Abstract
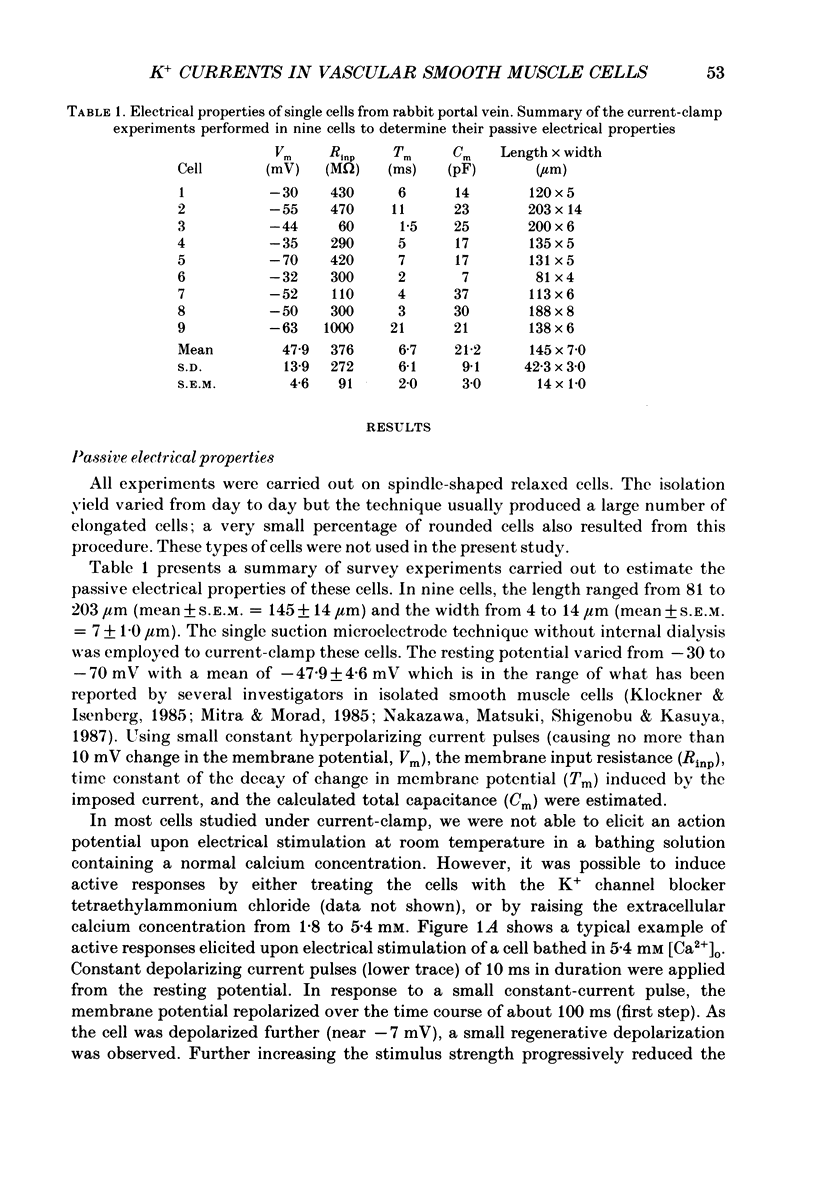
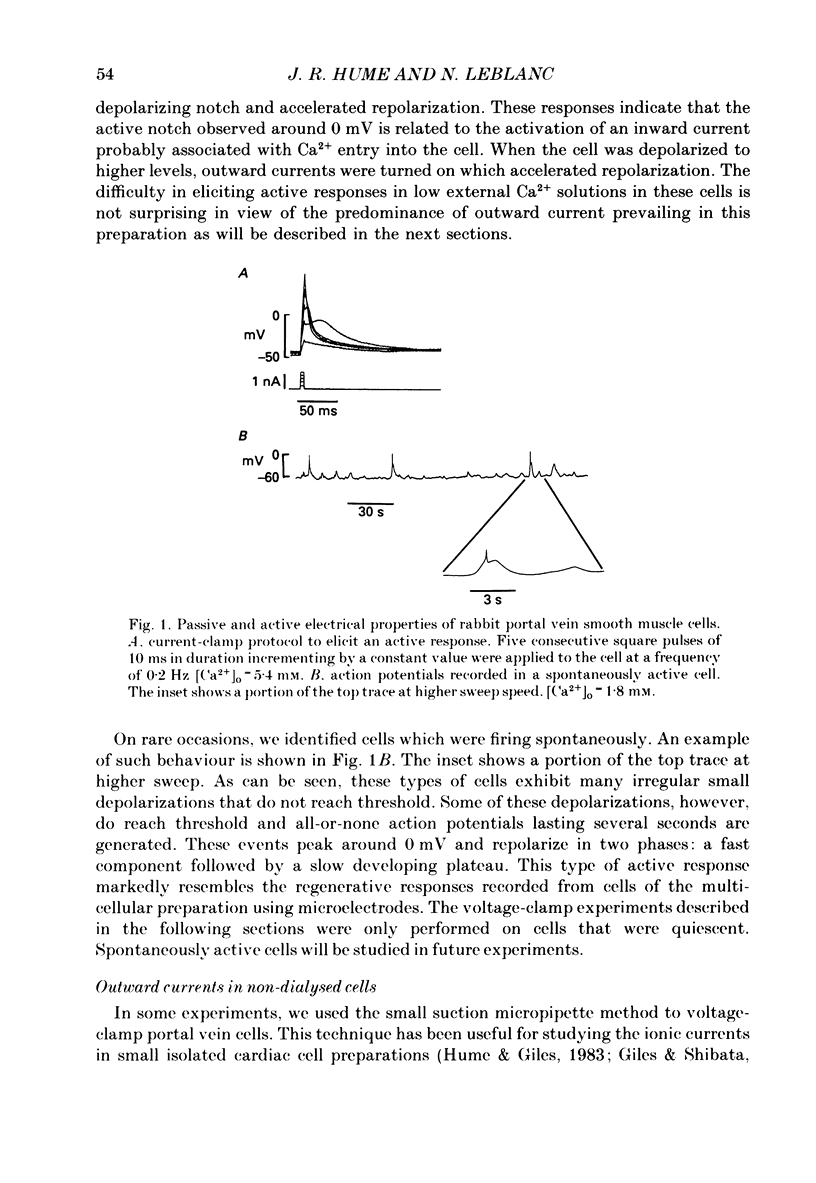
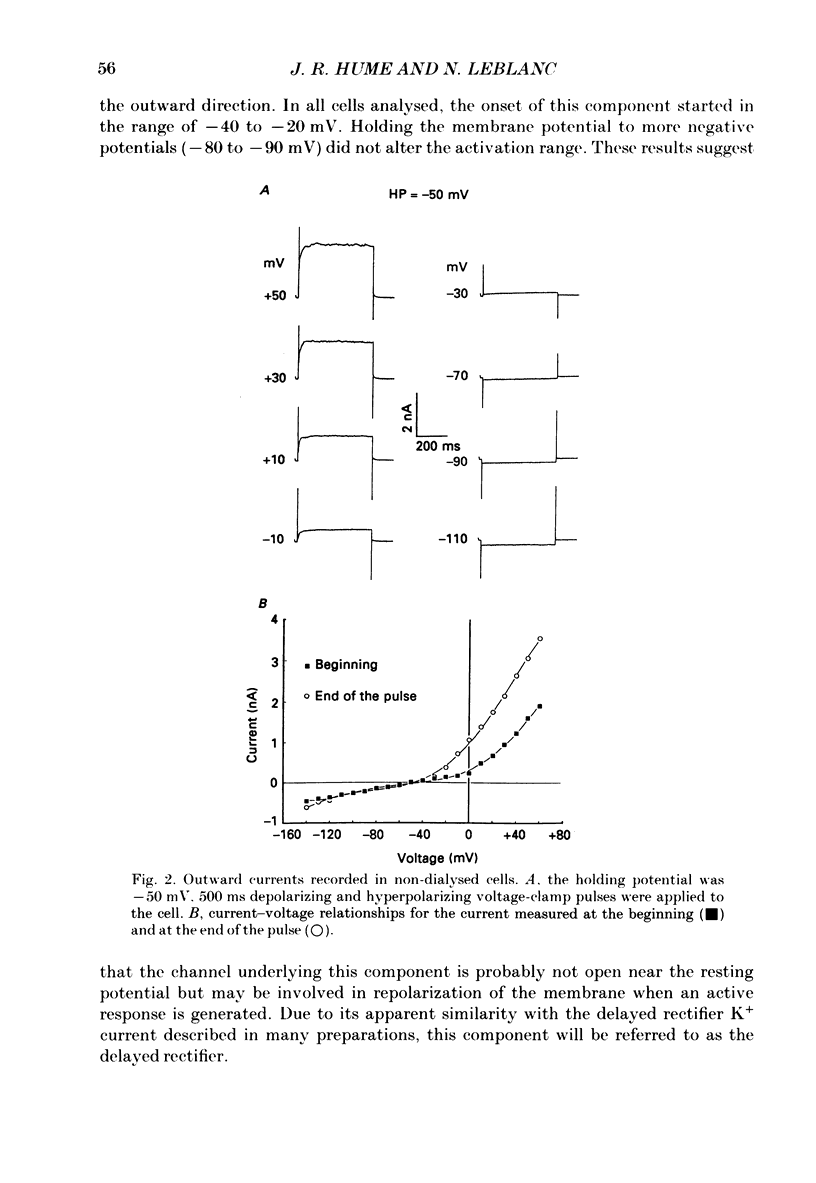
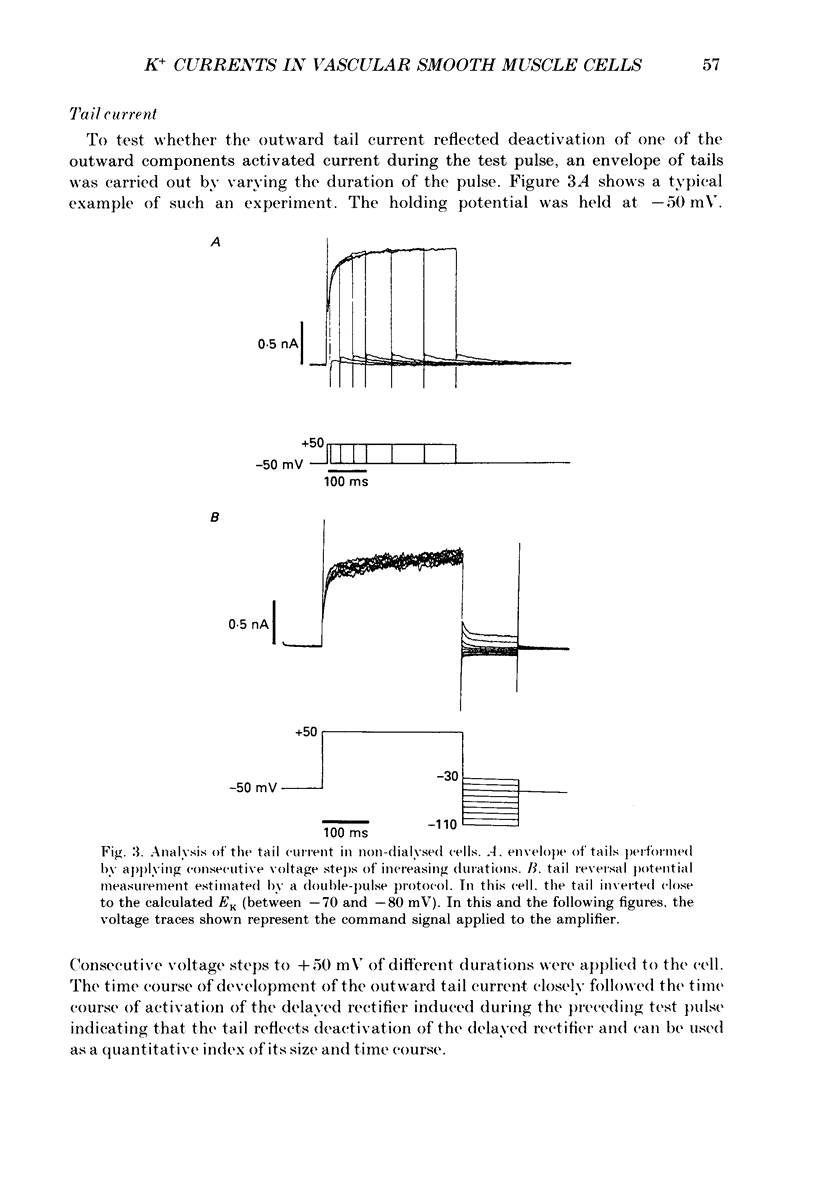
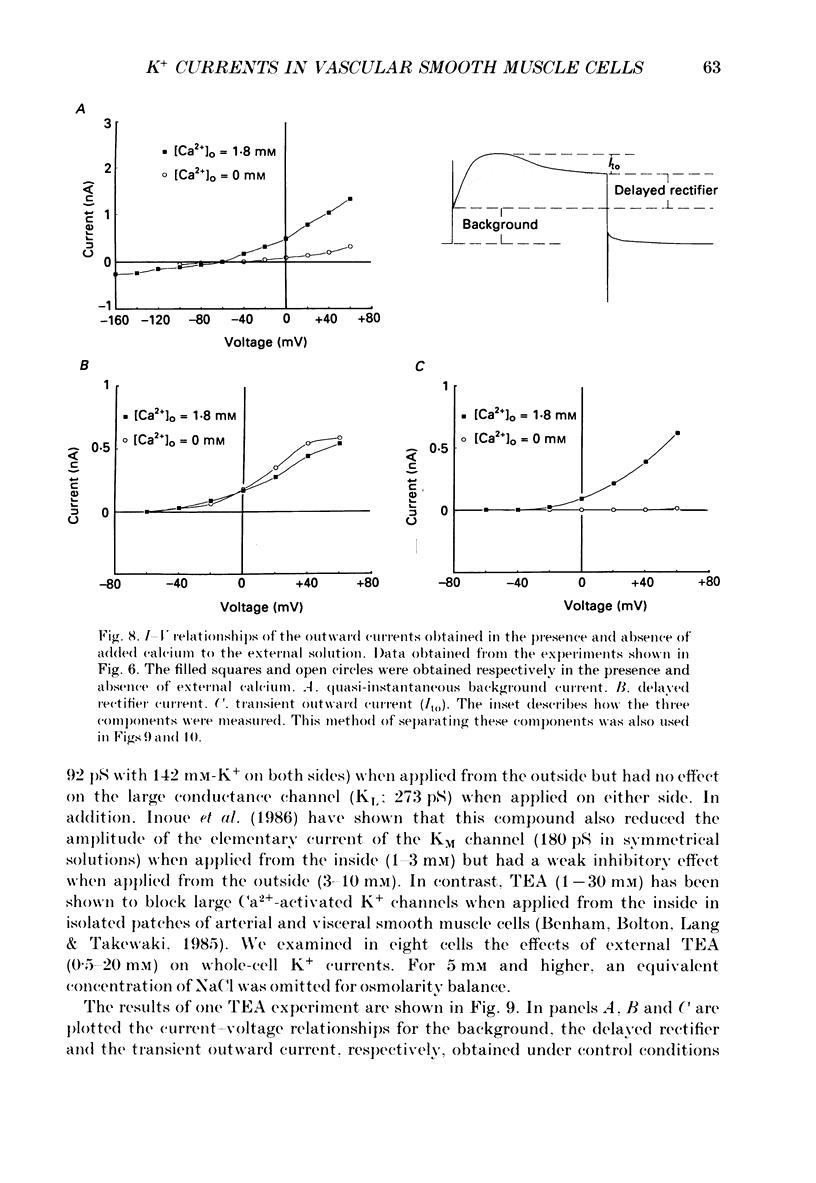
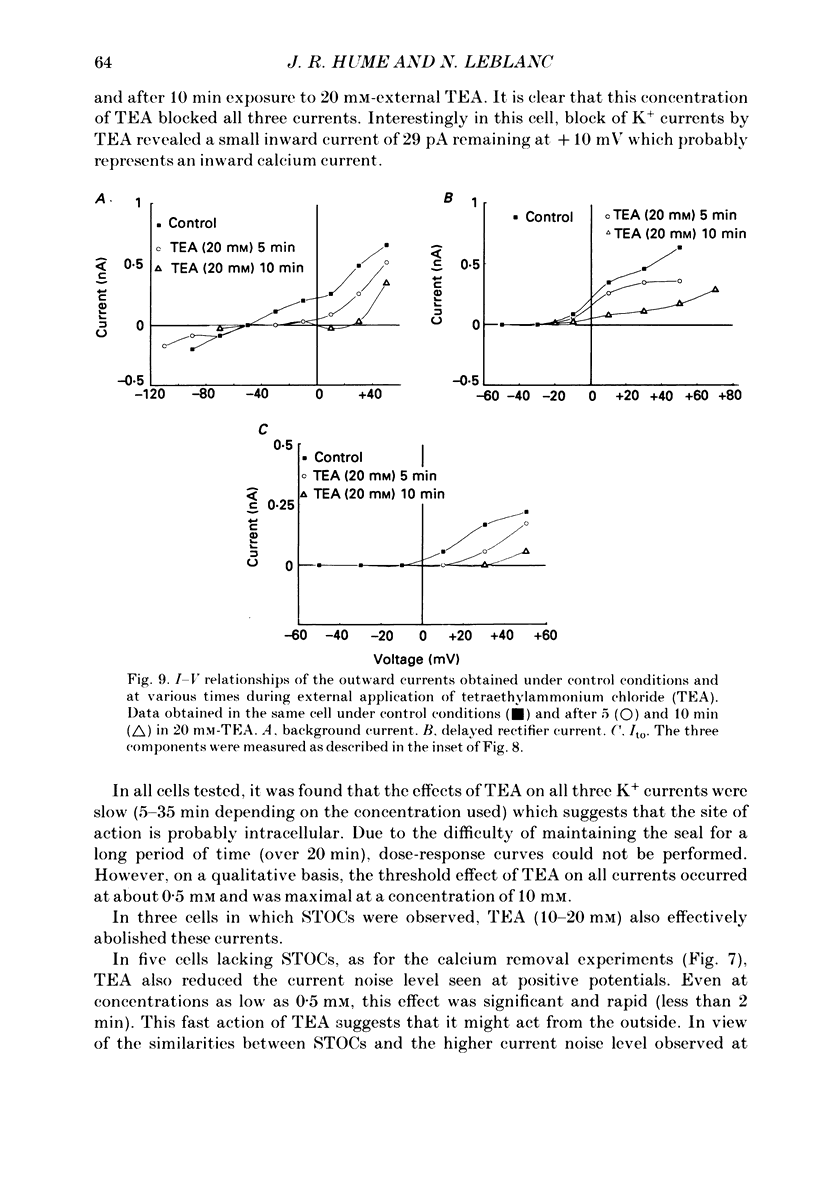
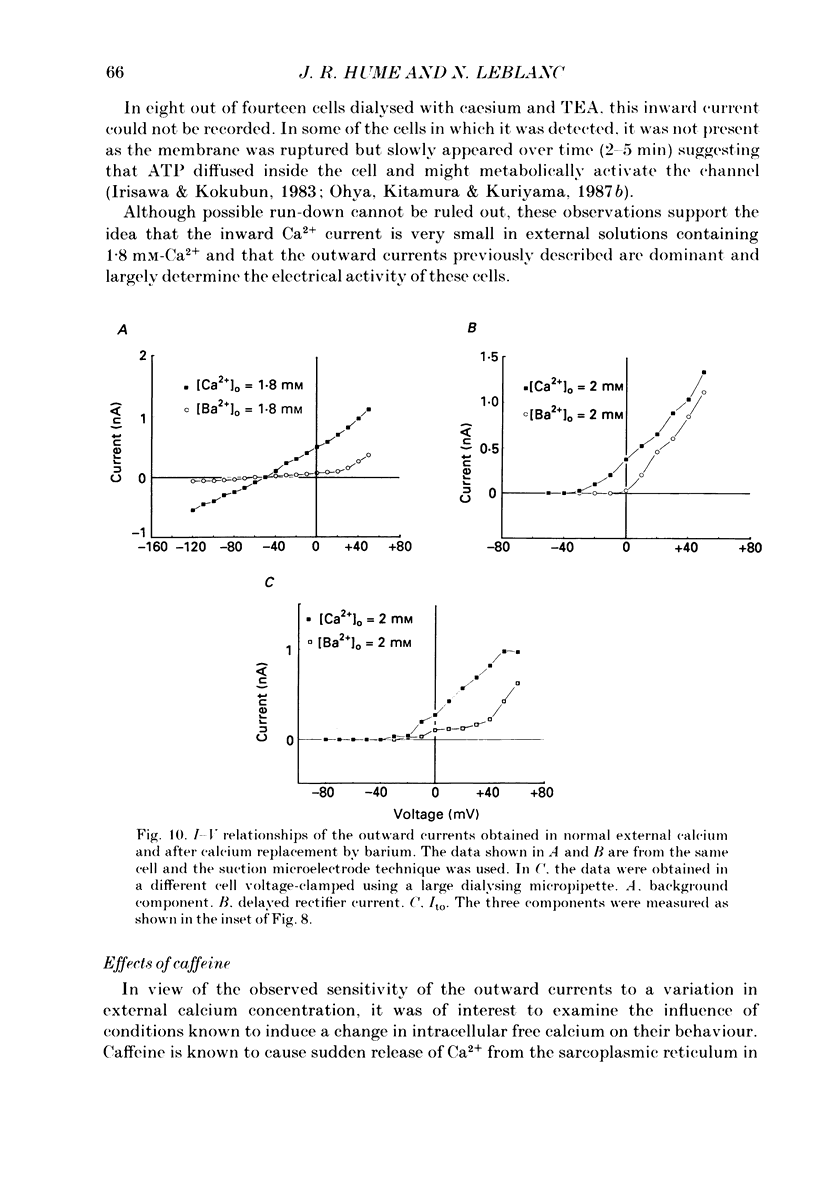
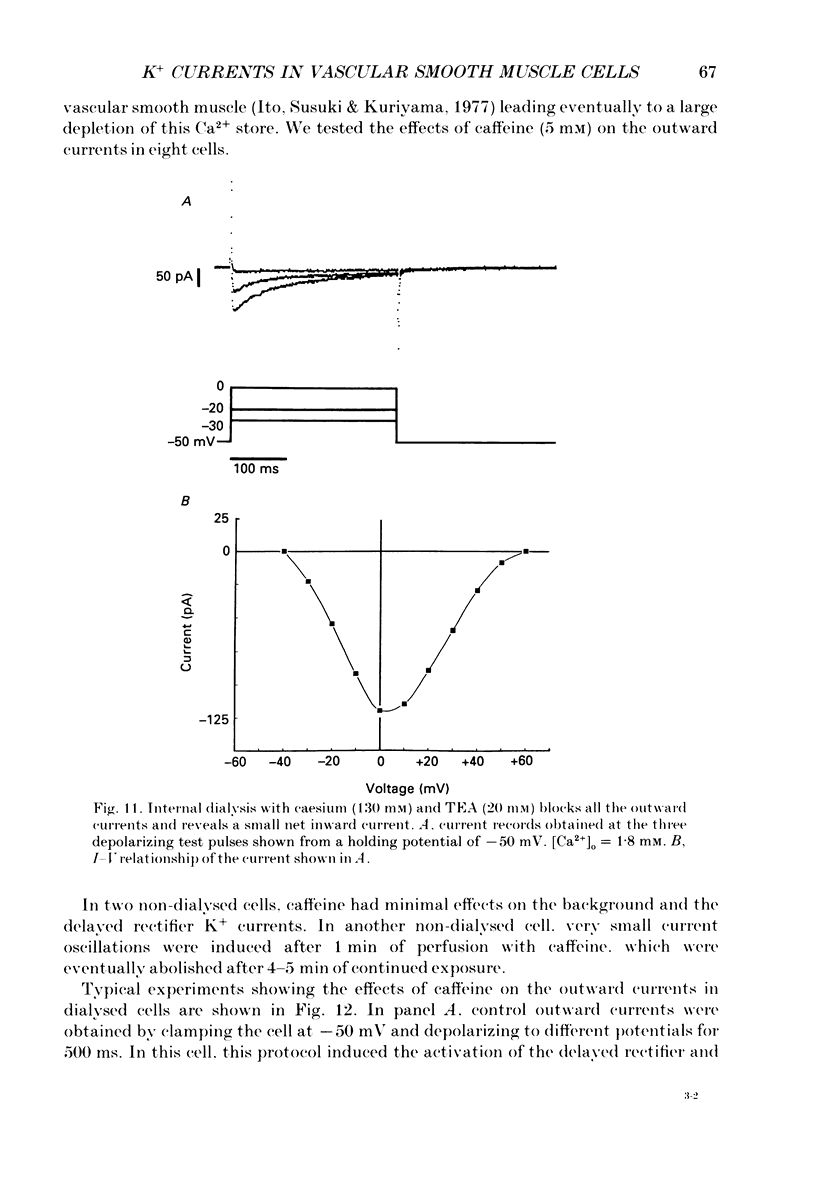
1. Single smooth muscle cells isolated from rabbit portal vein were voltage clamped at room temperature