Abstract
Sulfur mustard (HD) alkylates biomolecules such as proteins, generating specific biomarkers. This study employs steric hindrance, electronic effects, and solvent effects through an occupancy-removal strategy to synthesize regioisomers [N1-HETE]-His and [N3-HETE]-His, overcoming isomer separation challenges in conventional methods. Density functional theory (DFT) calculations revealed hexafluoroisopropanol (HFIP)’s critical role in directing HD’s regioselective alkylation: HFIP modulates steric and electronic environments to preferentially target N1 or N3 sites of histidine imidazole rings, with predictions validated experimentally. The method further enables selective detection of the isomers in HD-contaminated plasma via standard addition, advancing absolute quantification. This work not only establishes a precision synthesis platform for biomarkers but also elucidates HFIP’s unique role in imidazole regioselectivity, offering insights for medicinal chemistry and HD toxicology. These findings hold implications for HD exposure tracking, mechanism analysis, clinical diagnostics, and antidote development.
Subject terms: Mass spectrometry, Chemical tools, Reaction mechanisms, Synthetic chemistry methodology
Sulfur mustard (HD) is a highly reactive chemical warfare agent capable of alkylating proteins, however, the absolute quantification of HD-exposed biomarkers remains challenging. Here, the authors prepare N1/N3-histidine modified with 2-hydroxyethylthioethyl using precision synthesis strategies and employ them as external standards to identify the HD-exposed plasma.
Introduction
Sulfur mustard (HD), also referred to as sulfur mustard, whose synthesis, storage and use have been forbidden since 1997 with the entry into force of the chemical weapons convention (CWC), is a well-known vesicant agent that induces inflammation and vesiculation when exposed to living organisms1. It has been one of the most significant and widely employed harmful compounds in modern history, attributable to the accessibility of its raw materials, the simplicity of its preparation technology, its chemical stability, and its high toxicity2–5. This is particularly evident in its use as a chemical warfare agent during World War I and II. Furthermore, in subsequent conflicts, such as the Iran–Iraq War and ongoing tensions in the Middle East, HD has continued to result in a substantial number of casualties6–8. Furthermore, unexpected exposures to HD and related injuries have occasionally occurred9,10. To date, a significant quantity of abandoned chemical weapons containing HD remains in China, leading to reported accidental casualties in various situations11. Consequently, HD remains a considerable threat to human life and contemporary society12. Consequently, it is imperative to establish reliable, convenient, and sensitive methodologies for the retrospective analysis of exposure to HD, which is relevant for chemical weapon verification, forensic identification, and clinical diagnosis.
Currently, several methodologies have been developed for the analysis of HD adducts and metabolites. Certain HD metabolites that are formed in vivo serve as valuable biomarkers, including hydrolysis products13,14, oxidation products15,16, and β-lyase products17,18, which are typically excreted from the body within a few days, resulting in such low concentrations that direct analysis becomes impractical13. The deoxyribonucleic acid (DNA) damage induced by HD is subject to repair, and the resultant deoxynucleotide adducts can also be rapidly eliminated from the organism13. In contrast, HD protein adducts can persist in the human body for extended periods, which exhibit greater chemical stability and are extensively utilized for medium- and long-term detection of HD exposure11,19–25. Several retrospective methodologies for assessing HD exposure have been established based on the analysis of plasma samples26,27. Due to the fact that human serum albumin (HSA) is the most abundant protein in human plasma, the study of HD-HSA adducts has been conducted extensively28–39. The existing literature indicates that various approaches for retrospective analysis have been developed utilizing several biomarkers. Among these, [2-[(2-hydroxyethyl)thio]ethyl]-cysteine-proline (HETE-CP)29–33 and [2-[(2-hydroxyethyl)thio]ethyl]-cysteine-proline-phenylalanine (HETE-CPF)34–39, derived from the digestion of proteins using pronase and proteinase K, respectively, have garnered significant attention due to their high abundance.
In recent years, the detection methods for amino acid adducts resulting from HD exposure have garnered significant attention40–42. When employed as biomarkers for exposure to chemical warfare agents, such as HD, these adducts demonstrate sequence non-specificity. Consequently, the signals from adducts located at various sites can be integrated to enhance detection sensitivity. Furthermore, the detection of amino acid adducts is not constrained by the species being analyzed, allowing for cross-species detection capabilities. Additionally, a diverse array of amino acid adducts can be produced through a uniform experimental procedure, facilitating the simultaneous qualitative and quantitative assessment of multiple biomarkers. Amino acid adducts can be derived from the pronase hydrolysis of protein adducts, with limits of detection (LOD) values for mustard gas exposure in plasma based on these adducts reaching as low as 1.00 ng/mL through the application of ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC-TQ MS)42. However, there remains a notable deficiency in absolute quantitative detection methods for amino acid adducts. The absolute concentration of these adducts is crucial, as it not only indicates the extent of poisoning in affected individuals but also holds significant implications for understanding the mechanisms of poisoning, as well as for the clinical diagnosis and treatment of those exposed. Therefore, the total synthesis of well-defined amino acid-HD adducts facilitates cross-species detection, enhances detection sensitivity, and allows for absolute quantification of biomarkers43. Although the synthesis of several common amino acid-HD adducts and their corresponding analytical methods have been documented, the preparation of well-defined HD-histidine adducts presents a greater challenge44. The challenge presented by the analogous reactivity of the two nitrogen atoms situated at different positions within the imidazole ring of histidine resulted in the formation of a mixture of the N1- and N3-isomers of the target compound in a 1:1 ratio, which was obtained from the conventional reaction between tert-butyloxycarbonyl (Boc)-histidine methyl ester and 2-(2-tert-butyloxyethylthio)ethyl chloride. Although this method did not yield a N1, N3-di-alkylated histidine derivative and the separation of the isomers was theoretically feasible through column chromatography, the purification process proved to be quite challenging (Δ Retention Factor (ΔRf) < 0.05)40. Additionally, the absence of structurally defined compounds as standards complicates the discrimination between these two isomers45.
