Abstract
Background:
Eye function is vitally dependent on an adequate blood supply, primarily provided by the ophthalmic artery, an internal carotid artery branch. This review provides an overview of the vascular supply of the eye.
Methods:
A targeted search of PubMed / MEDLINE was performed using the terms “central retinal vein,” “central retinal artery,” “internal carotid artery,” “ophthalmic artery,” “ophthalmic vein,” “posterior ciliary arteries,” “retinal capillaries,” “vascular supply of the eye,” “ocular vascular supply,” “external carotid artery,” and “vortex vein”. Studies published between 1960 and 2024 were reviewed. Relevant references cited in these publications were also analyzed.
Results:
Overall, 62 publications were reviewed. The ophthalmic artery branches into several arteries—the central retinal artery supplies the retina, whereas the posterior ciliary arteries supply the posterior choroid and optic nerve. The anterior ciliary arteries mainly supply the conjunctiva, sclera, ciliary body, and iris. Extraocular muscles receive their primary blood supply from the muscular branches of the ophthalmic artery, lacrimal artery, and infraorbital artery. The lacrimal gland is perfused by the lacrimal artery. The eyelids receive blood from both the internal and external carotid arteries. The superficial vascular network of the medial eyelid skin is established primarily through anastomoses between the branches of the internal carotid artery. The superficial vascular network of the lateral upper and lower eyelids is primarily derived from branches emanating from the superficial temporal artery (a branch of the external carotid artery) and the lacrimal artery. Venous drainage follows a complex pathway, beginning with the central retinal vein and the vortex veins, then draining into the ophthalmic veins, and finally into the internal jugular vein.
Conclusions:
The eye features a complex arterial supply and venous drainage that can vary greatly among individuals. This complex vascular system is critical for the oxygenation and nutrition of ocular tissues and the maintenance of ocular health. The arterial and venous circulation coordinate to support different regions of the eye, including the retina, choroid, and optic nerve. Understanding this intricate vascular network is essential for the diagnosis and treatment of various ocular pathologies. Abnormalities in these pathways can cause substantial problems, including vision loss.
Key Words: blood vessel, eyes, central retinal vein, central retinal artery, internal carotid artery, ophthalmic arteries, retinal veins, ophthalmic vein, ciliary artery, uveas, capillary, vein
INTRODUCTION
The ophthalmic artery (OA) is the primary source of blood perfusion of the eye and orbit and originates as the first branch of the internal carotid artery (ICA) [1, 2]. The ICA arises from the common carotid artery within the cervical soft tissues, typically at the level of the C3 to C5 vertebrae [2]. The common carotid artery bifurcates into two main branches: the ICA and the external carotid artery (ECA). The ECA primarily supplies blood to the face, scalp, and cervical viscera, whereas the ICA delivers blood to the intracranial structures, including the eye and orbit via the OA [1].
In line with the Bouthillier classification system, the ICA is divided into different anatomical segments according to its course and branches [3]. These include the first (cervical) segment, which gives rise to minor branches; the second (petrous) segment, giving rise to the caroticotympanic and vidian arteries; the third (lacerum) segment; the fourth (cavernous) segment, which gives rise to the meningohypophyseal trunk and the inferolateral trunk; the fifth (clinoid) segment; the sixth (ophthalmic) segment, from which the OA and the superior hypophyseal artery arise; and the seventh (communicating) segment, which provides origin to the anterior choroidal artery and the posterior communicating artery. Thereafter, the ICA bifurcates into its terminal branches. The OA is classified as the fifth branch of the ICA [1-3]. Figure 1 displays magnetic resonance images of the OA arising from the ICA.
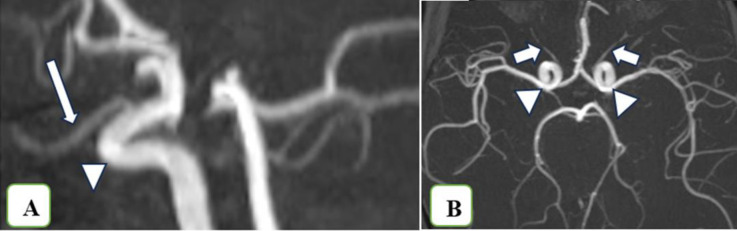
Figure 1.
Time-of-flight magnetic resonance imaging (1.5 Tesla; Siemens, Erlangen, Germany) in the sagittal (A) and axial (B) planes, demonstrating the ophthalmic artery (arrows) originating from the internal carotid artery (arrowheads).
Ophthalmic artery
The OA is the first intracranial branch of the ICA and arises immediately as the ICA exits the cavernous sinus [4-7]. The OA is anatomically divided into three portions according to its course: the intracranial, intracanalicular, and intraorbital parts [4-7]. Digital subtraction angiographic images of the segments of the OA are shown in Figure 2.
Figure 2.
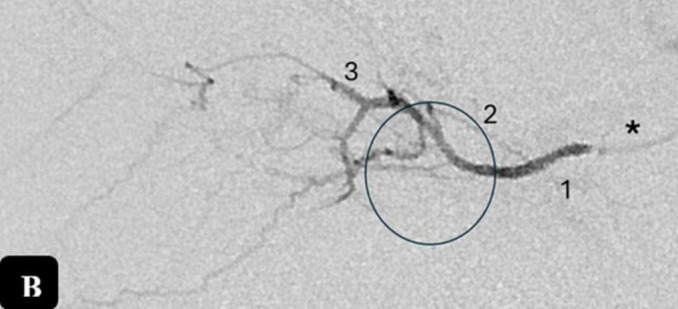
Digital subtraction angiography (Bi-plane Rotational Angiography, Artis Zee; Siemens, Erlangen, Germany) demonstrates the OA in the late arterial phase in the lateral projection (A) in a two-year-old patient. A lateral angiogram (B) was obtained by superselective contrast injection into the OA (*) in a 2-year-old patient with bilateral retinoblastoma (Group C based on the International Intraocular Retinoblastoma Classification) during intraarterial chemotherapy. The first segment (1) of the OA corresponds to the segment coursing inferolateral to the optic nerve after its origin from the supraclinoid ICA and piercing the dura. The second segment (2) bends around the optic nerve. From this segment the ocular complex arises, comprising the central retinal artery and ciliary arteries (black circles, A and B). The third segment (3) of the OA after its bend under or over the nerve, which courses medially and parallel to it. Normal choroidal blush (A, arrowheads); anterior falcine artery (A, arrow). Abbreviations: ICA, internal carotid artery; L, lacrimal artery; OA, ophthalmic artery.
Intracranial course and branches: The OA has a short intracranial course of approximately 0.5 to 0.95 mm before it pierces the dura and enters the optic canal [8, 9]. This region is particularly important in surgeries, specifically in the management of OA aneurysms [8]. The path of the OA is not linear between its origin and the optic canal, and it typically forms one or two distinct curves. The major branches in this area include the deep recurrent OA, an embryonic remnant of the dorsal OA that connects with a branch of the inferolateral trunk, and the superficial recurrent OA, which anastomoses with the marginal tentorial artery [8-10].
Intracanalicular course: After completing its intracranial course, the OA passes beneath the posterior edge of the falciform ligament, pierces the dura mater of the optic nerve—typically on its inferior and lateral sides—and enters the optic canal with the optic nerve. The optic canal itself is relatively short, measuring between 5 and 7 mm in length [10-12]. In approximately 6.7% of cases, the OA arises from the ICA anterior to the falciform ligament. In very rare occasions (<3%), the OA enters the orbit separate from the optic nerve through the superior orbital fissure or a distinct bony canal (duplicate optic canal) [10]. Within the optic canal, the optic nerve is attached to the surrounding dura by fibrous bands, which contain the small branches of the OA. In individuals sustaining canal fractures, tearing of these fibrous bands can disrupt the vessels they enclose, leading to ischemic damage to the optic nerve [12].
