabetes in pregnant women and diabetes and obesity in their offspring [1]. The pregnancy outcomes and complications caused by recurrent GDM may be more severe than those caused by first-time GDM. A study by Wang [2] showed that pregnant women with recurrent GDM had a higher risk of developing diabetes after delivery than those with first-time GDM. The recurrence of GDM is common, but there have been few studies on it in China. In international studies, only ethnicity is recognized as an important risk factor [3, 4]. In addition, the old age of pregnant women, overweight before pregnancy, insulin use in the previous pregnancy, family history of diabetes, weight gain during two pregnancies, inter-pregnancy interval (IPI), and oral glucose tolerance test (OGTT) levels in the second trimester of the previous pregnancy are considered to be related to GDM recurrence [5]. However, the conclusions are not consistent. With the adjustment of China’s fertility policy, the number of pregnant women who bear another child will increase significantly, and the problem of recurrent GDM is becoming increasingly prominent. How to identify the high-risk factors for recurrent GDM as soon as possible, especially the controllable factors, so as to take corresponding measures to avoid the recurrence is gradually taken seriously. Therefore, it is necessary to conduct a comprehensive analysis of the existing literature from a statistical perspective, in order to draw a clearer conclusion.
Methods
Retrieval methods
The search formula was designed by using the Boolean logic operators “AND”, “OR” to combine medical subject headings and text words “Diabetes, Gestational”, “Diabetes, Pregnancy Induced”, “Diabetes, Pregnancy Induced”, “Pregnancy Induced Diabetes”, “Gestational Diabetes” “Diabetes Mellitus, Gestational”, “Gestational Diabetes Mellitus”, “Recurrence”, “Recurrences”, “Recrudescence”, “Recrudescences”, “Relapse”, “Relapses”, “Risk Factors”, “Factor, Risk”, “Risk Factor”, “Social Risk Factors”, “Factor, Social Risk”, “Factors, Social Risk”, “Risk Factor, Social”, “Risk Factors, Social”, “Social Risk Factor”, “Health Correlates”, “Correlates, Health”, “Population at Risk”, “Populations at Risk”, “Risk Scores”, “Risk Score”, “Score, Risk”, “Risk Factor Scores”, “Risk Factor Score”, “Score, Risk Factor”. The following databases were comprehensively searched from the establishment of each database to January 2023 to collect domestic and foreign studies on risk factors of recurrent GDM: China National Knowledge Infrastructure, Wanfang Data, VIP Database for Chinese Technical Periodicals, PubMed, EMBASE, the Cochrane library, and Web of Science. The language was limited to Chinese and English.
Inclusion criteria
(1) Research type: Case-control study, cohort study; (2) Subjects: women who had a history of GDM were pregnant again; (3) Language: Chinese or English; (4) Following the IADPSG standards supported by IADPSG [6] and WHO [7]: 75 g glucose OGTT test is performed at 24–28 gestational weeks; If FPG ≥ 5.1mmol/L or 1hPG ≥ 10.0mmol/L or 2hPG ≥ 8.5mmol/L, then GDM is diagnosed; if FPG ≥ 7.0mmol/L, or 2hPG ≥ 11.1mmol/L, or HbA1c% ≥ 6.5% combined with other diabetes symptoms, then pregestational diabetes mellitus (PGDM) is diagnosed; (5) In univariate analysis, for continuous variables, the original data should include sample size (N), mean, and standard deviation (SD); For categorical variables, the original data should include the number of people (total), exposed events (event), and non-exposed events (noevent). In multivariate analysis, the original data should include odds ratio (OR), 95% confidence interval (CI), or can be derived from other known data.
Exclusion criteria
(1) Repeated literature (such as different databases, different languages) or reports from the same cohort; (2) Review, meta-analysis, system review, meeting abstract, case report, guidelines, animal experiments, literature in other languages (excluding Chinese and English); (3) The diagnostic criteria are unclear or inconsistent; (4) The literature quality score (NOS) is less than 6, or the data collection and analysis methods are not scientific or cannot be extracted; (5) The literature lacks clear descriptive data on target factors and fails to provide mean, odds ratio (OR), 95% confidence interval (CI), and cannot be derived from known data for statistical calculations.
Data extraction
Excel software was used to extract relevant data from included literature and establish: (1) Literature quality evaluation table; (2) Basic information table: including the name of the first author, publication year, literature source, research type, total sample size, case group sample size, and control group sample size; (3) Analysis data table: the name of the first author and the year of publication were used as labels; the continuous variables used N (sample size), mean value, SD value, the second categorical variable used total number of people, event (exposure event), noevent (non-exposure event), and the multivariate analysis used OR value, 95% CI value as target data to record accordingly; (4) For risk factors such as IPI, maternal weight gain, and neonatal birth weight, the units was converted to months, kilograms, and grams, respectively; (5) The selection of literature and data extraction were independently conducted by two researchers based on predetermined criteria. Disagreements between the two were resolved through discussion. If a consensus could not be reached, a third party was consulted for further resolution.
Literature quality evaluation
The Newcastle-Ottawa scale (NOS), which is applicable to case-control studies and cohort studies [8], was used to assess the quality of the included literature. An NOS score of ≥ 7 was considered high-quality, 4–6 moderate-quality, and ≤ 3 low-quality literature.
Statistical methods
Selection of research indicators
STATA/SE 15.1 software was used for data processing. The univariate analysis was conducted to compare baseline differences and extract study indicators. For continuous variables, the sample size (N), mean, and standard deviation (SD) were reported. For binary variables, the total number of participants, the number of events (exposed cases), and the number of non-events (non-exposed cases) were recorded. Weighted Mean Differences (WMDs) or pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated for each risk factor, and the results were visualized using forest plots. For multivariate analysis, logistic regression was employed to extract study indicators, including odds ratios (ORs) and their corresponding 95% CIs. Effect sizes (ESs) and 95% Cs for each risk factor were also calculated, and the results were presented in forest plots.
Heterogeneity test, effect model, and publication bias
Statistical analysis software STATA/SE 15.1 was used to analyze the risk factors of GDM recurrence. If the heterogeneity (I2) is ≥ 50%, the heterogeneity between studies is large. In this case, the random-effects model was adopted, and further sensitivity analysis was performed to explore the source of heterogeneity. If the heterogeneity (I2) is less than 50%, the heterogeneity between studies is small, and a fixed-effect model was used. Publication bias was evaluated by Egger’s test, where P > 0.05 indicated no significant publication bias and P ≤ 0.05 indicated possible publication bias.