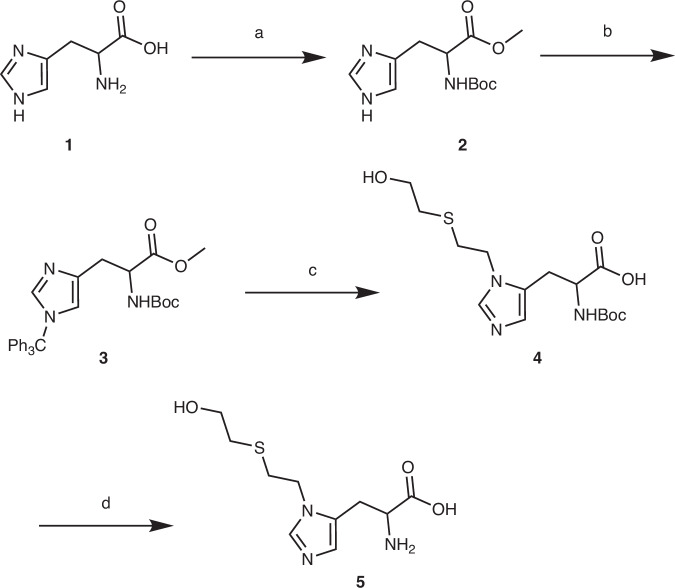
In prior research, we utilized the non-specificity of pronase to digest plasma samples exposed to HD, successfully identifying five amino acid adducts as biomarkers for retrospective assessment of HD exposure within a single analytical run42. Additionally, we developed a highly sensitive quantification method employing derivatization technology, which enhanced both the reliability and throughput of the analysis. In this study, we established a series of precision synthesis methods that successfully produced HD-histidine adducts, specifically N1-[2-[(2-hydroxyethyl)thio]ethyl]-Histidine ([N1-HETE]-His) and N3-[2-[(2-hydroxyethyl)thio]ethyl]-Histidine ([N3-HETE]-His), along with precise structural characterization (Scheme 1). This was achieved through the application of steric hindrance and occupancy-removal strategies, thereby circumventing the challenges associated with the separation of mixtures and the purification of targets that typically arise from simplistic synthesis methods.
Scheme 1.

Structures of HD, histidine and the corresponding biomarkers.
Results and discussion
Synthetic methodologies and strategies for [N1-HETE]-His
In the synthesis pathway of [N1-HETE]-His (refer to Supplementary Information (SI) and Scheme 2), the reaction between l-histidine (1) and an excess of sulfoxide chloride (2 equiv., added in two portions) in methanol over 16 hours resulted in the formation of histidine methyl ester hydrochloride salt with a yield of 95% (see Scheme 2, step a-1 and SI, Section 2.1.1). Subsequently, the synthesis of N-tert-butyloxycarbonyl (Boc)-protected histidine (2) was achieved with a yield of 69% in dry anhydrous methanol through a reaction with triethylamine (5 equiv.) and di-tert-butyl dicarbonate ((Boc)2O) (2.3 equiv.) at ambient temperature (see Scheme 2, step a-2). This was followed by a deprotection using potassium carbonate (K2CO3) under reflux conditions (see Scheme 2, step a-3)46. Notably, the step to protect the acyclic amino group with a Boc group was not specific. In addition to introducing Boc on this group, it also added to the N1 or N3 groups of the histidine ring. The pair of regioisomers obtained—one Boc group on the acyclic amino group and one on either N1 or N3 of the histidine ring—were observed by thin layer chromatography (TLC). The starting material (Rf 0) disappeared and the two products appeared (Rf 0.2 and 0.3). Treatment of this regioisomeric mixture with K2CO3 (0.1 equiv.) selectively cleaved the Boc groups attached to N1 and N3, but not the acyclic amino Boc group, providing the single product (2) ready for the next step of the synthesis (see SI, Section 2.1.1).
Scheme 2.
Synthesis route of [N1-HETE]-His. Reaction conditions: a 1. SOCl2, MeOH, 16 h; 2. (Boc)2O, Et3N, MeOH, 3 h; 3. K2CO3, MeOH, reflux, 2 h; b Ph3CCl, Et3N, toluene, 80 °C, 3 h; c 1. BrCH2CH2SCH2CH2OH, CaO, ACN, 50 °C, ultrasonic, 20 h; 2. DCM/TFA (9:1), 12 h; 3. 0.2 M NaOH, MeOH/H2O (9:1), 12 h; 4. (Boc)2O, K2CO3, MeOH, 3 h; d DCM/TFA (1:1), 6 h.
The modification of the N3 position of the histidine imidazole ring was achieved under standard reaction conditions utilizing triphenylmethyl chloride (commonly referred to as trityl chloride) and triethylamine47–50. Boc-histidine methyl ester (2) (1 equiv.) and trityl chloride (1 equiv.) were dissolved in toluene at ambient temperature. Following this, the reaction was subjected to basic conditions with triethylamine (1 equiv.) at 80 °C for several hours, leading to the conversion of the starting materials into the new compound. This transformation was monitored by thin-layer chromatography (TLC) employing a dichloromethane/methanol solvent system (97:3, v/v), which yielded a retention factor (Rf) of 0.3 (see Scheme 2, step b). The resulting product was purified through silica gel column chromatography using a dichloromethane-methanol eluent (97:3, v/v), resulting in the target N-Boc-N’-Trityl (Trt)-L-histidine methyl ester (3) with a yield of 97% (see SI, Section 2.1.2).
