Abstract
Introduction
Tinidazole shows potential as a first-line treatment for bacterial vaginosis (BV). However, its superiority to metronidazole remains uncertain. Therefore, this meta-analysis compares tinidazole versus metronidazole in patients with BV.
Methods
We systematically searched PubMed, Embase and Cochrane for studies comparing tinidazole and metronidazole in patients with BV. Statistical analyses were performed using R Studio 4.3.2. Heterogeneity was examined with the Cochran Q test and I2 statistics. Risk ratios (RR) with 95% confidence intervals (CI) were pooled across trials. Outcomes of interest were BV cure at the first and the second follow-up appointment, and adverse events such as nausea and bad or metallic taste.
Results
Five randomized controlled trials and 1 prospective observational study, reporting data on 1,036 patients were included in this meta-analysis. Among them, 511 (49%) received tinidazole and 525 (51%) received metronidazole. Follow-up ranged from 1 to 6 weeks. There was no significant difference between groups for BV cure at the first follow-up appointment (RR 1.03; 95% CI 0.92 to 1.14; I2 = 76%), cure at the second follow-up appointment (RR 1.05; 95% CI 0.80–1.38; I2 = 88%), nausea (RR 0.89; 95% CI 0.39–2.04; I2 = 83%), and bad or metallic taste (RR 0.74; 95% CI 0.12–4.45; I2 = 89%).
Conclusion
In patients with BV, tinidazole and metronidazole exhibit similar efficacy and safety, with equivalent cure rates and incidence of adverse events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00404-024-07899-z.
Keywords: Bacterial vaginosis, Tinidazole, Metronidazole, Meta-analysis
Introduction
Bacterial vaginosis (BV) is the most frequent cause of vaginitis in women at reproductive age. It is characterized by a disruption of the normal microbiota, but despite its high prevalence and six decades of research, its pathogenesis is not fully elucidated [1]. Numerous complications are associated with BV, such as sexually transmitted infections, including human immunodeficiency virus (HIV), pelvic inflammatory disease, risk of preterm birth, and other obstetric or gynecologic impairments [2, 3]. Approximately less than 50% of cases of BV are appropriately managed, one of the reasons for the low rate of successful treatments is due to the low adherence of patients to the treatment regimen [4].
Currently, nitroimidazoles are the class of medications preferably employed in the treatment of BV [5]. Published in 2021 by the Centers for Disease Control and Prevention of the United States (CDC), the STI Treatment Guidelines recommended Metronidazole as the first line treatment for BV with a dosage of 500 mg orally twice a day for 7 days. Whereas oral tinidazole is often prescribed as an alternative treatment, generally 2 g orally once daily for two days. [6] In this context, the discussion arises regarding the possibility of a shorter therapeutic regimen with tinidazole, contributing to greater patient adherence, potentially resulting in higher cure rates and reduced recurrence rates.
Despite several studies, the efficacy of tinidazole compared to metronidazole remains unclear [7–12]. Individual trials lack sufficient power to detect significant differences in cure outcomes and adverse events. Therefore, we aimed to conduct a systematic review and meta-analysis examining the efficacy and safety of tinidazole compared to metronidazole in patients with bacterial vaginosis to understand the optimal first-line treatment option for BV.
Material and methods
This systematic review and meta-analysis was performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [13]. As such, it was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42024541694).
Search strategy and data extraction
We systematically searched PubMed, Embase, and Cochrane Central Register of Controlled Trials from inception to april 2024 with the following search terms: (“vaginosis” OR “bacterial vaginosis” OR “Gardnerella infection” OR “Gardnerella vaginalis”) AND (“tinidazole” OR “tindamax”). The references from all included studies, previous systematic reviews and meta-analyses were also searched manually for any additional studies. Two authors (M.S. and H.F.) independently extracted the data following predefined search criteria and quality assessment. Disagreements were resolved by consensus between the authors.