Intraorbital course and branches: The OA enters the orbit at its apex, typically through the optic canal. Its intraorbital course is divided into three segments. Along the way, the artery changes direction at two key points: the junction between the first and second segments, and the junction between the second and third segments [12].
The first intraorbital segment of the OA extends from its entry into the orbit to the point at which it bends to form the second segment. This portion of the artery typically lies near the inferolateral aspect of the optic nerve, to which it adheres primarily to fat and loose connective tissue, although firm attachment to the nerve is uncommon. If the middle meningeal artery is the sole or primary source of blood supply, this segment of the OA is often reduced in caliber [12].
The second segment of the OA crosses the optic nerve to lie medially in most cases, passing over it in 83% of cases and under it in 17% of cases. In a study investigating this crossing pattern in both orbits of the same individual, the artery crossed over the optic nerve bilaterally in 70% of cases, the artery crossed under the nerve bilaterally in 5%, and in the remaining cases, the crossing pattern differed between the two orbits [12]. The second segment lies near the optic nerve but is loosely attached to its dural sheath [12].
The third segment of the OA courses anteriorly, medial to the optic nerve. Unlike the first two segments, it is not closely associated with the optic nerve. This segment traces anteriorly above the medial rectus muscle and below the superior oblique muscle, reaching the medial orbital wall near the anterior ethmoid foramen. The third segment is the most tortuous in most cases. It is typically anchored to the medial orbital wall by the short, thick trunk of the anterior ethmoidal artery (AEA). From there it continues anteriorly, adjacent to the medial orbital wall, passing beneath the trochlea, and then typically ascending and moving anteriorly to a position approximately midway between the medial palpebral ligament and the orbital rim. Occasionally, a tortuous loop is present just before the artery terminates [12].
The OA typically terminates at the superomedial angle of the orbital opening, where it bifurcates into the supratrochlear and dorsal nasal branches. However, in a histopathological study by Hayreh et al. [12], in 26% of the specimens, the main portion of the OA terminated at the level of the anterior ethmoid foramen. In these cases, the artery is bifurcated into two trunks: one forming the AEA and the other continuing as the OA toward the superomedial angle of the orbital orifice [12-14].
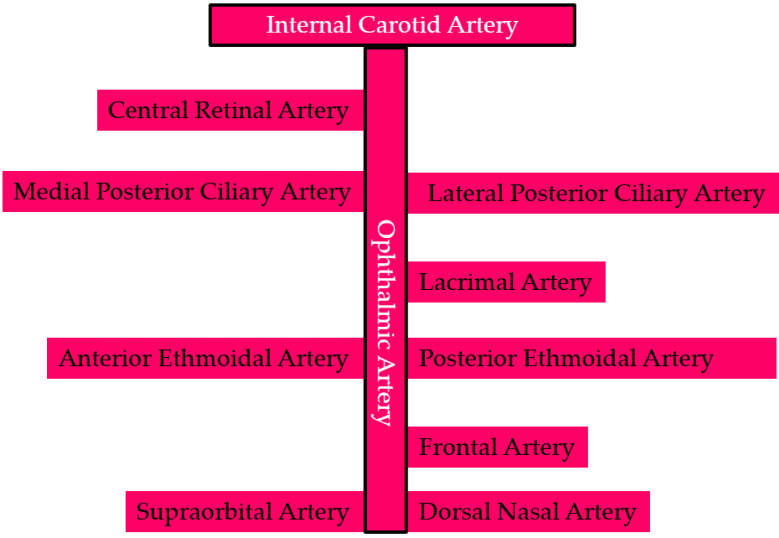
Because of the critical importance of the globe, the branches of the OA are commonly divided into two distinct groups: the ocular group, which supplies blood to the globe, and the orbital group, which supplies blood to non-ocular orbital structures. There is substantial inter-individual variability in the branching pattern of the OA, which is better described as a “common pattern” than a fixed one [10, 12, 14]. The ocular branches include the central retinal artery (CRA), the lateral posterior ciliary artery (PCA), and the medial PCA. These branches typically arise near the junction of the first and second segments of the OA, with no ocular branches arising distal to the second segment [10, 12, 14]. A schematic illustration of the OA and its branching pattern is shown in Figure 3.
Figure 3.
Schematic illustration of the ophthalmic artery and its branching pattern [8, 10, 12-19].
Central retinal artery: The CRA is the first branch of the OA in 77.5% of cases. It arises from the first segment of the intraorbital OA in 22.1% of cases, from the junction of the first and second segments in 58.7%, and from the second segment in 18.3%. The CRA arises independently in 37.5% of cases, in a common trunk with the medial PCA; with the lateral PCA in 11.5%; and with both the medial and lateral PCA in 1.9%. Following a tortuous course, the CRA pierces the dura mater of the optic nerve, typically at its inferomedial aspect, before entering the retina [8, 10, 12, 14]. In less than 2% of cases, the artery is duplicated. The length of the CRA ranges from 7 to 20 mm and the diameter is 0.1 to 0.6 mm. The CRA enters the optic nerve medially, approximately 10 mm posterior to the globe. Upon reaching the retina, it bifurcates into superior and inferior branches, which further subdivide into temporal and nasal branches [10, 12, 14-16]. Proximity of the CRA origin to the ciliary arteries produces the “choroidal brush” formed by the ciliary arteries, a recognized landmark for identifying the CRA [17]. Figure 4 presents an image from the arterial phase of fluorescein angiography showing the CRA and its branching pattern.
Figure 4.
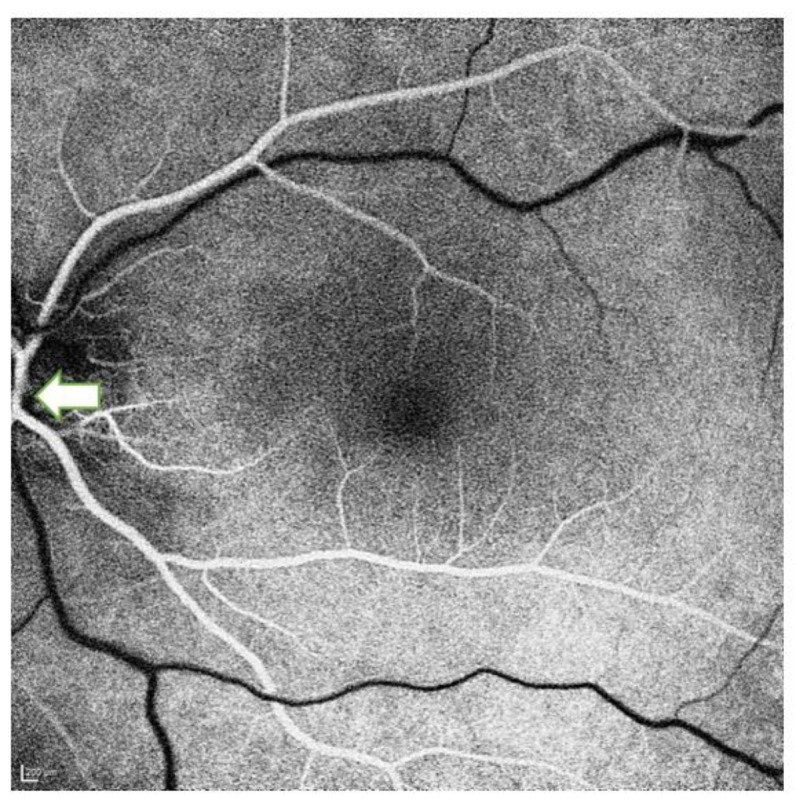
Arterial phase of fluorescein angiography in a 45-year-old healthy man, demonstrating the central retinal artery (white arrow) and its branching pattern.