N-Boc-N’-Trt-L-histidine methyl ester (3) (1 equiv.), 2-((2-bromoethyl)thio)ethan-1-ol (4–5 equiv.), and CaO (1.2 equiv.) were combined in a glass flask. The reaction chamber was evacuated to create a vacuum and subsequently flushed with argon three times to establish an inert atmosphere. Following this, acetonitrile was added to form a 0.2 M solution of compound (3). It is noteworthy that conventional methods involving heating and microwave irradiation in various solvents (such as acetone, acetonitrile, methyl sulfoxide (DMSO), dimethylformamide (DMF), dioxane, ethanol, hexafluoroisopropanol (HFIP), methanol, tetrahydrofuran (THF), toluene, etc.) complicate the synthesis of quaternary ammonium salts51–61. Despite heating to reflux or subjecting the mixture to microwave irradiation for an extended period of one week, only a minimal amount of quaternary ammonium salt was produced from the alkylation reaction, while a substantial quantity of starting materials remained unreacted. However, it was observed that utilizing a standard ultrasonic cleaning machine allowed for the processing of the reactants at an ultrasonic frequency of 40 kHz, which was monitored using LC–MS. Under ultrasonication, N-Boc-N’-Trt-L-histidine methyl ester (3) was completely converted within 24 h, resulting in the formation of an intermediate N-Boc-N’-Trt-L-histidine methyl ester quaternary ammonium salt, a derivative of the HD-histidine adduct, with a yield of 29% after concentration and separation via neutral alumina column chromatography (see Scheme 2, step c-1). Subsequently, the corresponding protecting groups were sequentially removed using excess trifluoroacetic acid (TFA) in DCM (see Scheme 2, step c-2) and sodium hydroxide (NaOH) in a methanol-water mixture (9:1, v/v) (see Scheme 2, step c-3) to obtain the highly polar target product (5)44. However, the high polarity of product (5) posed challenges for purification, leading to the implementation of a strategy involving Boc-derivatization followed by repurification to synthesize Boc-modified histidine (4) (see Scheme 2, step c-4). After deprotecting the Boc-derivative (4) with TFA in DCM, the highly pure target compound [N1-HETE]-His (5) was successfully synthesized with a yield of 55% (see Scheme 2, step d), resulting in an overall yield of 10% for the nine-step synthesis procedure (see SI, section 2.1.3).
Synthetic methodologies and strategies for [N3-HETE]-His
The regioselective synthesis of well-defined [N3-HETE]-His exhibits slight variations compared to the synthesis of [N1-HETE]-His, as depicted in Scheme 3 and the supplementary information (SI). l-Histidine (1) was modified at the N1 position of the histidine imidazole ring through a series of sequential reactions involving an excess of sulfoxide chloride (2 equiv., added in two portions) in methanol over a period of 16 h (see Scheme 3, step a-1). Subsequently, the reaction mixture was treated with 1,1’-carbonyldiimidazole (CDI, 1.5 equiv.) and potassium carbonate (K2CO3, 1.5 equiv.) in 1,2-dichloroethane (DCE) (see Scheme 3, step a-2). These reactions resulted in the formation of histidine methyl ester hydrochloride salt with a yield of 95.0% (see SI, Section 2.1.1) and led to the synthesis of the imidazo[1,5-c]pyrimidine derivative (6) with an 87% yield (see SI, Section 2.2.1)62.
Scheme 3.
Synthesis route of [N3-HETE]-His. Reaction conditions: a 1. SOCl2, MeOH, 16 h; 2. CDI, K2CO3, DCE, 2 h; b ClCH2CH2SCH2CH2OH, HFIP, 50 °C, ultrasonic, 10 h; c 1. 10 M KOH, MeOH, 80 °C, 72 h; 2. (Boc)2O, MeOH, 3 h; d DCM/TFA (1:1), 6 h.
2-((2-Chloroethyl)thio)ethan-1-ol, a monofunctional analog of sulfur mustard commonly referred to as a half mustard (2-chloroethyl ethyl sulfide, CEES) derivative, was employed to facilitate a regioselective alkylation reaction of the imidazo[1,5-c]pyrimidine derivative (6), utilizing 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as a specialized solvent. In contrast, the application of 2-((2-bromoethyl)thio)ethan-1-ol in conjunction with alternative solvents (such as methanol, ethanol, acetone, acetonitrile, dichloromethane (DCM), tetrahydrofuran (THF), among others) resulted in nonselective modifications. In this procedure, the N1-histidine cyclization compound (6, 1 equiv.) and 2-((2-chloroethyl)thio)ethan-1-ol (0.5 equiv.) were introduced into a reaction flask, which was subsequently subjected to an inert atmosphere by evacuating the flask with a pump and refilling it with argon three times. HFIP, which had been degassed through a process of alternating freezing, evacuation, and thawing, was then added as the solvent. The mixture was transferred to an ultrasonic reactor operating at a frequency of 40 kHz, and the alkylation reaction was monitored using thin-layer chromatography (TLC) analysis (see Scheme 3, step b). Upon completion of the reaction, a colorless oily compound (7) was obtained with a yield of 58% through extraction, concentration, and purification (see SI, Section 2.2.2).
The protected N3-histidine adduct (7) was dissolved in methanol. A 10 M potassium hydroxide (KOH) solution was introduced into the reaction system in four aliquots at 10-hour intervals. The protective group was removed by refluxing in a 10 M KOH solution for 72 h (see Scheme 3, step c-1)63. The target product (9) was subsequently obtained by neutralizing the solution to an approximate pH of 8.0 using 1 M HCl. Similar to [N1-HETE]-His (5), the excessive polarity of [N3-HETE]-His (9) presented challenges for purification. Consequently, the strategy of Boc-derivatization and repurification was once again implemented to yield Boc-modified histidine (8) (see Scheme 3, step c-2). Following the deprotection of the Boc-derivative (8) with trifluoroacetic acid (TFA) in DCM, the highly pure target compound [N3-HETE]-His (9) was successfully synthesized with a yield of 16% (see Scheme 3, step d), resulting in a total yield of 8% for the six-step synthesis procedure (see SI, Section 2.2.3).