Eligibility criteria
Inclusion in this meta-analysis was restricted to studies that met all the following eligibility criteria: (1) randomized controlled trials (RCTs) or nonrandomized trials; (2) comparing tinidazole to metronidazole; (3) enrolling patients with bacterial vaginosis; and (4) reporting at least one of the outcomes of interest. We excluded studies with (1) no control group; (2) population overlap; (3) in patients without bacterial vaginosis; or (4) combination of other drugs in the intervention or control group.
Endpoints and subanalyses
Efficacy outcomes included bacterial vaginosis cure at the first and second follow-up appointment. Adverse events such as nausea, bad or metallic taste, diarrhea, and headache were the safety outcomes of interest. The criteria for cure varied slightly among the studies and are reported in Table 1. Pre-specified sub-analyses included data restricted to (1) RCTs; (2) follow-up time (1, 2, and 4 weeks); and (3) tinidazole dose (500 mg, 1 g, and 2 g) for cure rates.
Table 1.
Baseline characteristics of included studies
| Study | Desgin | Cure criteria | Tinidazole dose | Metronidazole dose | Patients, no (T/M) | Follow-up |
|---|---|---|---|---|---|---|
| Gupta 2015 | RTC | Criterion of Nugent | 2 g single dose | 400 mg twice a day for 7 days | 86/84 | 2 weeks |
| Mohanty 1987 | Non-RTC | Negative culture for G. vaginalis and absence of at least three of the following criteria: homogeneous frothy vaginal discharge, vaginal pH ≥ 4.8-, amine odour on KOH treatment of discharge, "clue cells" in the absence of lactobacilli on Gram's staining | 2 g single dose | 2 g single dose | 82/98 | 1,2, 4 and 6 weeks |
| Raja 2016 | RTC | Criterion of Amsel | 500 mg once a day + placebo | 500 mg twice a day for 5 days | 57/57 | 1 and 4 weeks |
| Schwebke 2011 | RTC | Criterion of Nugent | 500 mg twice a day for 7 days or 1 g twice a day for 7 days | 500 mg twice a day for 7 days | 160/160 | 2 weeks |
| Thulkar 2012 | RTC | Criterion of Amsel | 2 g, single dose | 2 g, single dose | 86/86 | 1 and 4 weeks |
| Wang 2008 | RTC | Vulvovaginal discomfort symptoms disappear and vaginal secretion properties return to normal; vaginal pH < 4.5; and clue cells disappear in smears | 1 g once a day, for 3 days, double for the first time | 750 mg each time, once a day, for 7 days | 40/40 | 3–5 days after the end of the next menstruation |
RTC randomized controlled trial, T tinidazole, M metronidazole, mg milligram, g gram
Quality assessment
Quality assessment of RCTs was performed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials (RoB-2), in which studies are scored as high, low, or unclear risk of bias in 5 domains: selection, performance, detection, attrition, and reporting biases [14]. Nonrandomized studies were appraised with the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) tool. This tool categorizes studies as having low, moderate, serious, or critical risk of bias across 5 domains: confounding, selection, intervention classification, intervention deviation, missing data, outcome measurement, and selection of reported results [15]. Bias risk assessment was conducted independently by two authors (M.S. and H.F.). Discrepancies were resolved through consensus among the authors.
Statistical analysis
Treatment effects were compared using Risk Ratios (RR), with 95% confidence intervals (CI), for binary outcomes. Given the expected heterogeneity between studies, we adopted the DerSimonian and Laird random-effects model for all outcomes reported. We used the Cochran Q test and I2 statistics to assess for heterogeneity; P values inferior to 0.1 and I2 > 40% were considered significant for heterogeneity [16]. Leave-one-out analysis was also performed to test the robustness of the findings and to address high heterogeneity when present. R version 4.3.2 was used for statistical analysis [17].