Forest plot interpretation
For the risk factor analysis of continuous variables in univariate analysis, when the pooled diamond (WMD and 95% CI) is located on both sides of the 0 standard line, it means that the data is not statistically significant. If the pooled diamond (WMD and 95% CI) is located on the right side of the 0 standard line, it means that the factor is a risk factor. If it is located on the left side, it means that the factor is a protective factor.
For the risk factor analysis of categorical variables in univariate analysis, when the pooled diamond (RR or ES and 95% CI) is on both sides of the 1 standard line, the data is not statistically significant. If the pooled diamond (RR or ES and 95% CI) is on the right side of the 1 standard line, the factor is a risk factor, and if it is on the left side, the factor is a protective factor.
Similarly, in the multivariate analysis of risk factors, if the pooled diamond (representing the OR or ES and its 95% CI) crosses the reference line at 1, the result is not statistically significant. If the pooled diamond lies completely to the right of the 1 reference line, the factor is identified as a risk factor, while if it lies entirely to the left, the factor acts as a protective factor.
Calculation of recurrence rate
The recurrence rate of the case-control studies (P = number of recurrence / total sample size) and the standard error of recurrence rate (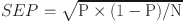 ) were calculated in Excel, where N was the total sample size. The data were processed by STATA/SE 15.1 software, and the P and SEP in each case-control study were extracted and combined for analysis. If I2 ≥ 50%, a random-effects model was adopted, and further sensitivity analysis was conducted to explore heterogeneity sources. If I2 < 50%, a fixed-effects model was used to calculate ES and 95% CI. At this point, ES was the pooled incidence rate after meta-analysis. Finally, Egger’s test was used to evaluate publication bias. P > 0.05 indicated that there was no significant publication bias, and P ≤ 0.05 indicated that there might be publication bias.
) were calculated in Excel, where N was the total sample size. The data were processed by STATA/SE 15.1 software, and the P and SEP in each case-control study were extracted and combined for analysis. If I2 ≥ 50%, a random-effects model was adopted, and further sensitivity analysis was conducted to explore heterogeneity sources. If I2 < 50%, a fixed-effects model was used to calculate ES and 95% CI. At this point, ES was the pooled incidence rate after meta-analysis. Finally, Egger’s test was used to evaluate publication bias. P > 0.05 indicated that there was no significant publication bias, and P ≤ 0.05 indicated that there might be publication bias.
Results
Retrieval results
1090 literatures were retrieved through the database search. After removing duplicates and screening according to inclusion and exclusion criteria, 19 related studies were finally included [2, 9–26], including 13 Chinese studies and 6 English studies. These studies involved 11,385 patients, among whom 2,462 experienced recurrent GDM. The basic characteristics of the literature are as follows (Table 1):
Table 1.
Basic information table
| Included studies | Year | Literature sources | Research design | Sample size | Recurrent group | Non recurrent group | NOS score |
|---|---|---|---|---|---|---|---|
| Aiyue Chen | 2019 | China | case-control study | 625 | 289 | 336 | 7 |
| Meijuan Jian | 2021 | China | case-control study | 380 | 145 | 235 | 8 |
| Ruixia Wu | 2021 | China | case-control study | 467 | 212 | 255 | 8 |
| Lai Cheng | 2021 | China | case-control study | 306 | 113 | 193 | 6 |
| Xinghe Wang | 2019 | China | case-control study | 500 | 127 | 373 | 8 |
| Jie Zhang | 2021 | China | case-control study | 269 | 132 | 137 | 7 |
| Jun Zhao | 2018 | China | case-control study | 187 | 108 | 79 | 6 |
| Yinyu Wang | 2018 | China | case-control study | 142 | 78 | 64 | 8 |
| Na Wang | 2019 | China | case-control study | 128 | 56 | 72 | 8 |
| LH Li | 2021 | China | cohort study | 238 | 37 | 78 | 8 |
| Hahn S | 2023 | Germany | case-control study | 159 | 115 | 44 | 6 |
| G Kotzaeridi | 2021 | Austria | cohort study | 706 | 68 | 52 | 6 |
| XH Wei | 2022 | China | case-control study | 128 | 65 | 63 | 7 |
| YS Liang | 2022 | China | case-control study | 376 | 166 | 210 | 6 |
| YM Wei | 2022 | China | cohort study | 6204 | 490 | 489 | 6 |
| Yi Zhou | 2021 | China | case-control study | 126 | 63 | 63 | 7 |
| Qiuhong Chen | 2018 | China | case-control study | 102 | 50 | 52 | 7 |
| Mei Liu | 2018 | China | cohort study | 222 | 76 | 66 | 7 |
| Ting Li | 2020 | China | case-control study | 120 | 72 | 48 | 7 |
Meta-analysis results
Calculation of recurrence rate
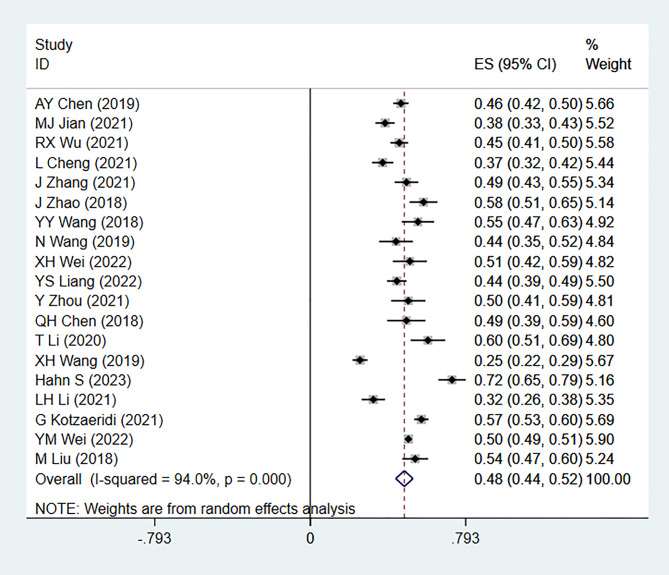
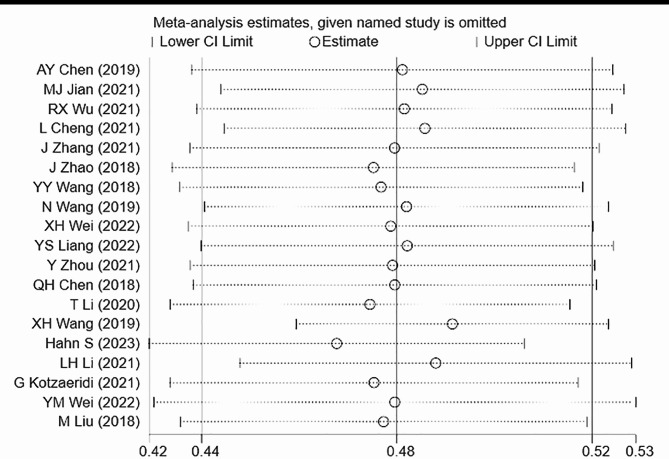
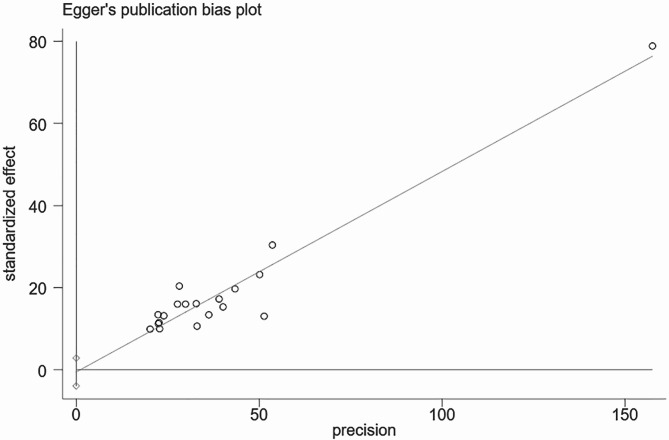
A total of 19 articles were included in this study. The analysis of GDM recurrence rate showed significant heterogeneity (I² = 94.0%, P = 0.000). Using a random-effects model, the pooled ES was 0.48 [95% CI (0.44, 0.52)], with the diamond plot positioned on the right side, indicating a GDM recurrence rate of 48%. This result was statistically significant. Egger’s test (P = 0.737) and comprehensive analysis indicated no publication bias. Sensitivity analysis did not identify any specific source of heterogeneity (Figs. 1, 2 and 3).