Density functional theory (DFT) calculations for the alkylation reaction involving imidazole
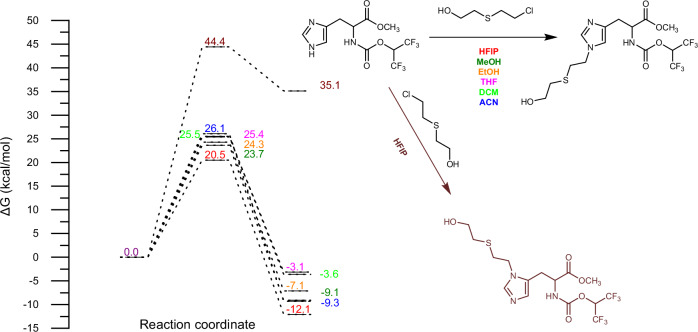
The regioselectivity of the alkylation reaction at either the N1 or N3 position of the histidine imidazole ring under various solvent conditions was elucidated through DFT calculations (see Fig. 1 and Supplementary Information (SI) for computational details). When 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) was utilized as the solvent, the activation barrier for the alkylation reaction at the N1 site of the imidazole ring was significantly reduced to 20.5 kcal/mol, in contrast to the higher barrier of 44.4 kcal/mol observed at the N3 site. Consequently, the alkylation reaction at the N1 position of the histidine imidazole ring was determined to be considerably more favorable. Furthermore, to investigate the influence of various solvents on the reaction occurring at the N1 site of the imidazole ring, a comprehensive examination of the alkylation reaction process was conducted using DFT calculations (refer to Fig. 1 and SI for computational details). The results indicated that HFIP effectively facilitates the alkylation reaction at the N1 site of the imidazole ring compared to other solvents, with the energy barrier shifting from 23.6 to 26.1 kcal/mol for solvents such as methanol (MeOH), ethanol (EtOH), tetrahydrofuran (THF), dichloromethane (DCM), acetonitrile (ACN), and acetone (AT).
Fig. 1. Investigation of the regioselectivity and solvent effects in histidine imidazole N-alkylation via DFT calculations.
Panel Description: (1) Reaction coordinate plot (Left). Shown is the free energy profile (ΔG, kcal/mol) for the N-alkylation of histidine's imidazole ring in various solvents (HFIP, MeOH, EtOH, THF, DCM, ACN). The x-axis represents the reaction progress, while the y-axis shows the free energy change. Solid/dashed lines denote reactants/transition states/products, with distinct colours corresponding to each solvent (HFIP: deep red and brown; MeOH: deep green; EtOH: orange; THF: pink; DCM: light green; ACN: deep blue). (2) Reaction depiction (Right). Reaction substrates/products: Highlighted are the imidazole ring structures of histidine before (reactant: black) and after N3-alkylation (product: black) in the solvent (HFIP: deep red; MeOH: deep green; EtOH: orange; THF: pink; DCM: light green; ACN: deep blue) and N1-alkylation (product: brown) in HFIP (brown).
The transition states were verified through intrinsic reaction coordinate (IRC) calculations and the analysis of imaginary vibrational modes, which connect the reactants and products. The structures of the reactants and products obtained from the IRC calculations served as initial structures for subsequent structural optimizations. Plots depicting total energy and the root-mean-squared (RMS) gradient norm along the IRC are presented in SI. The total energy plots indicate that, with the exception of 7_HFIP_N3, the product exhibits lower energy than the reactant. Furthermore, the analysis of the RMS gradient norm along the IRC reveals that the root mean square forces in Cartesian coordinates for all stationary points are approximately zero.
Chromatographic behaviors and application
As outlined in the instructional section, hydroxyethylthioethyl (HETE) adducts can be generated through the modification of human serum albumin (HSA) by HD, which may serve as biomarkers for the verification of HD exposure in forensic analyses. In our prior research, we identified five amino acid adducts—HETE-Lys, [N1-HETE]-His, [N3-HETE]-His, HETE-Asp, and HETE-Glu—produced from the pronase digestion of HD-exposed human serum albumin (HD-HSA) in plasma, which were selected as biomarkers for the retrospective detection of HD exposure42. In that study, HD-HSA was precipitated from plasma using acetone, subsequently digested with pronase, derivatized with propionic anhydride (PA), and analyzed via ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC-TQ MS). However, the structural and polar similarities between [N1-HETE]-His and [N3-HETE]-His posed significant challenges for their separation during liquid chromatography. This difficulty hindered the acquisition of highly pure [N1-HETE]-His or [N3-HETE]-His, thereby precluding the establishment of an absolute quantitative analysis method for these two HD-amino adducts in the absence of reference materials.
In this study, the aforementioned issues will be addressed through the implementation of precise synthesis strategies. Subsequently, we employed the synthesized [N1-HETE]-His (5) and [N3-HETE]-His (9) to identify significant biomarkers associated with HD-histidine adducts using the standard addition method (refer to Scheme 4). Previous reports have indicated that the first eluting isomer of HETE-His on a silica gel column is the N3-isomer, which exhibits lower polarity compared to the N1-isomer [44]. Conversely, the latter eluting isomer on the C18 column has been identified as [N3-HETE]-His, while the other is identified as [N1-HETE]-His40,42.
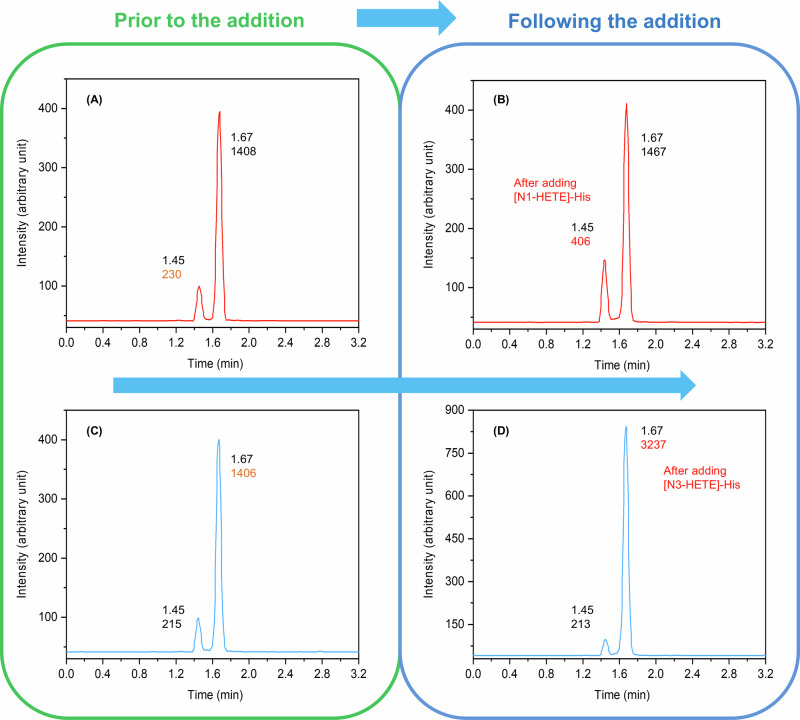
Scheme 4.