Results
Study selection and characteristics
As detailed in Fig. 1, the initial search yielded 225 results. After removal of duplicate records and ineligible studies, 12 remained and were fully reviewed based on inclusion criteria. Of these, a total of 6 studies were included, comprising 1036 patients from 5 randomized controlled trials (RCTs)[7, 9–12], and 1 non-randomized study [8]. A total of 511 (49%) patients received tinidazole and 525 (51%) received metronidazole.
Fig. 1.
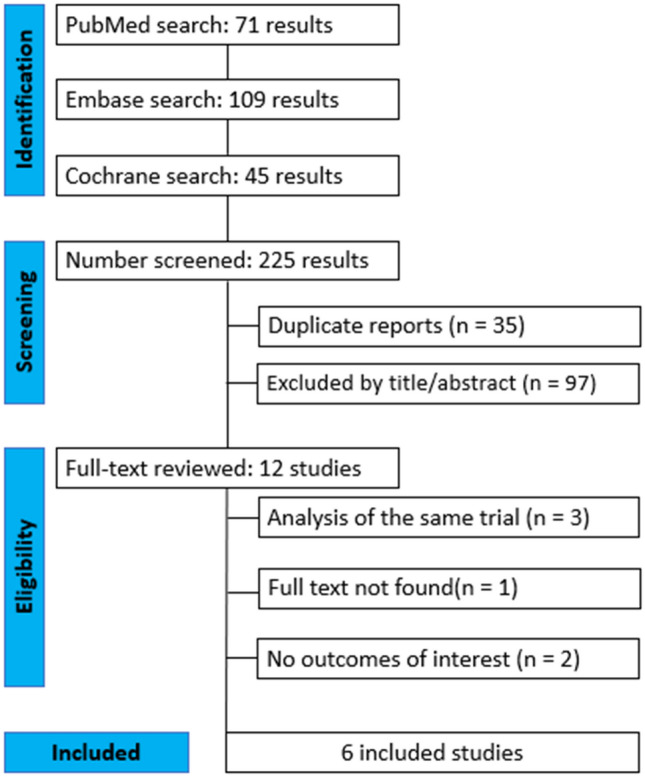
PRISMA flow diagram of study screening and selection
In this meta-analysis, the criteria used to assess the cure of bacterial vaginosis varied between the studies analyzed. Most of the studies chose to use the Amsel criteria, which are widely recognized in clinical practice, but Schwebke's study took a different approach, using the Nugent criteria.
Study characteristics are reported in Table 1.
Pooled analysis of all studies
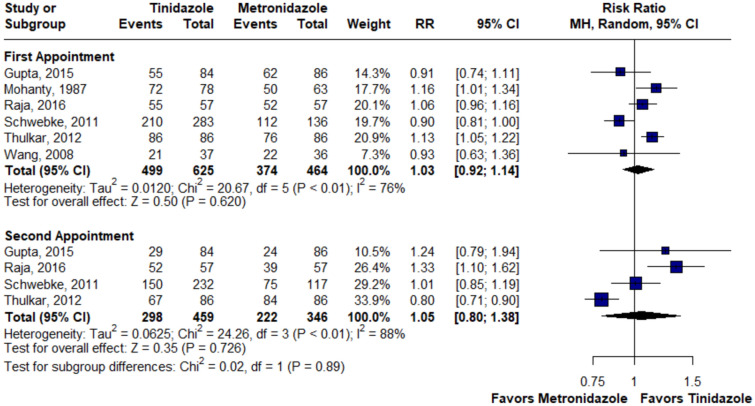
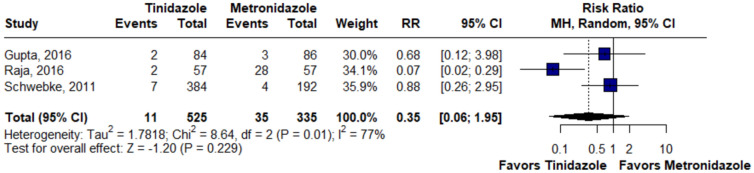
There was no statistically significant difference between the groups in terms of cure at the first (RR 1.03; 95% CI 0.92–1.14; p = 0.62; I2 = 76%; Fig. 2) and second appointment (RR 1.05; 95% CI 0.80–1.38; p = 0.72; I2 = 88%; Fig. 2), nausea (RR 0.89; 95% CI 0.39–2.04; p = 0.78; I2 = 83%; Fig. 3), bad or metallic taste (RR 0.74; 95% CI 0.12–4.45; p = 0.74; I2 = 89%; Fig. 4), diarrhea (RR 0.35; 95% CI 0.06–1.95; p = 0.22; I2 = 77%; Fig. 5), and headache (RR 1.24; 95% CI 0.58–2.63; p = 0.58; I2 = 45%; Fig. 6).