Posterior ciliary arteries: The PCAs, branches of the OA, are the primary vascular supply to the choroid. They are classified as medial or lateral according to their anatomical location. Their number varies from one to five, with two or three present in 80% of cases. Specifically, a single PCA (always the medial) is observed in 3% of cases, two in 39%, three in 48%, four in 8%, and five in 2%. The presence of a superior PCA varies in frequency [8, 10, 18]. Lateral PCAs are observed in 97% of eyes, with a single artery present in 75%, two arteries in 20%, and three arteries in 2% of cases. Medial PCAs are found in 100% of eyes, with one artery in 71% and two arteries in 29% of cases [19].
The PCAs bifurcate into two distinct branches. The short PCAs can range from ten to twenty in number, depending on the intraorbital subdivisions of the PCA before it enters the sclera. These short PCAs further divide into two vessel types: para-optic short PCAs and distal short PCAs [19]. Para-optic short PCAs are limited in number and small in size, penetrating the sclera near the optic nerve. In contrast, distal short PCAs represent most of the short PCAs; they penetrate the sclera bilaterally, slightly away from the optic nerve, and extend radially toward the equator of the eye. In particular, the temporal distal short PCAs traverse the sclera to enter the macular region. The second type of PCA branch—long PCAs—consists of two arteries, one medial and one lateral [19]. These long PCAs penetrate the eyeball along the horizontal plane on their respective medial and lateral sides, maintaining a distance from the distal short PCAs. Once inside the globe, they extend radially along the horizontal meridian toward the iris. This anatomical arrangement ensures a comprehensive vascular supply to both the anterior and posterior segments of the eye, facilitating essential functions such as nutrient delivery and waste removal [19].
After originating from the OA, the PCAs divide into several short branches that supply the proximal choroid and the optic nerve head (ONH). These branches pierce the sclera and continue as long PCAs that supply blood to the distal choroid, iris, and ciliary body. The long PCAs, together with the medial PCAs, are typically 0.5 to 0.7 mm in diameter. The PCAs also anastomose posterior to the lamina cribrosa, forming the arterial ring known as the circle of Zinn, which is critical for the blood supply to the ONH [8, 13, 18].
Lacrimal artery: The lacrimal artery (LA) primarily supplies the lacrimal gland, adjacent muscles, and surrounding periorbital tissues [8, 10, 14, 20]. It is approximately 0.7 mm in diameter. The main branches of the LA are the glandular artery (dedicated to the lacrimal gland), the lateral palpebral artery (supplying the lateral eyelids), the recurrent meningeal branch (a small branch that anastomoses with the middle meningeal artery), and the muscular branches (supplying the nearby extraocular muscles) [8, 10, 14, 20].
Muscular branches: The OA gives rise to the medial and lateral muscular arteries, which supply the extraocular muscles [21]. The medial branch is typically larger than the lateral [21].
Anterior ciliary arteries: The anterior ciliary arteries (ACAs), which branch from the muscular arteries, provide a vital blood supply to the anterior segment of the eye. Seven ACAs course along the extraocular muscles [21], supplying the rectus muscles, conjunctiva, and sclera. They also contribute to the great arterial circle of the iris by joining the long PCAs. Each rectus muscle is supplied by two ACAs, except for the lateral rectus, which is supplied by one ACA [21].
Ethmoidal arteries: The OA gives rise to two ethmoidal arteries: anterior (AEA) and posterior (PEA). The AEA is usually larger than the PEA and passes through the anterior ethmoid foramen to enter the cranial cavity, where it becomes the anterior meningeal artery. It supplies the ethmoid air cells, periosteum, and adjacent structures [10, 13]. The diameter of the AEA is approximately 0.6 mm. The PEA supplies the superior oblique muscle, the superior and medial recti, and the levator palpebrae superioris. It occasionally contributes to the blood supply of the nasal cavity, ethmoid air sinuses, sphenoid sinus (rarely), and falx cerebri and dura in the anterior fossa. It also nourishes the periosteum and areolar tissue in the orbit. The diameter of the PEA is approximately 0.4 mm [10, 13].
Supraorbital, frontal, and dorsal nasal arteries: These are distinct branches of the OA that supply only periorbital and extracranial tissues without vascularizing the globe or intracranial structures. The relatively small supraorbital artery travels along the superior aspect of the orbit, providing smaller muscular branches and nourishing areolar tissues before terminating in the scalp [8, 10]. The frontal and dorsal nasal arteries are the terminal branches of the OA, as it leaves the orbit, supplying the frontal scalp and nose, respectively [8, 10]. These arteries are crucial in supporting the superficial tissues of the face and periorbital region [8, 10].
This review provides a detailed summary of the ocular vasculature, emphasizing the arterial supply and venous drainage pathways that maintain the functional integrity of the eye and its adnexa.
METHODS
A detailed search of the PubMed / MEDLINE database covering the years 1960 to 2024 was performed using the following keywords: “central retinal vein,” “central retinal artery,” “internal carotid artery,” “ophthalmic artery,” “ophthalmic vein,” “posterior ciliary arteries,” “retinal capillaries,” “vascular supply of the eye,” “ocular vascular supply,” “external carotid artery,” and “vortex vein”. Relevant studies were identified, and additional articles were selected by examining the reference lists of included publications. Only English-language articles were considered for this review.
This narrative review was ethically approved at the department level. The figures shared in this article were sourced from our unit’s comprehensive patient documentation archive. Patients provided informed consent prior to the inclusion of figures in this review.
RESULTS
A review was performed on 62 articles identified by targeted searches using specific keywords and from the reference lists of these articles. Two senior ophthalmologists (O.K., A.O.S.) carefully reviewed all included publications. The arterial supply and venous drainage of the ocular structures were analyzed in detail, systematically examining each anatomical segment to ensure a thorough understanding and accurate representation of the ocular vascular network. The arterial supply and venous drainage of the major ocular structures are outlined in Table 1 [22-62].
Table 1.
| Ocular structures | Arterial supply | Venous drainage | |
|---|---|---|---|
| Optic nerve | CRA (branch of OA) and PCA | CRV and vortex veins drain into SOV/IOV | |
| Uvea | Choroid | Short PCA (multiple branches of OA) | Vortex veins drain into SOV/IOV |
| Ciliary Body | Long PCA and ACA | ACV and vortex veins | |
| Iris | MACI (formed by long PCA and ACA) | ACV and vortex veins | |
| Sclera and episclera | PCA and ACA | Episcleral veins draining into ACV | |
| Conjunctiva | ACA and palpebral branches of lacrimal and OA | Conjunctival veins draining into ACV | |
| Extraocular muscles | Muscular branches of OA, LA, and infraorbital artery | SOV and IOV | |
| Lacrimal gland | LA | Lacrimal veins drain into ophthalmic veins | |
| Eyelid | Branches of OA (supraorbital, supratrochlear, dorsal nasal arteries, and LA) and ECA (facial artery, superficial temporal artery, and infraorbital artery) | Ophthalmic and facial veins | |
Abbreviations: CRA, central retinal artery; OA, ophthalmic artery; PCA, posterior ciliary artery; CRV, central retinal vein; SOV, superior ophthalmic vein; IOV, inferior ophthalmic vein; ACA, anterior ciliary arteries; ACV, Anterior ciliary veins; MACI, major arterial circle of the iris; LA, lacrimal artery; ECA, external carotid artery.
