Abstract
Purpose
CDK4/6 inhibitors (CDK4/6i) represent the first-line therapy approach of choice for patients with hormone receptor-positive, HER2-negative advanced breast cancer (HR + /HER-ABC). Approximately 50% of HR + /HER2-ABC displays low HER2 expression (HER2 low). Recent data emerging from the DESTINY-Breast04 trial demonstrated practice-changing efficacy of the antibody–drug conjugate trastuzumab deruxtecan (T-DXd) in patients with low HER2 expression. Here, we aimed to analyze the impact of low HER2 expression on CDK4/6i therapy response in a well-characterized multicenter HR + /HER-ABC cohort.
Methods
Patients diagnosed with HR + /HER2-ABC who were treated with CDK4/6i in clinical routine between November 2016 and December 2020 at four certified German Breast Cancer Centers were retrospectively identified. The cohort was stratified according to graduation of positivity in HER2 immunohistochemistry (IHC; HER2 zero = IHC score 0 and HER2 low = IHC score 1 + , 2 + /fluorescence in situ hybridization negative). Subgroups were analyzed with regard to progression-free survival (PFS) following CDK4/6i initiation.
Findings
The study cohort comprised n = 448 patients. For n = 311 patients, HER2 status from the metastatic site was available. n = 91 (29.3%) cases were HER2 zero and n = 220 cases (70.7%) were HER2 low. There was no significant difference in PFS between the two groups (PFS: 17 months versus 18 months, log-rank p = 0.42). Further, we examined the influence of HER2 expression changes between primary and metastatic tissue (n = 171; HER2 gain/HER2 loss/HER2 stable expression) on CDK4/6i treatment response. Again, there was no significant difference between these three groups, respectively (PFS: 16 months versus 13 months versus 17 months, log-rank p = 0.86).
Conclusions
In our analysis, HER2 status did not have a significant impact on treatment response to CDK4/6i.
Keywords: HER2 low, CDK4/6 inhibitor, Breast cancer, Trastuzumab deruxtecan
Main
CDK4/6 inhibitors (CDK4/6i) in combination with endocrine therapy (ET) represent the therapeutic mainstay for patients with hormone receptor-positive, HER2-negative advanced breast cancer (HR + /HER2-ABC). Despite recent controversy regarding efficacy differences between the three different approved CDK4/6i, namely Palbociclib, Ribociclib, and Abemaciclib, the overall clinical benefit of CDK4/6i is uncontroversial as demonstrated in several randomized clinical trials and published real-world data [1–5].
However, patients with HR + /HER-ABC comprise a heterogenous population and a substantial patient proportion show disease progression on first-line CDK4/6i-based therapy. Beyond different histopathological subtypes, estrogen and progesterone receptor expression, histomorphological grading and Ki-67 index, the HER2 receptor exhibits varying expression levels across HR + /HER2-ABC. Research has shown, that approximately 50% of HR + /HER2-ABC displays low HER2 expression designated by graduation of positivity in HER2 immunohistochemistry (IHC; HER2 zero = IHC score 0 and HER2 low = IHC score 1 + , 2 + /fluorescence in situ hybridization negative) [6–9]. This is of the highest clinical relevance as data from the DESTINY-Breast04 trial demonstrated practice-changing efficacy of the antibody–drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) in patients with low HER2 expression [10]. Further, recent data from the DESTINY-Breast06 trial showed a statistically significant and clinically meaningful PFS benefit for T-DXd compared to treatment of physicians choice in patients who experienced disease progression on ET for HR + /HER2-ABC (NCT04494425;[11, 12]). Of note, this applies for both the group of patients with HER2 low and for patients with HER2 ultralow (IHC score 0 with membrane staining).
To date, few data exist to which extent HER2 expression impacts response to CDK4/6i. In the present study, we aimed to analyze the impact of HER2 expression on CDK4/6i therapy response in a well-characterized multicenter HR + /HER-ABC real-world data cohort.