Fig. 2.
There was no statistically significant difference in terms of cure between groups at the first and second appointments. MH Mantel–Haenszel method, CI Confidence Interval
Fig. 3.
There was no statistically significant difference in terms of nausea between groups. MH Mantel–Haenszel method, CI Confidence Interval
Fig. 4.
There was no statistically significant difference in terms of bad or metallic taste between groups. MH Mantel–Haenszel method, CI Confidence Interval
Fig. 5.
There was no statistically significant difference in terms of diarrhea between groups. MH Mantel–Haenszel method, CI Confidence Interval
Fig. 6.
There was no statistically significant difference in terms of headache between groups. MH Mantel–Haenszel method, CI Confidence Interval
Subanalyses in selected populations
In a subanalysis of RCT-only data, there was no statistically significant difference between groups in terms of cure at the first appointment (RR 1.00; 95% CI 0.88–1.14; p = 0.98; I2 = 80%; Supplementary Fig. 1) and at the second appointment (RR 1.05; 95% CI 0.80–1.38; p = 0.72; I2 = 88%; Supplementary Fig. 1). There were no statistically significant differences within 1 week (RR 1.06; 95% CI 0.94–1.19; p = 0.37; I2 = 70%; Supplementary Fig. 2), 2 weeks (RR 0.99; 95% CI 0.72–1.36; p = 0.95; I2 = 55%; Supplementary Fig. 2) and 4 weeks (RR 1.02; 95% CI 0.75–1.37; p = 0.91; I2 = 91%; Supplementary Fig. 2).
There were no statistically significant differences for doses of 0.5 g (RR 0.99; 95% CI 0.84–1.17; p = 0.88; I2 = 79%; Supplementary Fig. 3), 1 g (RR 0.89; 95% CI 0.79–1.01; p = 0.06; I2 = 0%; Supplementary Fig. 3) and 2 g (RR 1.08; 95% CI 0.95–1.24; p = 0.23; I2 = 66%; Supplementary Fig. 3). When only RCTs were considered for this subanalysis, the results remained not statistically significant (Supplementary Fig. 4).
Leave-one-out analysis
The leave-one-out analysis showed the robustness of the pooled results for the outcomes of nausea, diarrhea, and headache. For those outcomes, there was no significant variability of the effect size with the removal of each study (i.e. changing from favoring one side to another, or even becoming significant). For all outcomes, the removal of the study “Raja, 2016” reduced the heterogeneity to 0% (Supplementary Figs. 5–8).
Quality assessment
Individual bias assessment is reported in Supplementary Fig. 9 and 10. RCTs were assessed using Rob2 [14]. Five studies lost points in domains related to lack of information or loss to follow-up [7, 9–12]. A non-randomized study was evaluated using Robins-I and was considered to have a serious risk of bias due to the important confounding bias due to the inclusion of patients with trichomoniasis, along with bacterial vaginosis [8].
Discussion
In this systematic review and meta-analysis of 6 studies, including 1,036 patients, we compared efficacy and safety of tinidazole and metronidazole in patients with bacterial vaginosis. The main findings from the combined analysis were: (1) at the first and second follow-up consultation, the cure rate was not different between patients treated with tinidazole or metronidazole and (2) there was no statistically significant difference in adverse events between the tinidazole and metronidazole groups.