Fig. 1.
Forest plot for recurrence rate calculation
Fig. 2.
Heterogeneity test for recurrence rate calculation
Fig. 3.
Publication bias in recurrence rate calculation
Univariate analysis
In univariate analysis, except for ethnicity, the risk factors discussed in the included literature were as follows: the top 11 risk factors discussed in the included studies were IPI, age of second pregnancy, OGTT levels of previous pregnancy, age of previous pregnancy, BMI before second pregnancy, age of second pregnancy ≥ 35 years, insulin use in previous pregnancy, macrosomia in previous pregnancy, and weight before second pregnancy. We analyzed risk factors that were investigated in five or more studies, while risk factors with fewer investigations were not included in the analysis. The combined results showed that except for the use of insulin in the previous pregnancy (P = 0.424 > 0.05), the other 10 risk factors had statistically significant differences (Table 2).
Table 2.
Results of risk factors by univariate analysis
| Risk factors | Number of studies | Heterogeneity | Effect model | WMD/RR | 95%CI | P | ||
|---|---|---|---|---|---|---|---|---|
| I2% | P | |||||||
| Abnormal 1hPG or (and) 2hPG in previous pregnancy | 3 | 68.5 | 0.042 | random | 0.89 | (0.62, 1.68) | 0.540 | |
| Abnormal FPG + 1hPG or (and) 2hPG in previous pregnancy | 3 | 0 | 0.766 | fixed | 3.11 | (2.24, 4.32) | < 0.001 | |
| OGTT 1hPG in previous pregnancy | 10 | 54.8 | 0.019 | random | 0.74 | (0.60, 0.88) | < 0.001 | |
| OGTT 2hPG in previous pregnancy | 11 | 88.6 | 0.000 | random | 0.74 | (0.43, 1.06) | < 0.001 | |
| OGTT FPG in previous pregnancy | 11 | 71.9 | 0.000 | random | 0.17 | (0.10, 0.24) | < 0.001 | |
| Postpartum hemorrhage of previous pregnancy | 2 | 0.0 | 0.484 | fixed | 1.29 | (0.44, 3.83) | 0.664 | |
| Isolated FPG abnormal in previous pregnancy | 3 | 44.0 | 0.168 | fixed | 0.68 | (0.57, 0.80) | < 0.001 | |
| BMI at delivery of previous pregnancy | 2 | 12.3 | 0.286 | fixed | 0.39 | (-0.10, 0.89) | 0.119 | |
| Weight at delivery of previous pregnancy | 2 | 0.0 | 0.748 | fixed | -0.39 | (-0.79, 0.02) | 0.062 | |
| Gestational hypertension in previous pregnancy | 3 | 0.0 | 0.474 | fixed | 2.26 | (0.99, 5.13) | 0.052 | |
| Macrosomia of previous pregnancy | 5 | 32.0 | 0.208 | fixed | 1.91 | (1.39, 2.62) | < 0.001 | |
| Age at previous pregnancy ≥ 35 years | 3 | 80.8 | 0.005 | random | 1.86 | (1.05, 3.28) | 0.034 | |
| Age of previous pregnancy | 9 | 81.2 | 0.000 | random | 0.91 | (0.44, 1.38) | < 0.001 | |
| Cesarean section in previous pregnancy | 3 | 0.0 | 0.454 | fixed | 1.23 | (1.03, 1.47) | 0.019 | |
| Insulin use in previous pregnancy | 7 | 54.7 | 0.039 | random | 1.10 | (0.87, 1.39) | 0.424 | |
| Neonatal hypoglycemia in previous pregnancy | 2 | 0.0 | 0.901 | fixed | 2.26 | (0.57, 8.94) | 0.245 | |
| Neonatal weight in previous pregnancy | 4 | 92.0 | 0.000 | random | 87.63 | (-73.29, 248.54) | 0.286 | |
| 2 or more abnormal OGTT levels in previous pregnancy | 2 | 70.3 | 0.066 | random | 1.93 | (1.40, 2.66) | < 0.001 | |
| Weight gain during previous pregnancy | 5 | 81.9 | 0.000 | random | -0.16 | (-0.94, 0.61) | 0.680 | |
| BMI before previous pregnancy | 5 | 94.4 | 0.000 | random | 1.08 | (0.00, 2.15) | 0.050 | |
| Pre-pregnancy weight of second pregnancy | 2 | 0.0 | 0.624 | fixed | -0.57 | (-0.98, -0.17) | 0.005 | |
| Gestational week of previous pregnancy | 4 | 65.0 | 0.036 | random | -0.11 | (-0.26, 0.05) | 0.171 | |
| Preterm delivery in previous pregnancy | 3 | 0.0 | 0.499 | fixed | 1.61 | (0.97, 2.69) | 0.067 | |
| Cholesterol in first trimester of previous pregnancy | 2 | 0.0 | 0.714 | fixed | 0.15 | (0.12, 0.18) | < 0.001 | |
| Triglycerides in first trimester of previous pregnancy | 2 | 0.0 | 0.886 | fixed | 0.28 | (0.25, 0.31) | < 0.001 | |
| Height | 2 | 0.0 | 0.498 | fixed | -0.48 | (-1.29, 0.33) | 0.249 | |
| Body weight change between pregnancies | 3 | 98.0 | 0.000 | random | 1.31 | (0.07, 2.55) | 0.038 | |
| Change in BMI between pregnancies | 2 | 93.0 | 0.000 | random | 0.98 | (-0.44, 2.39) | 0.177 | |
| Inter-pregnancy interval | 11 | 94.5 | 0.000 | random | 2.18 | (0.19, 4.17) | 0.031 | |
| Number of pregnancies ≥ 3 | 2 | 0.0 | 0.764 | fixed | 1.09 | (0.85, 1.41) | 0.494 | |
| Number of production ≥ 3 | 2 | 0.0 | 0.512 | fixed | 0.96 | (0.66, 1.39) | 0.835 | |
| Triglycerides in second pregnancy | 3 | 73.1 | 0.024 | random | 0.59 | (0.10, 1.09) | 0.019 | |
| Low density lipoprotein in second pregnancy | 3 | 65.4 | 0.056 | random | 0.03 | (-0.22, 0.29) | 0.796 | |
| High density lipoprotein in second pregnancy | 3 | 62.8 | 0.068 | random | -0.02 | (-0.20, 0.17) | 0.853 | |
| IPI ≥ 5 years | 2 | 61.6 | 0.106 | random | 1.35 | (0.92, 1.99) | 0.121 | |
| Age of second pregnancy ≥ 35 years | 5 | 51.7 | 0.082 | random | 1.67 | (1.33, 2.08) | < 0.001 | |