Patients diagnosed with HR + /HER2-ABC who were treated with CDK4/6i in clinical routine between November 2016 and December 2020 at four certified German Breast Cancer Centers (Saarland University Medical Center, University Medical Center Charité Berlin, University Medical Center Bonn and University Medical Center Schleswig–Holstein, Campus Kiel) were retrospectively identified. A recent follow-up was conducted in January 2023.
The cohort was stratified according to graduation of positivity in HER2 immunohistochemistry (IHC; HER2 zero = IHC score 0 and HER2 low = IHC score 1 + , 2 + /fluorescence in situ hybridization negative). Subgroups were analyzed with regard to progression-free survival (PFS) following CDK4/6i initiation. Statistical analyses were performed using SPSS 28.0 (IBM, Armonk, USA) and GraphPad Prism software (GraphPad software, San Diego, CA, USA). Kaplan–Meier analysis and Cox regression were performed to analyze PFS and confounders on PFS.
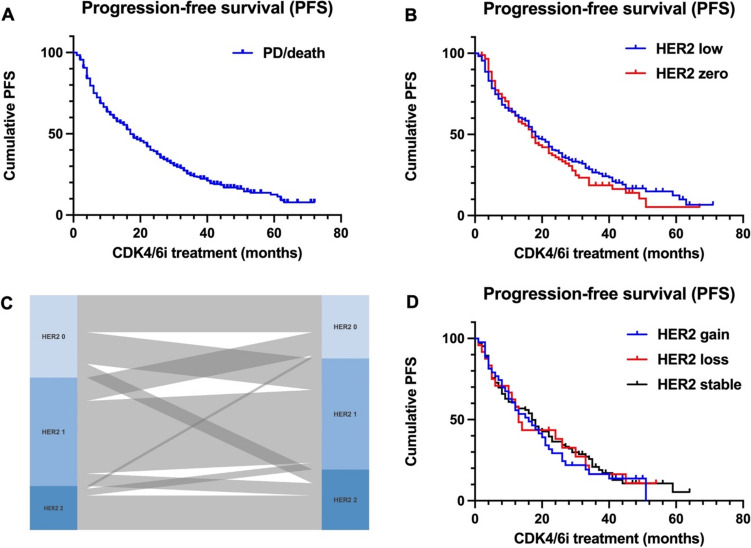
A detailed characterization of the study cohort has been published previously [5]. In total, n = 448 patients were included in the final analysis. n = 319 patients (71.3%) received Palbociclib, n = 114 patients (25.4%) received Ribociclib, and n = 15 patients (3.3%) received Abemaciclib. n = 165 (36.8%) patients exhibited primary metastatic breast cancer, and n = 283 (63.2%) patients had secondary metastatic disease (Table 1). HER2 expression status from metastatic tissue was available for n = 311 cases (69.4%). n = 91 (29.3%) cases were HER2 zero, n = 154 cases (49.5%) were HER2 1 + , and n = 66 cases (21.2%) were HER2 2 + / fluorescence in situ hybridization (FISH) negative (Table 1). The median progression-free survival (PFS) following CDK4/6i therapy initiation was 17 months in the entire population (Fig. 1A). The cohort was stratified according to graduation of positivity in HER2 IHC in HER2 zero (IHC score 0) and HER2 low (IHC score 1 + , 2 + /FISH negative). Examining the median PFS in relation to the HER2 status in metastatic tissue revealed no significant difference. The median PFS was 17 months for patients with HER2 zero and 18 months for patients with HER2 low status, respectively. The differences between the groups were not statistically significant (Fig. 1B; HER2 0 vs. HER2 low (Log-Rank p = 0.42)). Of note, the PFS following CDK4/6i therapy initiation in the study cohort is shorter compared to the pivotal trials. This is attributable to the real-world population and in particular CDK4/6i treatment in later lines of therapy (Table 1, [5]). Furthermore, we examined the influence of HER2 expression changes during metastatic evolution on PFS. For this purpose, patients who had secondary metastatic disease were divided into (i) patients with gained HER2 expression (initial HER2 zero and in metastatic tissue HER2 1 + or 2 + /FISH-negative; or initial HER2 1 + and in metastatic tissue HER2 2 + /FISH-negative), (ii) patients with constant HER2 receptor expression and (iii) patients with loss of HER2 expression. In n = 171 patients, HER2 receptor expression was present in both, primary and metastatic tissue. In this subgroup, n = 42 patients (24.5%) had HER2 receptor gain, n = 24 (14.1%) patients had loss of HER2 expression, and n = 105 patients (61.4%) displayed constant HER2 expression (Table 2, Fig. 1C). No PFS difference was observed between these three subgroups (16 months versus 13 months versus 17 months, log-rank p = 0.86; Fig. 1D).