Chromatograms before (A, C) and after the standard addition of [N1-HETE]-His (B) and [N3-HETE]-His (D) to the HD-exposed plasma following pronase digestion. UHPLC-TQ MS (MRM) conditions: a monitored transition of 260.1 → 105.0 in positive electrospray ionization (ESI) mode. Separation was achieved using a C18 Zorbax Eclipse Plus reversed phase column (1.8 μm, 150 × 2.1 mm I.D.) employing a gradient elution program, with the mobile phase comprising water and acetonitrile at a flow rate of 1.0 mL min−1.
By introducing 6.8 μL (200 ng/mL) of the well-defined [N1-HETE]-His aqueous solution to the HD-exposed sample, which had previously undergone purification and pronase digestion, we observed an increase in the peak area of the characteristic chromatographic peak with a retention time of 1.45 min. The response value increased from 230 to 406 (see Scheme 4A, B). Subsequently, upon the addition of 5.8 μL (500 ng/mL) of the well-defined [N3-HETE]-His aqueous solution, the peak area of the characteristic chromatographic peak with a retention time of 1.67 min exhibited a response value increase from 1406 to 3237 (see Scheme 4C, D). Consequently, it was determined that the first eluate from the C18 column corresponded to [N1-HETE]-His, which is slightly more polar, while the second eluate was identified as [N3-HETE]-His, which is slightly less polar. These findings are consistent with previously reported results40,42. This study successfully achieved the differentiation between the two biomarkers using the standard addition method for the first time.
Conclusions
In this study, a regionally specific alkylation methodology was developed for the synthesis of histidine adducts, which present considerable challenges for separation via liquid chromatography in the context of diagnosing exposure to HD, unless derivatization techniques are employed prior to analysis42. The target compounds, specifically the isomers [N1-HETE]-His and [N3-HETE]-His, were successfully synthesized. In contrast to the straightforward synthesis method previously reported44, this research employed strategies to introduce steric hindrance and facilitate the removal of occupying groups, thereby addressing the challenges associated with the separation and purification of isomers. The biomarkers were effectively distinguished from plasma samples contaminated with HD, thereby supporting the subsequent establishment of absolute quantitative detection methods for amino acid adducts. This advancement addresses the limitations of traditional quantitative analysis methods, which are incapable of directly detecting these biomarkers. It is well established that the absolute concentration of amino acid adducts not only reflects the extent of mustard gas adduction to plasma proteins and the degree of poisoning in exposed individuals but also holds significant implications for understanding the mechanisms of poisoning and for the clinical diagnosis and treatment of affected personnel. Furthermore, DFT was employed to systematically investigate the regioselectivity of N1 or N3 alkylation of the histidine imidazole ring, providing a foundational reference for the study of the regioselectivity of organic reactions involving imidazole compounds. The findings presented offer substantial reference value for the investigation of regioselective reactions involving the imidazole group, as well as for understanding the relationship between amino acid adducts and the exposure dose to HD, along with the associated damage mechanisms.
Methods
Reagents and methods
2-((2-bromoethyl)thio)ethan-1-ol (95% purity) and 2-((2-chloroethyl)thio)ethan-1-ol (98% purity) were provided by the Laboratory of Analytical Chemistry, Research Institute of Chemical Defense of Chinese People’s Liberation Army. The other reagents utilized in this study were procured from commercial suppliers and employed without modification. All glassware was thoroughly cleaned with detergent, rinsed with acetone, and subsequently dried in an oven at 125 °C prior to use. Reactions sensitive to moisture were conducted under an argon atmosphere, with sensitive reagents introduced via syringe and cannula techniques. Thin layer chromatography (TLC) was performed on pre-coated silica gel HSGF254 plates, and visualization was achieved using ultraviolet light (254 nm), iodine, potassium permanganate solution, or ammonium phosphomolybdate in alcohol. Column chromatography (CC) was executed using silica gel (300–400 mesh), sourced from Qingdao Haiyang Chemical. Nuclear magnetic resonance (NMR, 1H & 13C) spectra were acquired in CDCl3 or D2O at room temperature utilizing a Bruker Avance III HD 600 MHz apparatus.
Synthetic methods
The synthetic pathways for the well-defined compounds [N1-HETE]-His and [N3-HETE]-His are depicted in Scheme 2 and Scheme 3, respectively. Both schemes utilize commercially available l-histidine 1 as the initial substrate, albeit through distinct methodologies and strategies. Detailed experimental procedures are provided in the supplementary information (SI).
Computation methods
All calculations were conducted utilizing density functional theory (DFT) at the M06-2X level of theory, employing the Gaussian 16 software suite64. The def2-TZVP basis set was applied to all atoms involved in the study. The gas-phase geometries of all intermediates and transition states were fully optimized without imposing any symmetry constraints. Subsequent harmonic frequency calculations were performed to confirm that the local minima exhibited zero imaginary frequencies, while the transition states displayed one imaginary frequency. This process also facilitated the derivation of thermal corrections for Gibbs free energies. The transition states were validated through intrinsic reaction coordinate (IRC) calculations and the analysis of imaginary vibrational modes, which connected the reactants and products. For the calculation of single point energies, the double hybrid functional (B2PLYP method)65, known for providing more accurate energetic information, was employed alongside the cc-pVTZ basis set. The Gibbs free energies of all stationary points were determined by applying thermal corrections to the Gibbs free energy in the gas phase, in conjunction with the single point energy calculations. Detailed descriptions of the calculation procedures can be found in the Supplementary Information (SI).