| Age of second pregnancy | 10 | 59.0 | 0.009 | random | 0.91 | (0.49, 1.33) | < 0.001 | |
| Weight gain during pregnancy | 4 | 94.6 | 0.000 | random | -0.57 | (-2.28, 1.14) | 0.515 | |
| Pre-pregnancy BMI ≥ 25 | 4 | 25.7 | 0.257 | fixed | 1.52 | (1.31, 1.77) | < 0.001 | |
| Pre-pregnancy BMI | 8 | 76.7 | 0.000 | random | 1.41 | (0.97, 1.85) | < 0.001 | |
| Weight before pregnancy | 5 | 0.0 | 0.937 | fixed | 1.79 | (1.45, 2.14) | < 0.001 | |
| FPG in early pregnancy in second pregnancy | 5 | 66.8 | 0.017 | random | 0.22 | (0.10, 0.34) | < 0.001 | |
| Early pregnancy weight gain in second pregnancy | 2 | 0.0 | 0.646 | fixed | 0.09 | (-0.05, 0.23) | 0.197 | |
| Second trimester weight gain in second pregnancy | 2 | 0.0 | 0.906 | fixed | 0.48 | (0.17, 0.79) | 0.002 | |
| Total cholesterol in second pregnancy | 3 | 0.0 | 0.772 | fixed | 0.11 | (0.08, 0.14) | < 0.001 | |
Multivariate analysis
Multivariate analysis was performed using eight factors that were statistically significant, appeared in three or more studies, and were frequently discussed in univariate analysis. The combined results showed that, the second pregnancy age ≥ 35 years, OGTT 2hPG in previous pregnancy, OGTT 1hPG in previous pregnancy, the previous delivery of macrosomia, the second pregnancy BMI before pregnancy, were risk factors for GDM recurrence, and the difference was statistically significant (P < 0.05) (Table 3).
Table 3.
Results of risk factors by multivariate analysis
| Risk factors | Number of studies | Heterogeneity | Effect model | ES | 95%CI | P | |
|---|---|---|---|---|---|---|---|
| I2% | P | ||||||
| Body weight change during two pregnancies | 2 | 94.7 | 0.000 | random | 1.04 | (0.90, 1.40) | 0.297 |
| Abnormal FPG + 1hPG or (and) 2hPG in previous pregnancy | 2 | 0.0 | 0.853 | fixed | 2.34 | (1.11, 3.22) | 0.019 |
| OGTT 1hPG in previous pregnancy | 4 | 73.8 | 0.009 | random | 2.79 | (1.11, 1.78) | 0.005 |
| Macrosomia in previous pregnancy | 4 | 45.0 | 0.141 | fixed | 3.48 | (1.38, 3.18) | 0.001 |
| Age of previous pregnancy | 2 | 0.0 | 0.397 | fixed | 0.97 | (0.92, 1.27) | 0.334 |
| Cesarean section in previous pregnancy | 2 | 35.4 | 0.213 | fixed | 1.26 | (0.82, 2.50) | 0.207 |
| Insulin use in previous pregnancy | 4 | 13.8 | 0.324 | fixed | 10.44 | (1.80, 2.36) | < 0.001 |
| Weight change in previous pregnancy | 2 | 89.8 | 0.002 | random | 1.04 | (0.84, 1.77) | 0.297 |
| IPI | 4 | 66.2 | 0.031 | random | 0.32 | (0.93, 1.11) | 0.746 |
| Age of second pregnancy ≥ 35 | 5 | 96.5 | 0.000 | random | 3.02 | (1.24, 2.79) | 0.003 |
| Age of second pregnancy | 5 | 76.1 | 0.002 | random | 0.93 | (0.93, 1.21) | 0.350 |
| Pre-pregnancy BMI ≥ 25 | 2 | 0.0 | 0.576 | fixed | 3.65 | (1.46, 3.53) | 0.000 |
| HbA1c in the first trimester of second pregnancy | 2 | 59.4 | 0.116 | random | 1.04 | (0.63, 4.46) | 0.299 |
| HDL-C in the first trimester of second pregnancy | 2 | 37.5 | 0.206 | fixed | 0.04 | (0.97, 1.03) | 0.970 |
| LDL-C in the first trimester of second pregnancy | 2 | 37.2 | 0.207 | fixed | 1.27 | (0.98, 1.01) | 0.205 |
| Cholesterol in the first trimester of second pregnancy | 2 | 0.0 | 0.459 | fixed | 0.02 | (0.99, 1.01) | 0.985 |
| Triglycerides in first trimester of second pregnancy | 2 | 83.0 | 0.015 | random | 0.84 | (0.70, 2.41) | 0.400 |
| FPG in the first trimester of second pregnancy | 3 | 0.0 | 0.839 | fixed | 3.02 | (1.03, 1.17) | 0.002 |
| Family history of diabetes in lineal relative | 4 | 50.6 | 0.108 | random | 2.10 | (1.05, 3.87) | 0.036 |
| Pre-pregnancy BMI in second pregnancy | 4 | 97.3 | 0.000 | random | 2.23 | (1.04, 1.72) | 0.026 |
| FPG in previous pregnancy | 3 | 68.5 | 0.042 | random | 1.04 | (0.77, 2.35) | 0.296 |
| OGTT 2hPG in previous pregnancy | 5 | 77.2 | 0.002 | random | 2.75 | (1.11, 1.91) | 0.006 |
Discussions
In recent years, with the improvement of people’s living standards, changes in eating habits, universal screening of GDM, and changes in China’s fertility policy, the incidence of GDM is dramatically increasing. According to the latest diagnostic criteria, the incidence of GDM in China has reached 17.5-18.9% [27, 28]. After the two-children and three-children policies in China have been liberalized, the increasing prevalence of GDM has become a huge burden. GDM has brought health problems to both mothers and offspring, and the prevention of GDM is as important as the management of GDM. The pathogenesis of GDM is mainly related to enhanced insulin resistance and relatively insufficient insulin secretion caused by various reasons, among which, insulin resistance may persist for quite a long period of time [29]. The risk of recurrent GDM in the second pregnancy is significantly higher, and women with recurrent GDM have a significantly higher risk of developing type 2 diabetes in the future [30]. According to the results of this study, the recurrence rate of GDM and its risk factors were analyzed as follows.