As previously mentioned, the first choice treatment regimen recommended by the CDC for treating bacterial vaginosis is Metronidazole 500 mg orally twice daily for 7 days. Oral tinidazole is presented as an alternative regimen in dosages of 2 g once daily for 2 days or 1 g once daily for 5 days [6]. However, currently there are questions raised about the ideal drug for the treatment of BV, as studies indicate controversial data and even antimicrobial and pharmacokinetic advantages of tinidazole when compared to metronidazole [18]. In the study conducted by Tulkar et al., after one week of treatment with tinidazole, 100% of the patients were cured, whereas, among those who received metronidazole, only 88% were cured at the first follow-up consultation [11]. In contrast, Mohanty et al., reveals potential superiority of metronidazole for BV [8]. However, the majority of included studies revealed that there is no statistically significant difference between the two drugs in question [7, 9, 10]. These data corroborate the findings of our meta-analysis, where tinidazole and metronidazole are equivalent for the cure of BV.
Similarly, in the pooled analysis, our results did not show a difference between the groups regarding the cure rate at the second follow-up visit. This reinforces the equivalence of the two drugs in short and long-term BV cure outcomes. Therefore, when considering the best therapeutic option, it is important to take into account factors that directly impact therapy adherence, such as dosing frequency associated with patient preference [19]. With a longer elimination half-life and lower total clearance than metronidazole, tinidazole achieves higher serum concentrations after oral and intravenous administration, reducing the need for multiple daily doses and allowing for shorter therapy [18, 20, 21]. These pharmacokinetic aspects may favor greater treatment adherence and, consequently, better clinical outcomes in the management of bacterial vaginosis.
The daily dosage of tinidazole varied across the included studies, with regimens of 500 mg, 1 g, and 2 g. When analyzing the tinidazole dosages separately, no statistically significant differences in cure rates were observed between the different dosing regimens. Even when considering only RCTs for this subanalysis, the results remained without statistically significant differences. These findings suggest that, regardless of the dose used, tinidazole demonstrated similar efficacy, which may be relevant for choosing a more convenient treatment regimen based on factors such as patient adherence and reduction of adverse effects.
The follow-up period in the included studies ranged from 1 to 6 weeks, which can be considered relatively short. This period was sufficient to assess the initial efficacy of the treatments, but a longer follow-up could provide a more in-depth understanding of the durability of therapeutic effects and the recurrence rate of bacterial vaginosis. The recurrent nature of the condition suggests that short-term results may limit the assessment of the long-term benefits of the treatments. Therefore, studies with longer follow-up periods would be important to evaluate the persistence of therapeutic effects and symptom recurrence, further expanding the understanding of the long-term efficacy of the therapeutic regimens.
Is known, however, that tinidazole is not safe for use during pregnancy, and it is essential to understand that its use is being discussed here in the context of non-pregnant patients. Regarding adverse events, a metallic taste, gastrointestinal irritation, abdominal pain, diarrhea, and headache are commonly associated with the use of these medications [22–24]. Although frequent, these manifestations are characterized as mild to moderate in severity, allowing the drug to be considered safe when administered correctly [25]. Consistent with this meta-analysis, individual studies do not reveal differences in the safety profile of adverse events between patients receiving tinidazole and those receiving metronidazole [7, 10]. Among the analyzed side effects, nausea emerges as the most common, affecting 16% of the analyzed patients, followed by the metallic taste, affecting 11% of patients.
This study has several limitations. The daily dosage of tinidazole varied across the included studies, with regimens of 500 mg, 1 g, or 2 g, potentially affecting the comparability of efficacy results. Additionally, the follow-up period was relatively short, ranging from 1 to 6 weeks, which may limit the assessment of long-term treatment efficacy and recurrence rates. Another significant limitation is the lack of age restrictions in the included studies, which could contribute to variability in the results. Different age groups may present distinct physiological and hormonal characteristics that influence both the progression of bacterial vaginosis and the response to treatment. However, subgroup analyses based on age could not be performed due to the absence of stratified data in the included studies. This limitation highlights the need for future research to address this gap and provide more comprehensive analyses.