Discussion
Vascular Supply of the Eye by Structure
The primary vascular supply to the eyeball is the OA, which, through its branches, supplies the various layers and structures of the eye. The vascular supply and corresponding branches to these structures are outlined as follows [22-50].
Optic nerve
The ONH has a complex vascular supply that is not yet fully understood. Over the past five decades, researchers have investigated the contributions of the choroid, short PCAs, and CRA to the vascularization of the inner retinal, prelaminar, laminar, and retrolaminar regions of the ONH [22]. Short PCAs provide the primary arterial supply to the anterior optic nerve. The CRA predominantly supplies the retina and superficial layers of the anterior optic nerve. Pial arteries arising from the CRA, OA, and PCAs may contribute to the vascularization of the anterior optic nerve [23, 24].
The anterior optic nerve is divided into four distinct anatomical regions: the superficial nerve fiber layer (NFL) and the prelaminar, laminar, and retrolaminar regions, each with unique vascular characteristics [22-25]. The superficial NFL receives blood primarily from arterioles derived from the peripapillary branches of the retinal arteries.
These vessels, featuring non-fenestrated capillaries, are an integral part of the blood-ocular barrier because of the tight junctions between their endothelial cells [23, 25]. Notably, this region has no direct vascular contribution from the choroid. The prelaminar region is directly vascularized by branches of the short PCAs and vessels from the Zinn–Haller arterial circle. Although some branches of the short PCAs cross the choroid in route to the prelaminar region, they are not of choroidal origin but merely traverse the choroidal layer. The arterial supply of the laminar region is similar to that of the prelaminar region, with short PCAs as the primary source, either directly or via the Zinn–Haller arterial circle [23, 25]. Precapillary arterioles enter the outer lamina cribrosa and branch into an interseptal capillary network within this region. In addition, small arterioles from the peripapillary choroid may occasionally contribute to the laminar vascular supply. The retrolaminar region is mainly supplied by the branches of pial arteries and the short PCA system. The CRA does not normally supply the prelaminar or laminar regions, but may make minor contributions through the small branches within the retrolaminar optic nerve [23, 25].
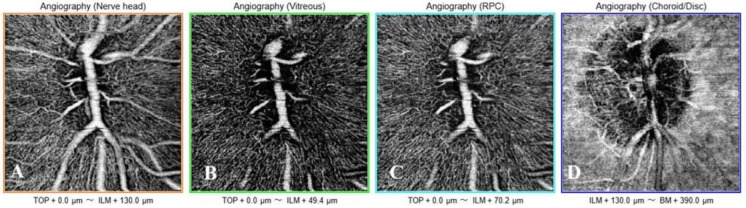
Optical coherence tomography (OCT) angiography (OCTA) is an advanced imaging modality that enables noninvasive visualization of large blood vessels and the microvasculature within the retina, ONH, and portions of the choriocapillaris by acquiring multiple scans of the same area [26]. Modern OCTA devices can capture detailed images of the macula and optic disc regions. The scans are divided into distinct layers, or slabs, to facilitate the detailed analysis, with segmentation methods varying across different OCTA systems [26]. The radial peripapillary capillary (RPC) slab focuses on the vasculature within the retinal NFL, extending from the internal limiting membrane to the posterior boundary of the retinal NFL. In contrast, the “choroidal” slab highlights the deeper retinal and choroidal vasculature in the parapapillary region, offering insights into the vascular structure beyond the retinal NFL [26]. Images of the full disc, vitreous, RPC, and choroid/disc slabs obtained using the OCTA device are shown in Figure 5.
Figure 5.
Optic nerve head optical coherence tomography angiography (OCTA) images (3 × 3 mm) obtained using the Triton™ DRI swept-source optical coherence tomography instrument (SS-OCTA, DRI OCT Triton, Topcon Corp., Tokyo, Japan) in a 22-year-old healthy woman. OCTA images show the nerve head (A), vitreous (B), RPC (C), and choroid/disc (D) slabs. Abbreviations: µm, micrometers; ILM, internal limiting membrane; BM, Bruch’s membrane; RPC, radial peripapillary capillary.
Retina
Retinal blood is supplied by two distinct vascular systems: the CRA and the choriocapillaris. The CRA nourishes the inner retina, whereas the choriocapillaris supports the retinal pigment epithelium and outer retina, which consists mainly of photoreceptors [27]. After passing along the lower edge of the optic nerve sheath, the CRA enters the eye at the center of the optic nerve. The CRA traverses the lamina cribrosa and enters the ONH slightly nasal to its center. Upon entry, it divides into superior and inferior branches. These branches further divide into nasal and temporal branches, which continue to bifurcate within the retinal NFL, supplying the inner retinal layers. Within the inner retina, vessels branch into three capillary networks that supply the inner retinal neurons. In contrast, the avascular photoreceptor layer relies on oxygen diffusion from the underlying choriocapillaris through the retinal pigment epithelium [27].
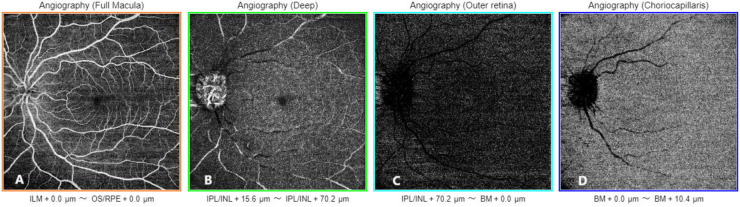
Our current understanding of the retinal vasculature comes from OCTA images and primate histological studies [28-30]. The human retina contains four distinct capillary plexuses, each with unique origins, distributions, physiological roles, and implications for disease pathophysiology [29]. The first layer, known as the RPC plexus, is localized around the optic disc and temporal arcades within the thick retinal NFL. This plexus is specialized to support the metabolic demands of the densely packed axons in this region [29, 30]. The superficial vascular plexus, supplied by the CRA, consists of larger vessels, including arteries, arterioles, capillaries, venules, and veins, located primarily in the ganglion cell layer [29, 30]. Beneath the superficial vascular plexus are two additional capillary networks, the intermediate (ICP) and deep capillary plexuses, located above and below the inner nuclear layer, respectively [30]. The ICP occupies the area from the inner two-thirds of the inner plexiform layer to the border between the inner nuclear and outer plexiform layers. This plexus develops during the perinatal period by angiogenic sprouting from the deep capillary plexus, driven by signals from amacrine cells [29, 31]. The ICP receives blood via vertical anastomoses with the superficial capillary plexus. Unlike the superficial capillary plexus, where capillaries are dispersed throughout the retinal NFL and ganglion cell layer, the ICP forms a compact and narrow laminar structure [29, 32]. Images of the superficial and deep capillary plexuses, outer retina, and choriocapillaris layers obtained by OCTA are shown in Figure 6.
Figure 6.