Recurrence rate
The pooled analysis demonstrated a GDM recurrence rate of 48%, which was statistically significant. However, substantial heterogeneity was observed among the studies. While sensitivity analysis did not identify specific sources of heterogeneity, several factors might have contributed to this variation, including: the limited number of included studies, relatively small sample sizes, the inclusion of some moderate-quality studies, and the combination of both case-control and cohort study designs.
Ethnicity
Previous foreign studies have shown that the recurrence rate of GDM is significantly related to ethnicity. In 2008, Kim [3] reviewed the literature from 1965 to 2006 and found that the recurrence rate of GDM ranged from 30 to 84%, with non-Hispanic whites having the lowest recurrence rate (< 40%) and other races (including Asians) having recurrence rates > 50%. Studies on GDM recurrence rates in Asians populations reported rates of 65.6% in Japan [31] and 45.0% in South Korea [32]. Nevertheless, the majority of data in this study were derived from Chinese and other Asian populations, with limited data on GDM recurrence from Caucasian populations. This limitation precluded a comprehensive analysis of racial/ethnic factors in GDM recurrence. Therefore, further studies incorporating diverse ethnic populations are warranted to elucidate the potential impact of ethnicity on GDM recurrence risk.
Age of pregnancy
In this study, univariate and multivariate analysis results showed that older pregnancy (age ≥ 35 years) increased the risk of recurrence of GDM, which was statistically significant. This was consistent with the results of most relevant studies. Age is one of the risk factors for GDM and is also a well-known risk factor for cardiovascular disease. As early as 2014, the Chinese Guidelines for the Diagnosis and Treatment of Gestational Diabetes Mellitus clearly stated that advanced age is a risk factor for GDM in Chinese pregnant women. The structure and function of the vascular system change with age, and dysfunctional endothelial cells can increase the risk of insulin resistance in patients, which in turn increases the risk of hypertension, type 2 diabetes and other metabolic syndromes. Lao’s study [33] showed that women over the age of 40 were 8.2 times more likely to have recurrent GDM when they became pregnant again than women aged 20–30. Al-Goblan’s study [34] also believed that with aging, the body’s ability to adjust blood glucose, pancreatic insulin secretion capacity, and the affinity between insulin receptor and insulin decreased, leading to abnormal glucose metabolism. Hence, GDM recurrence risk increases in older pregnant women.
BMI and weight before second pregnancy
The results of univariate and multivariate analysis showed that BMI and weight gain before pregnancy increased the risk of recurrence of GDM, which was consistent with the results of most studies. Weight and BMI were generally considered as high-risk factors for GDM, and obesity itself was an independent risk factor of diabetes mellitus [35]. In a cohort study by Kim et al. [36], the incidence of GDM was 2.3% in normal-weight pregnant women, 4.8% in overweight pregnant women, 5.5% in obese pregnant women, and 11.5% in severely obese pregnant women. They estimated that by reducing pre-pregnancy obesity, half of GDM cases could be prevented. Moon JH et al. [37] found that pregnant women with pre-pregnancy BMI ≥ 20.9 are twice as likely to develop GDM as those with pre-pregnancy BMI ≤ 19.1. Berkovitz et al. [38] found that pregnant women with BMI > 32.9 were 2.82 times more likely to develop GDM than those with BMI between 27.3 and 32.9, and 3.82 times more likely than those with BMI < 27.3. This may be because excessive fat tissue and various cytokines secreted by overweight or obese people before pregnancy may easily cause insulin resistance, promote islet β cells to work with high load to maintain blood sugar levels, and then damage islet function, eventually leading to elevated blood sugar and the occurrence of GDM. Meanwhile, obesity is easy to cause inflammation in the body, damages islet cell function, and increases the risk of GDM [39]. The results of this study also support that pre-pregnancy BMI and weight are the risk factors for GDM recurrence. Therefore, it is important to control weight and strengthen diet and exercise intervention for women with a history of GDM who have a need for a second pregnancy.