UHPLC-TQ MS target analysis
The prepared samples were subjected to analysis using a 1290 II Infinity series ultra-high performance liquid chromatograph system, which was connected to an Agilent Technologies Ultivo triple quadrupole mass spectrometer. A volume of 20.0 μL of the samples was separated utilizing a C18 pre-column (1.8 μm, 12.5 × 2.1 mm inner diameter (I.D.), from Agilent Technologies) in conjunction with a C18 Zorbax Eclipse Plus reversed phase column (1.8 μm, 150 × 2.1 mm I.D., from Agilent Technologies). The mobile phases employed were solvent A, consisting of 0.1% (v/v) formic acid in water, and solvent B, comprising 0.1% (v/v) formic acid in acetonitrile. The optimized gradient elution program commenced with 2% solvent B for 3.0 min, followed by a linear increase to 6% solvent B over 0.5 min, which was maintained for 3.5 min. Subsequently, the concentration of solvent B was increased to 20% within 0.5 min and held for an additional 4.5 min. The column was then washed with 95% solvent B for 3.5 min and re-equilibrated with 2% solvent B for 3 min. The column temperature was maintained at 30 °C, and a constant flow rate of 300 μL/min was employed. Mass spectrometric detection was performed using a triple quadrupole mass analyzer equipped with an electrospray ionization (ESI) source operating in positive ionization mode. Data were collected using MassHunter software (Agilent) in multiple reaction monitoring (MRM) mode, with a dwell time of 80 ms for each transition. The optimized parameters were determined: a gas temperature of 320 °C, a gas flow rate of 11 L/min, a nebulizer pressure of 241.3 kPa, a capillary voltage of 5000 V, a fragmentor voltage of 80 V, a collision energy of 15 V, and transitions for the precursor ion at 316.1 m/z and the product ion at 105.0 m/z.
Supplementary information
Acknowledgements
We sincerely acknowledge the generous support provided by the State Key Laboratory of NBC Protection for Civilian, which includes continuous research funding (Grant No. SKLNBC2021-04) and essential assistance in material characterization. We also appreciate the material and technical contributions made by the Research Institute of Chemical Defense of the Chinese People’s Liberation Army.
Author contributions
Long Wen: Writing—original draft, Investigation. Zhibin Shu: Methodology, Supervision, Conceptualization. Li Pan: Resources, Methodology, Formal analysis for DFT calculation. Bo Chen: Resources, Methodology, Investigation. Gang Qu: Resources, Methodology, Formal analysis for LC–MS and LC–HRMS. Shu Geng: Resources, Methodology, Formal analysis for NMR. Yuntao Yang: Project administration. Yan Jiang: Methodology, Conceptualization. Shilei Liu: Methodology, Conceptualization.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The data supporting the findings of this study are available within the paper and and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Long Wen, Zhibin Shu, Li Pan.
These authors jointly supervised this work: Zhibin Shu, Yan Jiang, Shilei Liu.
Contributor Information
Zhibin Shu, Email: johns0203@163.com.
Yan Jiang, Email: jiangyan199@126.com.
Shilei Liu, Email: liu_shilei@lacricd.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-025-01479-1.
References
- 1.Borak, J. & Sidell, F. R. Agents of chemical warfare: sulfur mustard. Ann. Emerg. Med.21, 303–308 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Balali-Mood, M. & Hefazi, M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fund. Clin. Pharmacol.19, 297–315 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Abbas, F. Report of the specialists appointed by the secretary-general of the United Nations to investigate allegations by the Islamic Republic of Iran concerning the use of chemical weapons. Mol. Ecol.15, 4577–4588 (1984). [PubMed] [Google Scholar]
- 4.Kilic, E., Ortatatli, M., Sezigen, S., Eyison, R. K. & Kenar, L. Acute intensive care unit management of mustard gas victims: the Turkish experience. Cutan. Ocul. Toxicol.37, 332–337 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Quillen, C. The Islamic state’s evolving chemical arsenal. Stud. Confl. Terror.39, 1019–1030 (2016). [Google Scholar]
- 6.Noort, D., Benschop, H. P. & Black, R. M. Biomonitoring of exposure to chemical warfare agents: a review. Toxicol. Appl. Pharmacol.184, 116–126 (2002). [PubMed] [Google Scholar]
- 7.Darvishi, B., Panahi, Y., Ghanei, M. & Farahmand, L. Investigating prevalence and pattern of long-term cardiovascular disorders in sulphur mustard-exposed victims and determining proper biomarkers for early defining, monitoring and analysis of patients’ feedback on therapy. Basic Clin. Pharmacol. Toxicol.120, 120–130 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Sezigen, S. et al. Victims of chemical terrorism, a family of four who were exposed to sulfur mustard. Toxicology Letters303, 9–15 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Prescott, T. Accidental sulfur mustard exposure from explosive ordnance in a UK military service person. BMJ Mil. Health167, 287–288 (2021). [DOI] [PubMed] [Google Scholar]
- 10.John, H., Koller, M., Worek, F., Thiermann, H. & Siegert, M. Forensic evidence of sulfur mustard exposure in real cases of human poisoning by detection of diverse albumin-derived protein adducts. Arch. Toxicol.93, 1881–1891 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Zhu, J. & Gao, C. On Chemical Warfare in War of Japan Aggression Against China and Post-War Issues (The Publishing House of Ordnance Industry, Beijing, 2010).
- 12.Xia, Z. Chemical Weapons: Defense and Destruction (Chemical Industry Press, Beijing, 2014).