Table 1.
Cohort characteristics
| (n) | (%) | |
|---|---|---|
| Primary metastatic disease | 165 | 36.8 |
|
Secondary metastatic disease CDK4/6i Palbociclib Ribociclib Abemaciclib |
283 319 114 15 |
63.2 71.3 25.4 3.3 |
| Disease site | ||
| Osseous | 334 | 74.6 |
| Hepatic | 133 | 29.7 |
| Pulmonary | 154 | 34.4 |
| Other (lymphatic, peritoneal, cerebral) | 219 | 48.9 |
| Therapy line | ||
| 1 | 278 | 62.1 |
| 2 | 86 | 19.2 |
| > 3 | 84 | 18.7 |
|
HER2 receptor status (metastatic disease) |
311 | |
| Her2 0 | 91 | 29.3 |
| Her2 1 + | 154 | 49.5 |
| Her2 2 + | 66 | 21.2 |
Fig. 1.
A Progression-free survival in Kaplan–Meier analysis following CDK4/6 inhibitor treatment in the entire study cohort (median PFS 17 months). B Median PFS depending on HER2 expression (metastatic site). Patients with HER2 zero had 17 months median PFS, patients with HER2 low (IHC score 1 + and 2 +) had 18 months median PFS. Log-Rank p = 0.42. C Sankey diagram illustrating HER2 expression changes. In n = 171 patients, HER2 receptor expression was available for both, primary and metastatic tissue. n = 42 patients (24.5%) had HER2 receptor gain, n = 24 (14.1%) patients had loss of HER2 expression, and n = 105 patients (61.4%) displayed constant HER2 expression. D PFS in Kaplan–Meier analysis following CDK4/6 inhibitor treatment showed no difference between patients with HER2 receptor gain/loss/stable expression (16 months versus 13 months versus 17 months, log-rank p = 0.86)
Table 2.
HER2 receptor switch
| HER2 status Primary Tumor | (n) | (%) | HER2 status Metastatic Tumor | (n) | (%) |
|---|---|---|---|---|---|
| HER2 IHC 0 | 60 | 35.1 | HER2 IHC 0 | 27 | 45.0 |
| HER2 IHC 1 + | 23 | 38.3 | |||
| HER2 IHC 2 + | 10 | 16.7 | |||
| HER2 IHC 1 + | 79 | 46.2 | HER2 IHC 0 | 17 | 21.5 |
| HER2 IHC 1 + | 53 | 67.1 | |||
| HER2 IHC 2 + | 9 | 11.4 | |||
| HER2 IHC 2 + | 32 | 18.7 | HER2 IHC 0 | 2 | 6.3 |
| HER2 IHC 1 + | 5 | 15.6 | |||
| HER2 IHC 2 + | 25 | 78.1 |
Data are presented for patients with secondary metastatic disease in which HER2 receptor expression data was available from primary breast cancer tissue (n = 171). HER2 receptor switch is illustrated in Fig. 1C
These data are in line with recently published data that demonstrated that approximately 38% of patients display changes of HER2 expression between primary tumor and metastasis [9, 13–15].