Inter-pregnancy interval
Based on our combined univariate and multivariate analyses, the association between prolonged IPI and increased risk of GDM recurrence was not statistically significant. Previous studies have been controversial about the association between IPI and GDM recurrence. In two small studies, short IPI (i.e., IPI ≤ 24 months) was shown to increase GDM recurrence [31, 40], which may be related to the failure to completely lose weight or insufficient recovery time of endocrine function after the end of the previous pregnancy. However, in the other two studies [41, 42], longer IPI increased the risk of GDM recurrence, which may be related to damaged islet β cells. Meanwhile, with the prolongation of IPI, physiological function and endocrine function of pregnant women decline, and the body is aging, which results in insulin insensitivity. A study from Australia [43] found that both short and long intervals between pregnancies increased the risk of GDM to some extent, with a U-shaped association between the two. The risk of developing GDM was significantly increased when the IPI was too short (< 18 months), and the difference was statistically significant. However, the relationship between long IPI and GDM was not statistically significant. The biological mechanism of IPI affecting the risk of GDM is still unclear. Some studies [44, 45] believe that women have a physiological ability to adapt to physical changes during pregnancy, and it can last for a period of time after delivery. Nonetheless, when the IPI is too long, this physiological adaptation will gradually weaken and disappear, thus increasing the risk of pregnancy complications such as GDM and gestational hypertension in the second pregnancy. On the other hand, women need enough time for physical recovery after delivery. If the IPI is too short and the recovery time is insufficient, the risk of adverse pregnancy outcomes such as GDM in second pregnancy will increase [46, 47]. In contrast, another study reports that extended IPI is inherently linked to advanced maternal age, a well-established risk factor for GDM. This interconnection creates a compound effect, as both increasing maternal age and longer IPI may synergistically contribute to elevated GDM risk [48].
Glucose status in previous pregnancy
According to the univariate and multivariate analysis of the results, elevated OGTT in the previous pregnancy can increase the risk of GDM recurrence, and the results were statistically significant. OGTT is a load test that can reflect the degree of insulin resistance and the degree of pancreatic β cell secretion in pregnant women [49–51]. The higher the blood glucose, the more severe the insulin resistance and the impaired islet β-cell function, and the higher the risk of GDM recurrence. The cohort study by Schwartz et al. [42] in 2016 found that OGTT blood glucose value in the second trimester of the previous pregnancy was a risk factor for GDM recurrence. The study by Lai Cheng [52] in 2021 found that after excluding other factors, 2hPGD > 9.1 mmol/L, abnormal 1hPG and/or 2hPG, and abnormal FPG + 1hPG and/or 2hPG in the previous pregnancy were independent risk factors for recurrent GDM (P < 0.05). Further analysis of the abnormal type of OGTT in recurrent GDM pregnant women showed that the abnormal type of OGTT in the previous pregnancy would also affect the recurrence of GDM. Compared with FPG abnormality alone, pregnant women with abnormal FPG + 1hPG and (or) 2hPG were 1.38 times more likely to have a higher level of glucose abnormality at the time of GDM recurrence. Abnormal OGTT ≥ 2 items in the previous pregnancy not only affects the recurrence rate of GDM, but also may increase the severity of recurrent GDM, especially in pregnant women with abnormal OGTT FPG combined with 1hPG or 2hPG in the previous pregnancy.
Macrosomia in previous pregnancy
According to univariate and multivariate analysis, macrosomia in previous pregnancy increased the risk of GDM recurrence (P < 0.05), which was consistent with most previous studies. According to the study by Santangeli L [53], the occurrence of macrosomia is linearly related to the hyperglycemia state during pregnancy. When the mean FPG ≤ 5.6 mmol/L, the incidence of macrosomia can be reduced to a level similar to that of non-GDM pregnant women [54, 55]. The mechanism may be that persistent maternal hyperglycemia enters the fetus through the placenta, causing fetal blood glucose to rise and stimulating fetal islet cells to secrete excess insulin. Both factors promote the synthesis of protein and fat in the fetus and inhibit lipolysis, which in turn leads to excessive development of the fetal body and high birth weight of the newborn [56]. In addition, higher maternal blood glucose levels lead to increased activity of multiple insulin-like growth factors and their receptors in both the mother and fetus, and increased nutrient exchange. At the same time, maternal hyperglycemia can also lead to an increase in the number of placental villi and blood vessels, the degree of capillarization and the surface area of blood vessels. These changes promote the exchange of blood glucose between mother and fetus, leading to the occurrence of macrosomia [57]. Therefore, the high blood glucose level of pregnant women with GDM is an important risk factor for macrosomia, and the history of macrosomia delivery also reflects the poor blood glucose control in the previous pregnancy, insulin resistance and severe impairment of islet β cell function, thus increasing the risk of recurrence of GDM.
Insulin use in previous pregnancy
The univariate analysis showed that insulin use in the previous pregnancy was not statistically significant in increasing the risk of GDM recurrence, which was contrary to some previous studies. It may be owing to the small number of included studies that adopted the new diagnostic criteria for GDM. The meta-analysis by Naama Schwartz [58] showed that the risk factors of glucose intolerance during pregnancy (such as insulin use) were the main predictors of GDM recurrence. When GDM pregnant women could not achieve ideal blood glucose levels by receiving nutrition and exercise guidance alone, insulin therapy was necessary. This treatment can reduce the risk of pregnancy complications such as gestational hypertension, macrosomia, and abortion events by enhancing glycemic control. Furthermore, appropriate insulin therapy supports optimal fetal growth, improve maternal and fetal prognosis, ensure the smooth delivery of the fetus, and reduce adverse pregnancy outcomes such as premature delivery and stillbirth [59]. However, when the previous pregnancy required insulin treatment, it is suggested that the blood sugar control is not good. Aiyue Chen [13] found that the OR value of insulin use in the previous pregnancy for GDM recurrence was 2.114, indicating that limited islet cell compensatory function increased the risk of GDM recurrence in the second pregnancy. Nevertheless, some studies [31] showed that there was no difference in the proportion of insulin use in the previous pregnancy between the recurrent and non-recurrent GDM groups (P > 0.05). Therefore, whether the use of insulin in the previous pregnancy is a risk factor for GDM recurrence needs to be analyzed with a larger sample size.
Conclusions
The results of this study showed that the recurrence rate of GDM was 48%. In women with a history of GDM, advanced pregnancy (age ≥ 35 years), increased BMI before second pregnancy, increased OGTT levels in the previous pregnancy, and the previous delivery of macrosomia may increase the risk of GDM recurrence, and the results were statistically significant. Women of reproductive age can prevent the recurrence of GDM by avoiding pregnancy at advanced age(≥35 years old), maintaining a healthy BMI, and managing blood glucose levels during previous pregnancies.
Advantages and innovations: In this study, the total sample size was expanded, and the reliability of data was improved by appropriate combinations. This study established a more reasonable literature inclusion criteria and exclusion criteria. In view of the contradictory results among different studies, more consistent results are obtained after comprehensive processing.
Limitations
Most of the risk factors analyzed in this paper were heterogeneous, but it was difficult to perform further subgroup analysis because of the small number of included studies. The evaluation of publication bias was also open to question. Some factors with less data were not included in this study. Since the diagnostic criteria were based on the IADPAG criteria established in 2011, the previous literature before 2011 and the literature that did not use this standard were excluded, which reduced the number of included studies to some extent. In addition, the studies in regions where the criteria were not adopted were excluded, resulting in the inclusion of studies mostly from China and the occurrence of population selection bias to a certain extent. Furthermore, most studies did not account for diabetes screening after the first birth, and it was possible that some of the women in subsequent pregnancies had type 2 diabetes, thereby increasing the number of members in the relapsed cohort and affecting the incidence.