Optical coherence tomography angiography (OCTA) images (12 × 12 mm) obtained using the Triton™ DRI swept-source optical coherence tomography instrument (SS-OCTA, DRI OCT Triton, Topcon Corp., Tokyo, Japan) in a 20-year-old healthy woman. The OCTA images show the superficial capillary plexus (A), deep capillary plexus (B), outer retina (C), and choriocapillaris (D) layers. Abbreviations: ILM, internal limiting membrane; OS, outer segment of photoreceptors; RPE, retinal pigment epithelium; µm, micrometers; IPL, inner plexiform layer; INL, inner nuclear layer; BM, Bruch’s membrane.
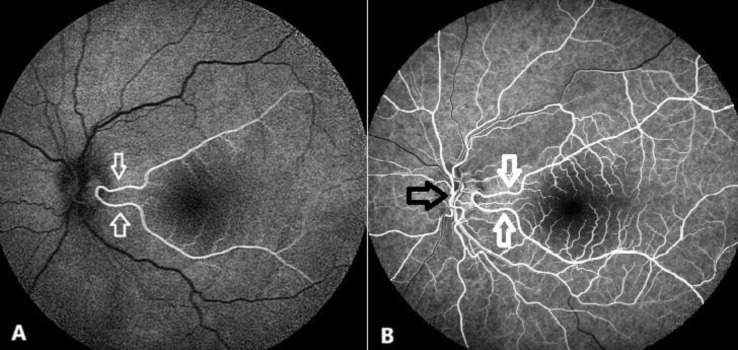
Cilioretinal arteries are notable for their potential contribution to retinal blood flow outside the CRA system. They are among the most commonly observed congenital anomalies of the retinal vasculature. Their prevalence has been reported to range from 6.9% to almost 50% [33, 34]. These arteries are more often unilateral and are predominantly temporal in location. Cilioretinal arteries arise from the PCA network and may originate directly from the choroid or the PCAs. They serve as a supplemental or alternative source of blood to the retina and are often important in supplying the macular region. They vary in size and extent, with some supplying limited retinal areas, and, in rare cases, extending to supply the entire retina [33]. A fluorescein angiographic image of a branching cilioretinal artery is presented in Figure 7 .
Figure 7.
Fluorescein angiography (Heidelberg Retinal Angiograph; Heidelberg Engineering, Inc., Dossenheim, Germany) in a 48-year-old healthy woman with a branching cilioretinal artery. The pre-arterial phase (A) depicts the branching cilioretinal artery (white arrows) as it fills simultaneously with choroidal perfusion, prior to central retinal artery filling. The early arteriovenous phase (B) reveals both the filled cilioretinal artery (white arrows) and the central retinal artery (black arrow).
Choroid, ciliary body, and iris
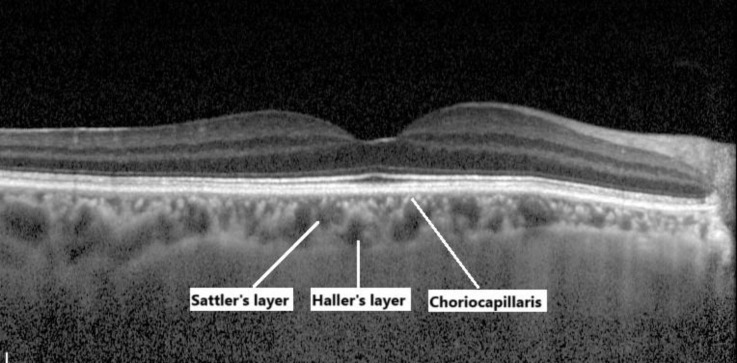
The choroid, a vascular and connective tissue layer of the uvea, lies between the sclera and retina and forms the posterior segment of the eye. Structurally, it consists of five sublayers arranged from the retinal side to the scleral side: the fifth (outermost) layer of Bruch’s membrane (basal lamina of the choriocapillaris), the choriocapillaris, Sattler’s layer, Haller’s layer, and the suprachoroidal layer [35]. The choriocapillaris, the innermost layer, contains fenestrated capillaries that are essential for nutrient and oxygen exchange. Sattler’s layer contains medium-sized arterioles and venules, whereas Haller’s layer, the outermost sublayer, is characterized by large-diameter arteries and veins (>100 µm) [35-37]. Figure 8 presents images of the choroidal layers acquired using spectral-domain OCT with enhanced-depth imaging mode.
Figure 8.
Spectral-domain optical coherence tomography (SD-OCT) with enhanced-depth imaging mode (Heidelberg Retinal SD-OCT; Heidelberg Engineering, Inc., Dossenheim, Germany), demonstrating the choroidal layers in a 58-year-old healthy woman.
The choroidal blood supply primarily originates from the short and long PCAs, with additional contributions from the ACAs. The short PCAs form a circular configuration around the optic disc, whereas the long PCAs enter in a triangular pattern extending from the posterior pole to the periphery. These vessels branch into the lobular choroidal arteries, which, despite their potential for anastomosis in vitro, functionally behave as terminal arteries [35, 38, 39]. Vascularization of the ciliary body and iris is intricately structured and mainly involves the ACAs and long PCAs, with additional input from their anastomoses with the anterior choroid [38, 39].
The perforating branches of the ACAs form a network known as the intramuscular circle, which is located in the posterior region of the ciliary muscle. This circle supplies blood to the outer and posterior parts of the ciliary muscle, the peripheral choroid, and parts of the iris. Meanwhile, the major arterial circle of the iris, which is mainly supplied by the long PCAs and is located in the stroma of the ciliary body, supplies the iris, anterior ciliary muscle, and ciliary processes [40, 41]. The ciliary processes are divided into three vascular zones, each served by its own arterioles and venules. The first zone, located at the anterior segment of the major processes, drains posteriorly via venules that bypass significant connections with other venules of the ciliary body. The second and third zones encompass the major and minor processes, draining through the venules along the edges of the ciliary processes. In addition, the minor arterial circle of the iris, located near the pupillary margin within the iris stroma, connects radially to the major circle [41].
Crystalline lens and cornea
As avascular structures, the crystalline lens and cornea rely on diffusion from the adjacent fluids to meet their nutritional and metabolic needs [42]. These essential nutrients are supplied primarily by aqueous humor, interstitial fluid from the peri-corneal vasculature, and in the case of the cornea, the tear film. This diffusion mechanism ensures the transparency and function of these critical optical components [42].
Sclera, episclera, and conjunctiva
The scleral stroma, being avascular, relies on the surrounding structures for its nutritional supply. Nutrients are primarily delivered by diffusion from the underlying choroid and vascular networks in Tenon’s capsule and the episclera. There is an intricate artery-to-artery anastomosis in these regions, where blood movement reflects an oscillatory pattern rather than a rapid flow, ensuring a steady nutrient supply to support the metabolic needs of the sclera [43]. The episclera and Tenon’s capsule are supplied primarily by the ACAs and long PCAs, with contributions from the conjunctival arteries at the limbus [43, 44]. These vessels form the episcleral arterial circle, a critical but often incomplete arterial network located approximately 4 mm from the limbus. This ensures a continuous blood supply to the sclera, episclera, Tenon’s capsule, and conjunctiva in the anterior segment of the eye despite fluctuations in intraocular pressure. In contrast, the sclera, episclera, and Tenon’s capsule in the posterior segment of the eye are supplied by the PCAs. Episcleral tissue and Tenon’s capsule are thinner in the posterior segment, resulting in a relative lack of vascularity in the superficial layers of the posterior sclera [43, 44].