With regard to ET response, experimental research has shown that HER2 expression promotes ET resistance [16–18]. However, clinical attempts to restore endocrine sensitivity by combined endocrine and HER2-directed therapy failed [19, 20]. Experimental data showed that CDK4/6i treatment response was independent of HER2 expression [21]. Similarly, in the clinical context of the current first-line regimen, HER2 status appears to exert no influence on treatment response, as demonstrated in the current study. In contrast to that, a recently published retrospective, multicentric observational study from Italy involving n = 428 patients demonstrated that HER2 low status was associated with worse PFS compared to HER2 zero (median PFS 23.6 months vs. 32.3 months; p = 0.014) [22]. However, only patients treated with first-line ET + /CDK4/6i were enrolled [22].
Ongoing scientific efforts aim to identify biomarkers to identify patients who do not benefit from CDK4/6i-based therapy. Potential contemporary alternative regimens in this treatment setting include conventional chemotherapy and ADCs e.g., T-DXd and Sacituzumab govitecan, both of which have been approved by the European Medicines Agency (EMA) for treatment of HR + /HER2-ABC after disease progression on ET and one to two lines of systemic therapy lines, respectively. Recent data from the DESTINY-Breast04 and DESTINY-Breast06 trials strongly encourage a consideration of this substance in the therapy algorithm of HR + /HER2-ABC [10–12].
To date, the use of the intensity of HER2 receptor expression as a potential predictive biomarker remains uncertain. Although the biological mechanism of action of ADC suggests a relationship between target receptor expression and treatment response, data on different ADCs demonstrated varying results [23, 24].
We acknowledge the limitation of our analysis (i.e., retrospective nature). Discrepancies among studies in HER2 low patients might be attributed to varying methods of HER2 status assessment, differences in patient populations (such as distinct patient and tumor characteristics), and diverse clinical management approaches (including different types of CDK4/6i, backbone endocrine therapy or differing subsequent lines of therapy).
However, in our multi-center HR + /HER2-ABC cohort, HER2 expression status had no impact on response to CDK4/6i therapy. Our data are consistent with previously published studies in which HER2 expression status also had no prognostic value on CDK4/6i therapy response [10, 11]. Therefore, HER2 expression status might not be a biomarker that defines a subset of HR + /HER2-ABC in terms of therapy response to CDK4/6i. Further studies are needed in this regard.
Abbreviations
- CDK4/6i
CDK4/6 inhibitors
- HR + /HER-ABC
Hormone receptor-positive, HER2-negative advanced breast cancer
- T-DXd
Trastuzumab deruxtecan
- IHC
Immunohistochemistry
- PFS
Progression-free survival
- ADC
Antibody–drug conjugate
- ET
Endocrine therapy
- EMA
European medicines agency
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by DJR, VK, CM and ACR. Data analysis was performed by CM and DJR. The manuscript was written by DJR, VK, CM and ACR. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. DJR is supported by the BONFOR program of the Medical Faculty of the University of Bonn (grant ID 2021-1A-14). ACR is supported by the Clinician Scientist Program in Evolutionary Medicine of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant ID 413490537). CM is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant ID MU 4812/2–1:2).
Data availability
The datasets generated and/or analyzed during the current study are available on request from the authors.
Declarations
Conflict of interest
None declared.
Ethics approval and consent to participate
This research study was conducted retrospectively from data obtained for clinical purposes. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Trial registration numbers were as follows: 35/20 (Ethics Committee of the Saarland Physicians’ chamber), 317/21 (Ethics Committee of the Bonn University Hospital), and B302/21 (Ethics Committee of the Christian-Albrechts-University Kiel).
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Damian J. Ralser, Verena Kiver, Carolin Müller and Anna-Christina Rambow have contributed equally.