Outlook
With the full opening of China’s fertility policy, the proportion of pregnant women with the risk of GDM recurrence is gradually increasing, and how to prevent GDM recurrence should be paid attention to. Multi-regional, multi-center, and large sample size studies are warranted to further identify other risk factors. It is also hoped that the summary and analysis of this study can provide a reference for the development of strategies to prevent and reduce the recurrence rate of GDM.
Acknowledgements
Not applicable.
Abbreviations
- GDM
Gestational diabetes mellitus
- IPI
Inter-pregnancy interval
- NOS
Newcastle-Ottawa scale
Author contributions
Conceptualization: Yuan Lu; Methodology: Yuan Lu, Yu Long; Formal analysis and investigation: Yuan Lu, Jianchun Huang, Jian Yan; Writing - original draft preparation: Yuan Lu, Jianchun Huang; Writing - review and editing: Yu Long; Funding acquisition: Jian Yan; Resources: Meirong He, Chunlan Yuan; Supervision: Qingfang Wei, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that they did not receive any funding from any source.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuan Lu and Jianchun Huang contributed equally to this work and should be considered as co-first authors.
References
- 1.Feng R, Shao H, Zhu H, Xie J. Meta-analysis of risk factors of gestational diabetes mellitus. Maternal Child Health Care China. 2014;29(17):2824–7. [Google Scholar]
- 2.Wang X, Liu M, Zhang M, Lou J. Recurrence rate of gestational diabetes mellitus, risk factors of recurrence and maternal and infant prognosis of recurrent gestational diabetes mellitus. Med Innov China. 2019;16(25):44–7. [Google Scholar]
- 3.Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314–9. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz N, Nachum Z, Green MS. The prevalence of gestational diabetes mellitus recurrence–effect of ethnicity and parity: a metaanalysis. Am J Obstet Gynecol. 2015;213(3):310–7. [DOI] [PubMed] [Google Scholar]
- 5.Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin Perinatol. 2007;31(3):176–84. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal MM. Gestational diabetes mellitus: an update on the current international diagnostic criteria. World J Diabetes. 2015;6(6):782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnostic criteria and classification. Of hyperglycaemia first detected in pregnancy: a world health organization guideline. Diabetes Res Clin Pract. 2014;103(3):341–63. [DOI] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 9.Jian M. Risk factors for recurrent gestational diabetes mellitus. 硕士. Inner Mongolia Medical University; 2022.
- 10.Wang Y. Clinical study on the risk factors for gestational diabetes mellitus in successive pregnancies. Shanghai Jiao Tong University; 2018.
- 11.Wang N. Recurrence of gestational diabetes in primiparous women in northern Zhejiang, China: risks factors and implications. 硕士. Zhejiang University; 2020.
- 12.Zhang J, Yang C, Han Y, Zhou W, Shi H. Risk factors analysis of recurrence of gestational diabetes mellitus in pregnant women. Chin Nurs Manage. 2021;21(04):498–502. [Google Scholar]
- 13.Chen A, Yuan J, Guo Y. Analysis of risk factors for recurrent gestational diabetes mellitus. New Med. 2019;50(09):696–9. [Google Scholar]
- 14.Wu R, Gong J, Liu Y, Liu Y. Risk factors analysis of recurrent gestational diabetes mellitus. J Mol Imaging. 2021;44(04):729–32. [Google Scholar]
- 15.Li T. Influence of clinical characteristics of previous gestational diabetes mellitus on recurrent diabetes mellitus in second pregnancy. Electron J Practical Gynecologic Endocrinol. 2020;7(20):63–4. [Google Scholar]
- 16.Cheng L, Yang M, Shao J. Effect of clinical features of previous gestational diabetes of women and the influence of their recurrent of gestational diabetes mellitus. Chin J Family Plann. 2021;29(1):193–7. [Google Scholar]
- 17.Chen Q. Precautions and analysis of patients with gestational diabetes mellitus (GDM) during childbirth. Diabetes New World. 2018;21(18):28–9. [Google Scholar]
- 18.Liu M, Wang X, Zhang M, Li W. Analysis of recurrence rate of gestational diabetes mellitus,risk factors for recurrence and maternal and infant prognosis of recurrent gestational diabetes mellitus. J Clin Res. 2018;35(5):845–8. [Google Scholar]
- 19.Zhao J, Lv L, Xu L, Yuan L, Wen J, Yin W, Huang C. Blood glucose and associated risk factors of women with history of gestational diabetes mellitus. Chin J Woman Child Health Res. 2018;29(09):1158–61. [Google Scholar]
- 20.Zhou Y. Risk factors of recurrent gestational diabetes mellitus. Women’s Health. 2021;4:111. [Google Scholar]
- 21.Li L, Shi G, Zhang X, Wang H, He S. Analysis and Intervention of Factors Affecting Abnormal Postpartum Glucose Tolerance and Gestational Recurrence in Gestational Diabetes. Evid Based Complement Alternat Med 2021, 2021:8470944. [DOI] [PMC free article] [PubMed] [Retracted]
- 22.Hahn S, Körber S, Gerber B, Stubert J. Prediction of recurrent gestational diabetes mellitus: a retrospective cohort study. Arch Gynecol Obstet. 2023;307(3):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotzaeridi G, Blätter J, Eppel D, Rosicky I, Falcone V, Adamczyk G, Linder T, Yerlikaya-Schatten G, Weisshaupt K, Henrich W et al. Recurrence of gestational diabetes mellitus: to assess glucose metabolism and clinical risk factors at the beginning of a subsequent pregnancy. J Clin Med 2021, 10(20). [DOI] [PMC free article] [PubMed]
- 24.Wei X, Hu R, Gao Y, Yu Z, Zhang X. Risk Factors for Recurrence of Gestational Diabetes Mellitus and Its Correlation with Maternal and Infant Prognosis. Evid Based Complement Alternat Med 2022, 2022:7237777. [DOI] [PMC free article] [PubMed] [Retracted]