Variations in tinidazole dosage, follow-up duration, and the lack of age-based stratification were carefully considered during the interpretation of the results. Furthermore, sensitivity analyses were conducted to ensure the robustness of the findings, minimizing the potential impact of biases introduced by individual studies [16].
Conclusion
In summary, our results reveal that there is no significant difference between the groups regarding the cure of bacterial vaginosis at the first and second follow-up visit and adverse events such as nausea, bad or metallic taste, headache and diarrhea. Considering the similar efficacy and safety of tinidazole and metronidazole, these findings suggest the potential of tinidazole as a first-line treatment for bacterial vaginosis. In this regard, its use should be determined at the discretion of physicians and patients through shared decision-making based on individualized considerations of the risks and benefits of treatment, in addition to drug availability. It is also important to consider the contraindications for the use of each drug, such as the non-recommendation of using tinidazole in pregnant women.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BV
Bacterial vaginosis
- CDC
Centers for Disease Control and Prevention of the United States
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCT
Randomized controlled trial(s)
- RR
Risk-ratio
Author contributions
Milene Vitória Sampaio Sobral contributed to the conception and design of the study. The preparation of the material was carried out by Milene Vitória Sampaio Sobral, Livia Kneipp Rodrigues, Victor Gonçalves Soares, Clara de Andrade Pontual Peres and Maria Julia Gonzaga Pascoalin, Data collection was carried out by João Lucas de Magalhães Leal Moreira, Livia Kneipp Rodrigues, Lubna Al-Sharif and Maria Julia Gonzaga Pascoalin. The analyzes were carried out by Victor Gonçalves Soares and João Lucas de Magalhães Leal Moreira. The first draft of the manuscript was written by all authors. General supervision of the manuscript was carried out by Fernando Augusto Barreiros and Marina Ayabe Gomes de Moraes. All authors read and approved the final manuscript.
Funding
The authors have not disclosed any funding.
Data availability
Data are available upon request.
Declarations
Conflict of interest statement
The authors Milene Vitória Sampaio Sobral, Victor Gonçalves Soares, João Lucas de Magalhães Leal Moreira, Livia Kneipp Rodrigues, Hilária Saugo Faria, Clara de Andrade Pontual Peres, Lubna Al-Sharif, Maria Julia Gonzaga Pascoalin,1 Fernando Augusto Barreiros and Marina Ayabe Gomes de Moraes have no relevant financial or non-financial interests to disclose. All authors report no relationships that could be construed as a conflict of interest. The authors have no competing interests to declare that are relevant to the content of this article. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coudray MS, Madhivanan P (2020) Bacterial vaginosis—a brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol 245:143–148. 10.1016/j.ejogrb.2019.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin HL et al (1999) Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180(6):1863–1868. 10.1086/315127 [DOI] [PubMed] [Google Scholar]
- 3.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P (2003) Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 189(1):139–147. 10.1067/mob.2003.339 [DOI] [PubMed] [Google Scholar]
- 4.Sobel JD, Vempati YS (2024) Bacterial vaginosis and vulvovaginal candidiasis pathophysiologic interrelationship. Microorganisms 12(1):108. 10.3390/microorganisms12010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mtshali A, Ngcapu S, Govender K, Sturm AW, Moodley P, Joubert BC (2022) In vitro effect of 5-nitroimidazole drugs against trichomonas vaginalis clinical isolates. Microbiol Spectr. 10.1128/spectrum.00912-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C. for D. C. and Prevention (2021) ‘Sexually Transmitted Infections Treatment Guidelines’, https://www.cdc.gov/std/treatment-guidelines/bv.htm. Accessed 20 Jan 2024