The conjunctiva is vascularized by the marginal tarsal arcades, the peripheral tarsal arcades, and the ACAs. The marginal tarsal arcades mainly supply the palpebral conjunctiva and fornices, whereas the peripheral tarsal arcades contribute to the forniceal conjunctiva and extend to form the posterior conjunctival arteries, which supply the bulbar conjunctiva. The ACAs, which originate from the OA, are also important in the blood supply of the bulbar conjunctiva [44-46]. There is considerable overlap in the vascular supply, with connections between the posterior conjunctival arteries and the ACAs, particularly in the limbal region, that create a watershed zone approximately 3 to 4 mm from the limbus, where the two vascular systems meet and interact [44-46].
Extraocular muscles
Extraocular muscles receive their primary blood supply from the muscular branches of the OA, LA, and infraorbital artery. The OA gives rise to two principal muscular branches, specifically, the superior and inferior muscular branches [47]. The primary sources of the muscular branches are as follows: the LA for the inferolateral muscular trunk, the supraorbital artery for the levator palpebrae superioris, the distal segment of the OA for the superior rectus, the LA for the lateral rectus, and the inferomedial muscular trunk for the medial rectus [48]. The infraorbital artery, a terminal branch of the maxillary artery, gives rise to muscular branches of the inferior rectus and inferior oblique muscles [49].
Lacrimal gland
The lacrimal gland is perfused by the LA, a branch of the OA. This artery traverses the posteromedial aspect of the orbital lobe. The palpebral lobe is vascularized by branches extending to the orbital lobe [50].
Eyelid
The eyelids receive blood from both the ICA and ECA [51].
Medial eyelid: The superficial vascular network of the eyelid skin is established primarily through anastomoses between the branches of the ICA [52]. These include the terminal branches of the OA, such as the supraorbital, supratrochlear, and dorsal nasal arteries, as well as the LA. Moreover, vessels from the external carotid system contribute to this network, including fine branches derived from the facial artery (which extends obliquely towards the nose and inner canthus), the angular artery (a continuation of the facial artery), the superficial temporal artery, and the infraorbital artery [52]. Among the four major branches of the superficial temporal artery, the frontal branch is particularly important in the vascularization of the eyelid region. The transverse facial and frontal branches of the superficial temporal artery establish anastomoses with their contralateral counterparts and with the supraorbital artery, which also have other functions [52]. The terminal branches of the OA form the medial marginal arcades of the upper and lower eyelids, which are located deeper within the lid, just anterior to the tarsus [52]. The inferior marginal vessel is a consistent, dichotomous branch of the superior marginal vessel. It descends vertically for approximately 10 mm, passing under the canaliculi and the medial canthal tendon before returning to a superficial position and becoming integrated into the lower marginal arcade. The inferior arcade is primarily supplied by a branch of the facial artery, which converges with the inferior marginal arcade at its medial extremity [52]. Furthermore, the infraorbital artery, which originates from the maxillary artery in the pterygopalatine fossa, provides blood to the medial lower eyelid through its entrance into the orbit via the posterior aspect of the inferior orbital fissure [52].
Lateral eyelid: The superficial vascular network of the lateral upper and lower eyelids is primarily derived from branches emanating from the superficial temporal artery (a branch of the ECA) and the LA [52]. Terminal branches of the LA perfuse the lateral marginal arcades of the upper and lower eyelids. The LA initially supplies the lacrimal gland before traversing the orbital septum, where it forms the two lateral palpebral arteries. Two branches from the internal maxillary artery provide primary anastomoses with the inferior marginal arcade. These branches emerge from the pterygopalatine fossa as the anterior deep temporal artery and the infraorbital artery [52].
Venous Drainage of the Eye
The central retinal vein (CRV) exits the eye through the lamina cribrosa and courses along the optic nerve, draining blood from the retina and optic nerve. After leaving the optic nerve, the CRV usually joins the superior ophthalmic vein (SOV), although in some cases, it may join the inferior ophthalmic vein (IOV). In rare cases, this vein drains directly into the cavernous sinus [12].
Blood drains from the distinct choroidal lobules through small veins that rarely merge directly with others [53]. These veins often form large fascicles that run parallel to the equator of the eye. At the equator, these veins converge into the vortex veins, which collect blood in a flask-shaped ampulla. There are usually three to eight vortex veins per eye, although most individuals have four or five [53-55]. Some people may have a larger-than-usual ampulla, called a vortex vein varix, which can be up to 6 mm in diameter [53, 56, 57]. This structure can in some cases resemble a choroidal tumor [57]. The superior vortex veins drain into the SOV, whereas the inferior vortex veins drain into the IOV, with collateral connections between the two venous systems [45]. The vortex veins deliver blood from the choroid, ciliary body, and iris to the ophthalmic veins, contributing to the overall venous return of the eye [40, 41, 58].
The pattern of venous drainage from the extraocular muscles is analogous to that of the arterial supply, ultimately draining into the SOV and IOV [47]. Blood from the lacrimal gland drains into the lacrimal vein, which subsequently empties into the ophthalmic vein [50]. Venous drainage of the eyelid is accomplished by the pre-tarsal and post-tarsal veins [59]. On the lateral aspect of the eye, anterior to the tarsal plate, the superficial temporal vein and the lacrimal vein receive blood from the orbital tissues [59]. Medially, the blood is drained into the angular and ophthalmic veins. Posterior to the tarsal plates, venous outflow is directed to the ophthalmic vein, the deeper tributaries of the anterior facial vein, and the pterygoid venous plexus [59].
The SOV traces posteriorly along the OA and exits the orbit through the superior orbital fissure to join the cavernous sinus [12]. The IOV typically divides into two branches. One branch exits the orbit through the inferior orbital fissure and drains into the pterygoid venous plexus, whereas the other passes through the superior orbital fissure and drains either into the SOV or directly into the cavernous sinus [60]. The cavernous sinus is part of the dural venous sinus system and drains through the superior and inferior petrosal sinuses and the sigmoid sinus, ultimately draining into the internal jugular vein, which eventually carries it via the superior vena cava to the right atrium of the heart [60-62]. However, in some people, there are alternative venous drainage pathways, such as the pterygoid plexus. This plexus can drain into either the internal or external jugular vein, providing an alternative route for venous return from the orbit [62].
This narrative review summarizes the findings from several studies to provide a condensed overview of ocular arterial and venous circulation. Its major strength is its broad coverage of the existing literature, including various imaging techniques and their contributions to the understanding of ocular circulation. Nevertheless, several limitations can be noted. First and foremost, the subjective nature of narrative reviews may result in the inadvertent omission of relevant sources. In addition, our review is limited by the techniques and technologies used in the existing studies, which may render some findings obsolete, and new developments could be inadequately addressed. Moreover, the lack of quantitative analysis or systematic data interpretation precludes any comprehensive conclusions. Further research must address the gaps in knowledge revealed in this review. In particular, the use of more sophisticated imaging techniques, such as OCTA, will undoubtedly result in a more detailed understanding of ocular vasculature. Studies with broad participation from different age groups and geographical regions will increase the accuracy and generalizability of the current findings on the vascularization of the eye.