- 25.Liang Y, Gong J, Chen X, Li G, Lin X, He J, Chen Y, Wu R. Risk factors for recurrence of gestational diabetes mellitus in Southern Chinese women: a retrospective study. Ginekol Pol. 2023;94(5):350–7. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, Juan J, Su R, Song G, Chen X, Shan R, Li Y, Cui S, Fan S, Feng L, et al. Risk of gestational diabetes recurrence and the development of type 2 diabetes among women with a history of gestational diabetes and risk factors: a study among 18 clinical centers in China. Chin Med J (Engl). 2022;135(6):665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu WW, Fan L, Yang HX, Kong LY, Su SP, Wang ZL, Hu YL, Zhang MH, Sun LZ, Mi Y, et al. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care. 2013;36(7):2038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Yang H, Zhu W, Yang H, Li H, Yan J, Zhang C. International association of diabetes and pregnancy study group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J (Engl). 2014;127(20):3553–6. [PubMed] [Google Scholar]
- 29.Li C. Comparative analysis of multiple pregnancy outcomes in patients with gestational diabetes mellitus. China Foreign Med Treat. 2009;28(35):30–1. [Google Scholar]
- 30.Bernstein J, Lee-Parritz A, Quinn E, Ameli O, Craig M, Heeren T, Iverson R, Jack B, McCloskey L. After gestational diabetes: impact of pregnancy interval on recurrence and type 2 diabetes. Biores Open Access. 2019;8(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nohira T, Kim S, Nakai H, Okabe K, Nohira T, Yoneyama K. Recurrence of gestational diabetes mellitus: rates and risk factors from initial GDM and one abnormal GTT value. Diabetes Res Clin Pract. 2006;71(1):75–81. [DOI] [PubMed] [Google Scholar]
- 32.Kwak SH, Kim HS, Choi SH, Lim S, Cho YM, Park KS, Jang HC, Kim MY, Cho NH, Metzger BE. Subsequent pregnancy after gestational diabetes mellitus: frequency and risk factors for recurrence in Korean women. Diabetes Care. 2008;31(9):1867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lao TT, Ho LF, Chan BC, Leung WC. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care. 2006;29(4):948–9. [DOI] [PubMed] [Google Scholar]
- 34.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papachatzi E, Dimitriou G, Dimitropoulos K, Vantarakis A. Pre-pregnancy obesity: maternal, neonatal and childhood outcomes. J Neonatal Perinat Med. 2013;6(3):203–16. [DOI] [PubMed] [Google Scholar]
- 36.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100(6):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, Park KS, Jang HC, Cho NH. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015;100(9):3548–55. [DOI] [PubMed] [Google Scholar]
- 38.Berkovitz A, Biron-Shental T, Pasternak Y, Sharony R, Hershko-Klement A, Wiser A. Predictors of twin pregnancy after ovarian stimulation and intrauterine insemination in women with unexplained infertility. Hum Fertil (Camb). 2017;20(3):200–3. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Shui J, Wang X, Lu Y, Shen M. Analysis of influencing factors of gestational diabetes in older pregnant women. J Practical Gynecologic Endocrinol. 2022;9(19):24–6. [Google Scholar]
- 40.Major CA, deVeciana M, Weeks J, Morgan MA. Recurrence of gestational diabetes: who is at risk? Am J Obstet Gynecol. 1998;179(4):1038–42. [DOI] [PubMed] [Google Scholar]
- 41.Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabet Med. 2013;30(4):452–6. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz N, Green MS, Yefet E, Nachum Z. Modifiable risk factors for gestational diabetes recurrence. Endocrine. 2016;54(3):714–22. [DOI] [PubMed] [Google Scholar]
- 43.Gebremedhin AT, Tessema GA, Regan AK, Pereira GF. Association between interpregnancy interval and pregnancy complications by history of complications: a population-based cohort study. BMJ Open. 2021;11(12):e046962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340(8):589–94. [DOI] [PubMed] [Google Scholar]
- 45.Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re-evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkvist A, Rasmussen KM, Habicht JP. A new definition of maternal depletion syndrome. Am J Public Health. 1992;82(5):691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129(3):408–15. [DOI] [PubMed] [Google Scholar]
- 48.Gebremedhin AT, Regan AK, Ball S, Betrán AP, Foo D, Gissler M, Håberg SE, Malacova E, Marinovich ML, Pereira G. Effect of interpregnancy interval on gestational diabetes: a retrospective matched cohort study. Ann Epidemiol. 2019;39:33–e3833. [DOI] [PubMed] [Google Scholar]
- 49.Huang X. Observation on the effect of enhanced blood glucose control on pregnancy outcomes of mothers with gestational diabetes mellitus and their infants. Maternal Child Health Care China. 2016;31(24):5312–4. [Google Scholar]
- 50.Wang N, Zhou H. Effect of early screening and intervention of gestational diabetes mellitus on maternal and infant perinatal outcomes. Maternal Child Health Care China. 2015;30(30):5130–1. [Google Scholar]
- 51.Li F. Correlation between blood glucose control level of gestational diabetes mellitus and maternal and infant pregnancy outcomes. Contemp Med. 2018;24(33):178–9. [Google Scholar]
- 52.Cheng L, Yang M, Shao J. Effect of clinical features of previous gestational diabetes of women and the influence of their recurrent of gestational diabetes mellitus. Chin J Family Plann. 2021;29(01):193–7. [Google Scholar]
- 53.Santangeli L, Sattar N, Huda SS. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):438–48. [DOI] [PubMed] [Google Scholar]
- 54.Langer O, Conway DL. Level of glycemia and perinatal outcome in pregestational diabetes. J Matern Fetal Med. 2000;9(1):35–41. [DOI] [PubMed] [Google Scholar]
- 55.Cyganek K, Skupien J, Katra B, Hebda-Szydlo A, Janas I, Trznadel-Morawska I, Witek P, Kozek E, Malecki MT. Risk of macrosomia remains glucose-dependent in a cohort of women with pregestational type 1 diabetes and good glycemic control. Endocrine. 2017;55(2):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20. [DOI] [PubMed] [Google Scholar]
- 57.Chen M, Yan S, Gao G, Cao H, Lei X, Zhang W, Ren S, Wang Y, Zhang B, Han Y, et al. Association analysis of abnormal glucose metabolism during pregnancy and macrosomia. Chongqing Med. 2019;48(23):4128–30. [Google Scholar]
- 58.Schwartz N, Nachum Z, Green MS. Risk factors of gestational diabetes mellitus recurrence: a meta-analysis. Endocr 2016, 53(3):662–71. [DOI] [PubMed]
- 59.Xu S, Zhen M, Long X. Effect and safety analysis of insulin therapy on pregnancy outcome in pregnant women with gestational diabetes mellitus. Guangzhou Med J. 2021;52(06):39–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.