- 7.Gupta M, Sharma A, Gupta G (2015) A comparative study of oral seven day of metronidazole versus tinidazole in bacterial vaginosis. Indian J Public Health Res Dev 6(2):40. 10.5958/0976-5506.2015.00070.4 [Google Scholar]
- 8.K. C. Mohanty and R. Deighton (1987) ‘Comparison of 2 g single dose of metronidazole, nimorazole and tinidazole in the treatment of vaginitis associated with Gardnerella vaginalis’. [Online]. Available: http://jac.oxfordjournals.org/. Accessed 20 Jan 2024 [DOI] [PubMed]
- 9.Raja I, Basavareddy A, Mukherjee D, Meher B (2016) Randomized, double-blind, comparative study of oral metronidazole and tinidazole in treatment of bacterial vaginosis. Indian J Pharmacol 48(6):654. 10.4103/0253-7613.194843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwebke JR, Desmond RA (2011) Tinidazole vs metronidazole for the treatment of bacterial vaginosis. Am J Obstet Gynecol 204(3):211.e1-211.e6. 10.1016/j.ajog.2010.10.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thulkar J, Kriplani A, Agarwal N (2012) A comparative study of oral single dose of metronidazole, tinidazole, secnidazole and ornidazole in bacterial vaginosis. Indian J Pharmacol 44(2):243. 10.4103/0253-7613.93859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Xiaodan Q, Hong X, Heyong Y (2008) Comparasion of clinical efficacy of 5-nitroimidazole drugs in the treatment of bacterial vaginosis. Natl Med J China 88:2201–2203 [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 10.1136/bmj.b2535 [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 15.Sterne JAC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT et al., ‘Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023)’, Cochrane, 2023. Available from www.training.cochrane.org/handbook. Accessed 20 Jan 2024
- 17.Rs Team (2020) ‘RStudio: Integrated Development for R. RStudio’. http://www.rstudio.com/. Accessed 20 Jan 2024
- 18.Männistö P et al (1984) Concentrations of metronidazole and tinidazole in female reproductive organs after a single intravenous infusion and after repeated oral administration. Infection 12(3):197–201. 10.1007/BF01640899 [DOI] [PubMed] [Google Scholar]
- 19.Losi S, Berra CCF, Fornengo R, Pitocco D, Biricolti G, OrsiniFederici M (2021) The role of patient preferences in adherence to treatment in chronic disease: a narrative review’. Drug Target Insights. 10.33393/dti.2021.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattila J, Männistö PT, Mäntylä R, Nykänen S, Lamminsivu U (1983) Comparative pharmacokinetics of metronidazole and tinidazole as influenced by administration route. Antimicrob Agents Chemother 23(5):721–725. 10.1128/AAC.23.5.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood BA, Monro AM (1975) ‘Pharmacokinetics of tinidazole and metronidazole in women after single large oral doses. Sex Transm Infect. 10.1136/sti.51.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czeizel AE, Kazy Z, Vargha P (2003) Oral tinidazole treatment during pregnancy and teratogenesis. Int J Gynecol Obstet 83(3):305–306. 10.1016/S0020-7292(03)00259-5 [DOI] [PubMed] [Google Scholar]
- 23.Karrar HR et al (2021) Metronidazole-induced metallic taste: a systematic review and meta-analysis. J Pharm Res Int. 10.9734/jpri/2021/v33i58A34120 [Google Scholar]
- 24.Ayinde O, Ross JDC (2023) The frequency and duration of side-effects associated with the use of oral metronidazole; a prospective study of VITA trial participants. Int J STD AIDS 34(12):897–902. 10.1177/09564624231179505 [DOI] [PubMed] [Google Scholar]
- 25.Mikamo H, Yuasa A, Wada K, Crawford B, Sugimoto N (2016) Optimal treatment for complicated intra-abdominal infections in the era of antibiotic resistance: a systematic review and meta-analysis of the efficacy and safety of combined therapy with metronidazole. Open Forum Infect Dis. 10.1093/ofid/ofw143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.