References
- 1.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S et al (2021) Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol Off J Eur Soc Med Oncol 32(8):1015–1024 [DOI] [PubMed] [Google Scholar]
- 3.Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women With HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol Off J Am Soc Clin Oncol 35(25):2875–2884 [DOI] [PubMed] [Google Scholar]
- 4.Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A (2020) Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet Lond Engl 395(10226):817–827 [DOI] [PubMed] [Google Scholar]
- 5.Müller C, Kiver V, Solomayer EF, Wagenpfeil G, Neeb C, Blohmer JU et al (2023) CDK4/6 Inhibitors in Advanced HR+/HER2–Breast cancer: a multicenter real-world data analysis. Breast Care Basel Switz 18(1):31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lal P, Salazar PA, Hudis CA, Ladanyi M, Chen B (2004) HER-2 testing in breast cancer using immunohistochemical analysis and fluorescence in situ hybridization: a single-institution experience of 2,279 cases and comparison of dual-color and single-color scoring. Am J Clin Pathol 121(5):631–636 [DOI] [PubMed] [Google Scholar]
- 7.Fehrenbacher L, Cecchini RS, Geyer CE, Rastogi P, Costantino JP, Atkins JN et al (2020) NSABP B-47/NRG oncology phase III randomized Trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol Off J Am Soc Clin Oncol 38(5):444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22(8):1151–1161 [DOI] [PubMed] [Google Scholar]
- 9.Corti C, Giugliano F, Nicolò E, Tarantino P, Criscitiello C, Curigliano G (2023) HER2-low breast cancer: a new subtype? Curr Treat Options Oncol 24(5):468–478 [DOI] [PubMed] [Google Scholar]
- 10.Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E et al (2022) Trastuzumab deruxtecan in previously treated HER2-Low advanced breast cancer. N Engl J Med 387(1):9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardia A, Barrios C, Dent R, Hu X, O’Shaughnessy J, Yonemori K, et al. Abstract OT-03–09: Trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: A randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021 Feb 15;81(4_Supplement):OT-03–09-OT-03–09.
- 12.Curigliano G, Hu X, Dent RA, Yonemori K, Barrios CH, O’Shaughnessy J, et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). J Clin Oncol. 2024 Jun 10;42(17_suppl):LBA1000–LBA1000.
- 13.Bergeron A, Bertaut A, Beltjens F, Charon-Barra C, Amet A, Jankowski C et al (2023) Anticipating changes in the HER2 status of breast tumours with disease progression-towards better treatment decisions in the new era of HER2-low breast cancers. Br J Cancer 129(1):122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M et al (2021) Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 7(1):137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stueber TN, Weiss CR, Woeckel A, Haeusler S (2019) Influences of adjuvant treatments in hormone receptor positive breast cancer on receptor conversion in recurrent breast cancer. Arch Gynecol Obstet 299(2):533–541 [DOI] [PubMed] [Google Scholar]
- 16.Johnston SRD (2005) Clinical trials of intracellular signal transductions inhibitors for breast cancer–a strategy to overcome endocrine resistance. Endocr Relat Cancer 12(Suppl 1):S145-157 [DOI] [PubMed] [Google Scholar]
- 17.Johnston SRD (2009) Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer 9(Suppl 1):S28-36 [DOI] [PubMed] [Google Scholar]
- 18.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA et al (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res Off J Am Assoc Cancer Res 16(5):1486–1497 [DOI] [PubMed] [Google Scholar]
- 19.Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 27(33):5538–5546 [DOI] [PubMed] [Google Scholar]
- 20.Leary A, Evans A, Johnston SRD, A’Hern R, Bliss JM, Sahoo R et al (2015) Antiproliferative effect of Lapatinib in HER2-Positive and HER2-Negative/HER3-High breast cancer: results of the presurgical randomized MAPLE Trial (CRUK E/06/039). Clin Cancer Res Off J Am Assoc Cancer Res 21(13):2932–2940 [DOI] [PubMed] [Google Scholar]
- 21.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ et al (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res BCR 11(5):R77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zattarin E, Presti D, Mariani L, Sposetti C, Leporati R, Menichetti A et al (2023) Prognostic significance of HER2-low status in HR-positive/HER2-negative advanced breast cancer treated with CDK4/6 inhibitors. NPJ Breast Cancer 9(1):27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klümper N, Ralser DJ, Ellinger J, Roghmann F, Albrecht J, Below E et al (2023) Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res Off J Am Assoc Cancer Res 29(8):1496–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakach E, Sacks R, Kalinsky K (2022) Trop-2 as a therapeutic target in breast cancer. Cancers 14(23):5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available on request from the authors.