- 13.Xu, H. et al. Four sulfur mustard exposure cases: overall analysis of four types of biomarkers in clinical samples provides positive implication for early diagnosis and treatment monitoring. Toxicol. Rep.1, 533–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi, M. et al. Simultaneous determination of sulfur mustard and related oxidation products by isotope-dilution LC-MS/MS method coupled with a chemical conversion. J. Chromatogr. B1028, 42–50 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Popiel, S., Nawała, J., Dziedzic, D., Gordon, D. & Dawidziuk, B. Study on the kinetics and transformation products of sulfur mustard sulfoxide and sulfur mustard sulfone in various reaction media. Int. J. Chem. Kinet.50, 75–89 (2018). [Google Scholar]
- 16.Nie, Z. et al. Monitoring urinary metabolites resulting from sulfur mustard exposure in rabbits, using highly sensitive isotope-dilution gas chromatography-mass spectrometry. Anal. Bioanal. Chem.406, 5203–5212 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Li, C., Chen, J., Liu, Q., Xie, J. & Li, H. Simultaneous quantification of seven plasma metabolites of sulfur mustard by ultra high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B917-918, 100–107 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Lin, Y. et al. Gas chromatographic–tandem mass spectrometric analysis of β-lyase metabolites of sulfur mustard adducts with glutathione in urine and its use in a rabbit cutaneous exposure model. J. Chromatogr. B945-946, 233–239 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Black, R. M., Clarke, R. J., Harrison, J. M. & Read, R. W. Biological fate of sulphur mustard: Identification of valine and histidine adducts in haemoglobin from casualties of sulphur mustard poisoning. Xenobiotica27, 499–512 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Noort, D. et al. Retrospective detection of exposure to sulfur mustard: Improvements on an assay for liquid chromatography-tandem mass spectrometry analysis of albumin/sulfur mustard adducts. J. Anal. Toxicol.28, 333–338 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Yue, L. et al. Abundance of four sulfur mustard-DNA adducts ex vivo and in vivo revealed by simultaneous quantification in stable isotope dilutionultrahigh performance liquid chromatography-tandem mass spectrometry. Chem. Res. Toxicol.27, 490–500 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Y. et al. Simultaneous determination of four sulfur mustard-DNA adducts in rabbit urine after dermal exposure by isotope-dilution liquid chromatographytandem mass spectrometry. J Chromatogr B961, 29–35 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Steinritz, D. et al. Medical documentation, bioanalytical evidence of an accidental human exposure to sulfur mustard and general therapy recommendations. Toxicol. Lett.244, 112–120 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Nie, Z. et al. Determination of the N-terminal valine adduct in rabbit hemoglobin after skin exposure to sulfur mustard. Sci. Sin. Vitae41, 884–889 (2011). [Google Scholar]
- 25.Orlova, O. I., Savel’eva, E. I. & Khlebnikova, N. S. Methods for the detection of sulfur mustard metabolites in biological materials: an analytical review. J. Anal. Chem.68, 1–11 (2012). [Google Scholar]
- 26.Steinritz, D. et al. Alkylated epidermal creatine kinase as a biomarker for sulfur mustard exposure: comparison to adducts of albumin and DNA in an in vivo rat study. Arch. Toxicol.95, 1323–1333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmeisser, W. et al. Transthyretin as a target of alkylation and a potential biomarker for sulfur mustard poisoning: electrophoretic and mass spectrometric identification and characterization. Drug Test. Anal.14, 80–91 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Lueling, R. et al. Sulfur mustard alkylates steroid hormones and impacts hormone function in vitro. Arch. Toxicol.93, 3141–3152 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Gandor, F., Gawlik, M., Thiermann, H. & John, H. Evidence of sulfur mustard exposure in human plasma by LC-ESI-MS-MS detection of the albumin derived alkylated HETE-CP dipeptide and chromatographic investigation of its cis/trans isomerism. J. Anal. Toxicol.39, 270–279 (2015). [DOI] [PubMed] [Google Scholar]
- 30.John, H. et al. Procedures for analysis of dried plasma using microsampling devices to detect sulfur mustard-albumin adducts for verification of poisoning. Anal. Chem.88, 8787–8794 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Chen, B. et al. A sensitive quantification approach for detection of HETE-CP adduct after benzyl chloroformate derivatization using ultra-high-pressure liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem.411, 3405–3415 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Richter, A., Siegert, M., Thiermann, H. & John, H. Alkylated albumin-derived dipeptide C(-HETE)P derivatized by propionic anhydride as a biomarker for the verification of poisoning with sulfur mustard. Anal. Bioanal. Chem.413, 4907–4916 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John, H., Richter, A. & Thiermann, H. Evidence of sulfur mustard poisoning by detection of the albumin-derived dipeptide biomarker C(-HETE)P after nicotinylation. Drug Test. Anal.13, 1593–1602 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Andacht, T. M. et al. Enhanced throughput method for quantification of sulfur mustard adducts to human serum albumin via isotope dilution tandem mass spectrometry. J. Anal. Toxicol.38, 8–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, C. et al. An improved method for retrospective quantification of sulfur mustard exposure by detection of its albumin adduct using ultra-high pressure liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem.407, 7037–7046 (2015). [DOI] [PubMed] [Google Scholar]
- 36.John, H. et al. Optimized verification method for detection of an albumin sulfur mustard adduct at Cys34 using a hybrid quadrupole time-of-flight tandem mass spectrometer after direct plasma proteolysis. Toxicol. Lett.244, 103–111 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Pantazides, B. G. et al. A quantitative method to detect human exposure to sulfur and nitrogen mustards via protein adducts. J. Chromatogr. B1121, 9–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegert, M. et al. Methionine329 in human serum albumin: a novel target for alkylation by sulfur mustard. Drug Test. Anal.11, 659–668 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Avigo, L. et al. Analytical methods based on liquid chromatography for the analysis of albumin adducts involved in retrospective biomonitoring of exposure to mustard agents. Anal. Bioanal. Chem.416, 2173–2188 (2024). [DOI] [PubMed] [Google Scholar]