CONCLUSIONS
The eye has a sophisticated and highly variable vascular network, with both arterial supply and venous drainage varying among individuals. This intricate system is essential for the proper delivery of oxygen and nutrients to the ocular tissues, assuring their optimal function and structural integrity. The arterial system, including branches such as the central retinal and ciliary arteries, precisely supplies the different eye regions, such as the retina, choroid, optic nerve, and anterior segment structures. Meanwhile, venous drainage via the CRV, vortex veins, and ophthalmic veins facilitates the efficient removal of deoxygenated blood and metabolic waste. The interplay between the arterial and venous systems is critical for maintaining the physiological health of metabolically active tissues such as the retina. A comprehensive understanding of the vascular anatomy of the eye and its functional variability is essential for the diagnosis and treatment of ocular diseases. Advances in diagnostic imaging and vascular-targeted therapies further highlight the need to understand ocular circulation. By deepening our understanding of this vascular architecture, clinicians and researchers can develop more precise therapeutic approaches, improving patient outcomes and protecting visual function.
ETHICAL DECLARATIONS
Ethical approval:
This narrative review was ethically approved at the department level. The figures shared in this article were sourced from our unit’s patient documentation archive. Patients provided informed consent prior to the inclusion of figures in this review.
Conflict of interest:
None.
FUNDING
None.
ACKNOWLEDGMENTS
None.
References
- 1.Arumugam S, Subbiah NK. A Cadaveric Study on the Course of the Cervical Segment of the Internal Carotid Artery and Its Variations. Cureus. 2020 ;12(4):e7663. doi: 10.7759/cureus.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolman DN, Moraff AM, Heit JJ. Anatomy of the Intracranial Arteries: The Internal Carotid Artery. Neuroimaging Clin N Am. 2022 ;32(3):603–615. doi: 10.1016/j.nic.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996 ;38(3):425–32; discussion 432-3. doi: 10.1097/00006123-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bird B, Stawicki SP. StatPearls [Internet] Treasure Island (FL): 2025. Anatomy, Head and Neck, Ophthalmic Arteries. [PubMed] [Google Scholar]
- 5.Cotofana S, Lachman N. Arteries of the Face and Their Relevance for Minimally Invasive Facial Procedures: An Anatomical Review. Plast Reconstr Surg. 2019 ;143(2):416–426. doi: 10.1097/PRS.0000000000005201. [DOI] [PubMed] [Google Scholar]
- 6.Tayebi Meybodi A, Borba Moreira L, Lawton MT, Eschbacher JM, Belykh EG, Felicella MM, Preul MC. Interdural course of the ophthalmic artery in the optic canal. J Neurosurg. 2020 ;132(1):277–283. doi: 10.3171/2018.6.JNS18856. [DOI] [PubMed] [Google Scholar]
- 7.Duma SR, Ghattas S, Chang FCF. Internal Carotid Artery Occlusion Causing Acute Cranial Neuropathies. J Stroke Cerebrovasc Dis. 2019 ;28(4):e5–e6. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Michalinos A, Zogana S, Kotsiomitis E, Mazarakis A, Troupis T. Anatomy of the Ophthalmic Artery: A Review concerning Its Modern Surgical and Clinical Applications. Anat Res Int. 2015;2015:591961. doi: 10.1155/2015/591961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayreh SS, Dass R. THE OPHTHALMIC ARTERY: I ORIGIN AND INTRA-CRANIAL AND INTRA-CANALICULAR COURSE. Br J Ophthalmol. 1962 Feb;46(2):65–98. doi: 10.1136/bjo.46.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrini P, Cardia A, Fraser K, Lanzino G. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg. 2007 Jan;106(1):142–50. doi: 10.3171/jns.2007.106.1.142. [DOI] [PubMed] [Google Scholar]
- 11.Huynh-Le P, Natori Y, Sasaki T. Surgical anatomy of the ophthalmic artery: its origin and proximal course. Neurosurgery. 2005 Oct;57(4 Suppl):236–41; discussion 236-41. doi: 10.1227/01.neu.000177442.96517.3d. [DOI] [PubMed] [Google Scholar]
- 12.Hayreh SS. Orbital vascular anatomy. Eye (Lond) 2006 Oct;20(10):1130–44. doi: 10.1038/sj.eye.6702377. [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS, Dass R. THE OPHTHALMIC ARTERY: II INTRA-ORBITAL COURSE. Br J Ophthalmol. 1962 Mar;46(3):165–85. doi: 10.1136/bjo.46.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayreh SS. THE OPHTHALMIC ARTERY: II BRANCHES. Br J Ophthalmol. 1962 Apr;46(4):212–47. doi: 10.1136/bjo.46.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond) 2013 Jun;27(6):688–97. doi: 10.1038/eye.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SINGH S, DASS R. The central artery of the retina I Origin and course. Br J Ophthalmol. 1960 Apr;44(4):193–212. doi: 10.1136/bjo.44.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toma N. Anatomy of the Ophthalmic Artery: Embryological Consideration. Neurol Med Chir (Tokyo) 2016 Oct 15;56(10):585–591. doi: 10.2176/nmc.ra.2015-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res. 2001 Sep;20(5):563–93. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 19.Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004 Mar;45(3):749–57; 748. doi: 10.1167/iovs.03-0469. [DOI] [PubMed] [Google Scholar]
- 20.Lang J, Kageyama I. The ophthalmic artery and its branches, measurements and clinical importance. Surg Radiol Anat. 1990;12(2):83–90. doi: 10.1007/BF01623328. [DOI] [PubMed] [Google Scholar]
- 21.Gupta N, Motlagh M, Singh G. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing ; 2023 . Anatomy, Head and Neck, Eye Arteries. [PubMed] [Google Scholar]
- 22.Arnold A. Vascular supply of the optic nerve head: implications for optic disc ischaemia. Br J Ophthalmol. 2023 May;107(5):595–599. doi: 10.1136/bjo-2022-322254. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nerve head. Can J Ophthalmol. 2008 Jun;43(3):308–12. doi: 10.3129/i08-042. [DOI] [PubMed] [Google Scholar]
- 24.Onda E, Cioffi GA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol. 1995 Jul;120(1):92–102. doi: 10.1016/s0002-9394(14)73763-8. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS. Blood supply of the optic nerve head. Ophthalmologica. 1996;210(5):285–95. doi: 10.1159/000310727. [DOI] [PubMed] [Google Scholar]
- 26.Rao HL, Pradhan ZS, Suh MH, Moghimi S, Mansouri K, Weinreb RN. Optical Coherence Tomography Angiography in Glaucoma. J Glaucoma. 2020 Apr;29(4):312–321. doi: 10.1097/IJG.0000000000001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Smith LEH. Retinal Vasculature in Development and Diseases. Annu Rev Vis Sci. 2018 Sep ;: 101–122. doi: 10.1146/annurev-vision-091517-034018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Hormel TT, Jia Y, Jian Y, Hwang TS, Bailey ST, Pennesi ME, Wilson DJ, Morrison JC, Huang D. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res. 2021 Jan;80:100878. doi: 10.1016/j.preteyeres.2020.100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad C, Baleine M, Motulsky E. An OCT-A Analysis of the Importance of Intermediate Capillary Plexus in Diabetic Retinopathy: A Brief Review. J Clin Med. 2024 Apr ;13(9):2516. doi: 10.3390/jcm13092516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep. 2017 Feb;7:42201. doi: 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usui Y, Westenskow PD, Kurihara T, Aguilar E, Sakimoto S, Paris LP, Wittgrove C, Feitelberg D, Friedlander MS, Moreno SK, Dorrell MI, Friedlander M. Neurovascular crosstalk between interneurons and capillaries is required for vision. J Clin Invest. 2015 Jun;125(6):2335–46. doi: 10.1172/JCI80297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavia C, Mecê P, Nassisi M, Bonnin S, Marie-Louise J, Couturier A, Erginay A, Tadayoni R, Gaudric A. Retinal Capillary Plexus Pattern and Density from Fovea to Periphery Measured in Healthy Eyes with Swept-Source Optical Coherence Tomography Angiography. Sci Rep. 2020 Jan 30;10(1):1474. doi: 10.1038/s41598–020-58359-y. doi: 10.1038/s41598-020-58359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider M, Molnar A, Angeli O, Szabo D, Bernath F, Hajdu D, Gombocz E, Mate B, Jiling B, Nagy BV, Nagy ZZ, Peto T, Papp A. Prevalence of Cilioretinal Arteries: A systematic review and a prospective cross-sectional observational study. Acta Ophthalmol. 2021 May;99(3):e310–e318. doi: 10.1111/aos.14592. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo C, Kilian R, Savastano MC, Fossataro C, Savastano A, Rizzo S. A case of cilioretinal artery occlusion: Diagnostic procedures. Am J Ophthalmol Case Rep. 2023 Oct;32:101949. doi: 10.1016/j.ajoc.2023.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Kaser-Eichberger A, Fan W, Platzl C, Schrödl F, Heindl LM. The structure and function of the human choroid. Ann Anat. 2024 Jun;254:152239. doi: 10.1016/j.aanat.2024.152239. [DOI] [PubMed] [Google Scholar]
- 36.Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, Fujimoto JG, Duker JS. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013 Sep;120(9):1901–8. doi: 10.1016/j.ophtha.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lejoyeux R, Benillouche J, Ong J, Errera MH, Rossi EA, Singh SR, Dansingani KK, da Silva S, Sinha D, Sahel JA, Freund KB, Sadda SR, Lutty GA, Chhablani J. Choriocapillaris: Fundamentals and advancements. Prog Retin Eye Res. 2022 Mar;87:100997. doi: 10.1016/j.preteyeres.2021.100997. [DOI] [PubMed] [Google Scholar]
- 38.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012 Sep;31(5):377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018 Nov;67:30–55. doi: 10.1016/j.preteyeres.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Iovino C, Peiretti E, Braghiroli M, Tatti F, Aloney A, Lanza M, Chhablani J. Imaging of iris vasculature: current limitations and future perspective. Eye (Lond) 2022 May;36(5):930–940. doi: 10.1038/s41433-021-01809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk R, Rohen JW. Scanning electron microscopic study on the vasculature of the human anterior eye segment, especially with respect to the ciliary processes. Exp Eye Res. 1990 Dec;51(6):651–61. doi: 10.1016/0014-4835(90)90049-z. [DOI] [PubMed] [Google Scholar]
- 42.Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008 Apr;19(2):125–33. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson PG, Young RD. Scleral structure, organisation and disease A review. Exp Eye Res. 2004 Mar;78(3):609–23. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 44.Meyer PA. Patterns of blood flow in episcleral vessels studied by low-dose fluorescein videoangiography. Eye (Lond). 1988;2 ( Pt 5):533–46. doi: 10.1038/eye.1988.104. [DOI] [PubMed] [Google Scholar]
- 45.Steven P, Gebert A. Conjunctiva-associated lymphoid tissue - current knowledge, animal models and experimental prospects. Ophthalmic Res. 2009;42(1):2–8. doi: 10.1159/000219678. [DOI] [PubMed] [Google Scholar]
- 46.Shumway CL, Motlagh M, Wade M. StatPearls [Internet] StatPearls Publishing; 2025 Jan–: Treasure Island (FL); 2023 . Anatomy, Head and Neck, Eye Conjunctiva. [PubMed] [Google Scholar]
- 47.Shumway CL, Motlagh M, Wade M. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing ; 2022 . Anatomy, Head and Neck, Eye Extraocular Muscles. [PubMed] [Google Scholar]
- 48.Erdogmus S, Govsa F. Arterial vascularization of the extraocular muscles on its importance for orbital approaches. J Craniofac Surg. 2007 Sep;18(5):1125–32. doi: 10.1097/scs.0b013e3180cc2c71. [DOI] [PubMed] [Google Scholar]
- 49.René C. Update on orbital anatomy. Eye (Lond) 2006 Oct;20(10):1119–29. doi: 10.1038/sj.eye.6702376. [DOI] [PubMed] [Google Scholar]
- 50.Singh S, Basu S. The Human Lacrimal Gland: Historical Perspectives, Current Understanding, and Recent Advances. Curr Eye Res. 2020 Oct;45(10):1188–1198. doi: 10.1080/02713683.2020.1774065. [DOI] [PubMed] [Google Scholar]
- 51.Turvey TA, Golden BA. Orbital anatomy for the surgeon. Oral Maxillofac Surg Clin North Am. 2012 Nov;24(4):525–36. doi: 10.1016/j.coms.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker SM, Linberg JV. Vascular anatomy of the eyelids. Ophthalmology. 1994 Jun;101(6):1118–21. doi: 10.1016/s0161-6420(94)31212-7. [DOI] [PubMed] [Google Scholar]
- 53.Spaide RF. CHOROIDAL BLOOD FLOW: Review and Potential Explanation for the Choroidal Venous Anatomy Including the Vortex Vein System. Retina. 2020 Oct;40(10):1851–1864. doi: 10.1097/IAE.0000000000002931. [DOI] [PubMed] [Google Scholar]
- 54.Kutoglu T, Yalcin B, Kocabiyik N, Ozan H. Vortex veins: anatomic investigations on human eyes. Clin Anat. 2005 May;18(4):269–73. doi: 10.1002/ca.20092. [DOI] [PubMed] [Google Scholar]
- 55.Lim MC, Bateman JB, Glasgow BJ. Vortex vein exit sites. Scleral coordinates. Ophthalmology. 1995 Jun;102(6):942–6. doi: 10.1016/s0161-6420(95)30930-x. [DOI] [PubMed] [Google Scholar]
- 56.Osher RH, Abrams GW, Yarian D, Armao D. Varix of the vortex ampulla. Am J Ophthalmol. 1981 Nov;92(5):653–60. doi: 10.1016/s0002-9394(14)74657-4. [DOI] [PubMed] [Google Scholar]
- 57.Gündüz K, Shields CL, Shields JA. Varix of the vortex vein ampulla simulating choroidal melanoma: report of four cases. Retina. 1998;18(4):343–7. doi: 10.1097/00006982-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Woodlief NF. Initial observations on the ocular microcirculation in man I The anterior segment and extraocular muscles. Arch Ophthalmol. 1980 Jul;98(7):1268–72. doi: 10.1001/archopht.1980.01020040120019. [DOI] [PubMed] [Google Scholar]
- 59.Downie LE, Bandlitz S, Bergmanson JPG, Craig JP, Dutta D, Maldonado-Codina C, Ngo W, Siddireddy JS, Wolffsohn JS. CLEAR - Anatomy and physiology of the anterior eye. Cont Lens Anterior Eye. 2021 Apr;44(2):132–156. doi: 10.1016/j.clae.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Azzam D, Cypen S, Tao J. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing ; 2023 . Anatomy, Head and Neck: Eye Ophthalmic Vein. [PubMed] [Google Scholar]
- 61.Cheung N, McNab AA. Venous anatomy of the orbit. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):988–95. doi: 10.1167/iovs.02-0865. [DOI] [PubMed] [Google Scholar]
- 62.Bergen MP. A literature review of the vascular system in the human orbit. Acta Morphol Neerl Scand. 1981 Dec;19(4):273–305. [PubMed] [Google Scholar]