- 40.Hemme, M., Fidder, A., van der Riet-van Oeveren, D., van der Schans, M. J. & Noort, D. Mass spectrometric analysis of adducts of sulfur mustard analogues to human plasma proteins: approach towards chemical provenancing in biomedical samples. Anal. Bioanal. Chem.413, 4023–4036 (2021). [DOI] [PubMed] [Google Scholar]
- 41.John, H., Hormann, P., Schrader, M. & Thiermann, H. Alkylated glutamic acid and histidine derived from protein-adducts indicate exposure to sulfur mustard in avian serum. Drug Test. Anal.14, 1140–1148 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Chen, B. et al. Simultaneous quantification of multiple amino acid adducts from sulfur mustard-modified human serum albumin in plasma at trace exposure levels by ultra-high performance liquid chromatography-triple quadrupole mass spectrometry after propionyl derivatization. J. Chromatogr. A1678, 463354 (2022). [DOI] [PubMed] [Google Scholar]
- 43.de Bruin-Hoegée, M. et al. Verification of exposure to chemical warfare agents through analysis of persistent biomarkers in plants. Anal. Methods15, 142–153 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Noort, D., Hulst, A. G., Trap, H. C., de Jong, L. P. & Benschop, H. P. Synthesis and mass spectrometric identification of the major amino acid adducts formed between sulphur mustard and haemoglobin in human blood. Arch. Toxicol.71, 171–178 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Chen, B. et al. A proteomics strategy for the identification of multiple sites in sulfur mustard-modified HSA and screening potential biomarkers for retrospective analysis of exposed human plasma. Anal. Bioanal. Chem.414, 4179–4188 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Lim, D., Gründemann, D. & Seebeck, F. P. Total synthesis and functional characterization of selenoneine. Angew. Chem. Int. Ed.58, 15026–15030 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Fletcher, A. R., Jones, J. H., Ramage, W. I. & Stachulski, A. V. The use of the N(π)-phenacyl group for the protection of the histidine side chain in peptide synthesis. J. Chem. Soc.1, 2261–2267 (1979). [Google Scholar]
- 48.Kim, B. M., Park, J. S. & Cho, J. H. Preparation of N(π)-alkyl- histamine and histidine derivatives through efficient alkylation followed by deprotection using activated silica gel. Tetrahedron Lett.41, 10031–10034 (2000). [Google Scholar]
- 49.Kerscher-Hack, S., Renukappa-Gutke, T., Höfner, G. & Wanner, K. T. Synthesis and biological evaluation of a series of N-alkylated imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors. Eur. J. Med. Chem.124, 852–880 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Torikai, K., Yanagimoto, R. & Watanabe, L. A. N(π)-2-naphthylmethoxymethyl-protected histidines: Scalable, racemization-free building blocks for peptide synthesis. Org. Process Res. Dev.24, 448–453 (2020). [Google Scholar]
- 51.Maton, C. et al. Continuous synthesis of peralkylated imidazoles and their transformation into ionic liquids with improved (electro)chemical stabilities. ChemPhysChem13, 3146–3157 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Kim, D. J., Oh, K. H. & Park, J. K. A general and direct synthesis of imidazolium ionic liquids using orthoesters. Green Chem16, 4098–4101 (2014). [Google Scholar]
- 53.Zicmanis, A. & Anteina, L. Dialkylimidazolium dimethyl phosphates as solvents and catalysts for the Knoevenagel condensation reaction. Tetrahedron Lett.55, 2027–2028 (2014). [Google Scholar]
- 54.Maton, C., Van Heckeb, K. & Stevens, C. V. Peralkylated imidazolium carbonate ionic liquids: synthesis using dimethyl carbonate, reactivity and structure. New J. Chem.39, 461–468 (2015). [Google Scholar]
- 55.Finger, L. H., Guschlbauer, J., Harms, K. & Sundermeyer, J. N-heterocyclic olefin-carbon dioxide and -sulfur dioxide adducts: structures and interesting reactivity patterns. Chem. Eur. J.22, 16292–16303 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Jayachandra, R. & Reddy, S. R. A remarkable chiral recognition of racemic Mosher’s acid salt by naturally derived chiral ionic liquids using 19F NMR spectroscopy. RSC Adv.6, 39758–39761 (2016). [Google Scholar]
- 57.Goossens, K. et al. Anisotropic, organic ionic plastic crystal mesophases from persubstituted imidazolium pentacyanocyclopentadienide salts. Chem. Mater.31, 9593–9603 (2019). [Google Scholar]
- 58.Penn, K. R., Anders, E. J. & Lindsay, V. N. G. Expedient synthesis of bis(imidazolium) dichloride salts and bis(NHC) complexes from imidazoles using DMSO as a key polar additive. Organometallics40, 3871–3875 (2021). [Google Scholar]
- 59.Berg, I. et al. Self-assembled monolayers of N-heterocyclic olefins on Au(111). Angew. Chem. Int. Ed.62, e202311832 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Both, N. F., Spannenberg, A., Jiao, H., Junge, K. & Beller, M. Bis(N-heterocyclic carbene) manganese(I) complexes: efficient and simple hydrogenation catalysts. Angew. Chem. Int. Ed.62, e202307987 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Rowsey, R. A., Hilgar, J. D. & Romero, N. A. Silylimidazolium hexafluorophosphate salts as synthetic precursors to N-heterocyclic carbene pentafluorophosphorus adducts. Org. Lett.26, 4750–4755 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guillen, F., Brégeon, D. & Plaquevent, J.-C. (S)-Histidine: the ideal precursor for a novel family of chiral aminoacid and peptidic ionic liquids. Tetrahedron Lett.47, 1245–1248 (2006). [Google Scholar]
- 63.Hergueta, A. R., López, C., Fernández, F., Caamaño, O. & Blanco, J. M. Synthesis of two enantiomerically pure precursors of cyclobutane carbocyclic nucleosides. Tetrahedron14, 3773–3778 (2003). [Google Scholar]
- 64.Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc.119, 215–241 (2008). [Google Scholar]
- 65.Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys.124, 034108 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper and and its supplementary information files.