using the whole-cell configuration of the patch-clamp technique. These cells exhibited a mean resting potential of -47.9 mV and a mean input resistance of 376 M omega. 2. Using small tip diameter micropipettes (to avoid dialysis of the cells), depolarizing voltage-clamp pulses from a holding potential of -50 mV elicited two distinct outward currents: a quasi-instantaneous background current and a time-dependent current that did not appear to inactivate (delayed rectifier). Upon return to the holding potential, an outward tail current decaying back to the holding current was observed. 3. The time course of development of the tail current as estimated from envelopes of tail current protocols followed the kinetics of activation of the delayed rectifier elicited during the preceding test pulse. The tail current reversed close to the equilibrium potential for K+ ions indicating that it is mainly carried by potassium ions. 4. Using large tip diameter micropipettes to internally dialyse the cells (EGTA = 0.1 mM; ATP = 5 mM), two additional outward currents having transient kinetics were revealed: a smooth transient outward current (Ito) and spontaneous transient outward currents (STOCs). Ito was found to be mainly selective for K+ ions and exhibited voltage-dependent inactivation with half-maximal availability near -40 mV. 5. Removal of calcium from the bathing solution significantly reduced the background current and abolished both Ito and STOCs. The delayed rectifier current appeared to be insensitive to this procedure. The two types of transient outward currents were never recorded when EGTA was elevated to 5 mM inside the micropipette whereas the background and delayed rectifier currents were not affected. These results suggested that Ito and the spontaneous transient outward currents are activated by internal calcium. 6. External application of TEA (0.5-20 mM) blocked all four outward currents. Calcium replacement by barium significantly reduced the background current and Ito, and had small effects on the delayed rectifier current. When potassium was replaced with caesium (130 mM) and TEA (20 mM) inside the pipette, none of the outward currents described was ever observed. In about 60% of the cells dialysed with this solution a small inward Ca2+ current was revealed. 7. External application of caffeine (5 mM) abolished STOCs in cells in which this activity was present under control conditions. In cells lacking this type of activity under control conditions caffeine induced and later abolished this type of current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





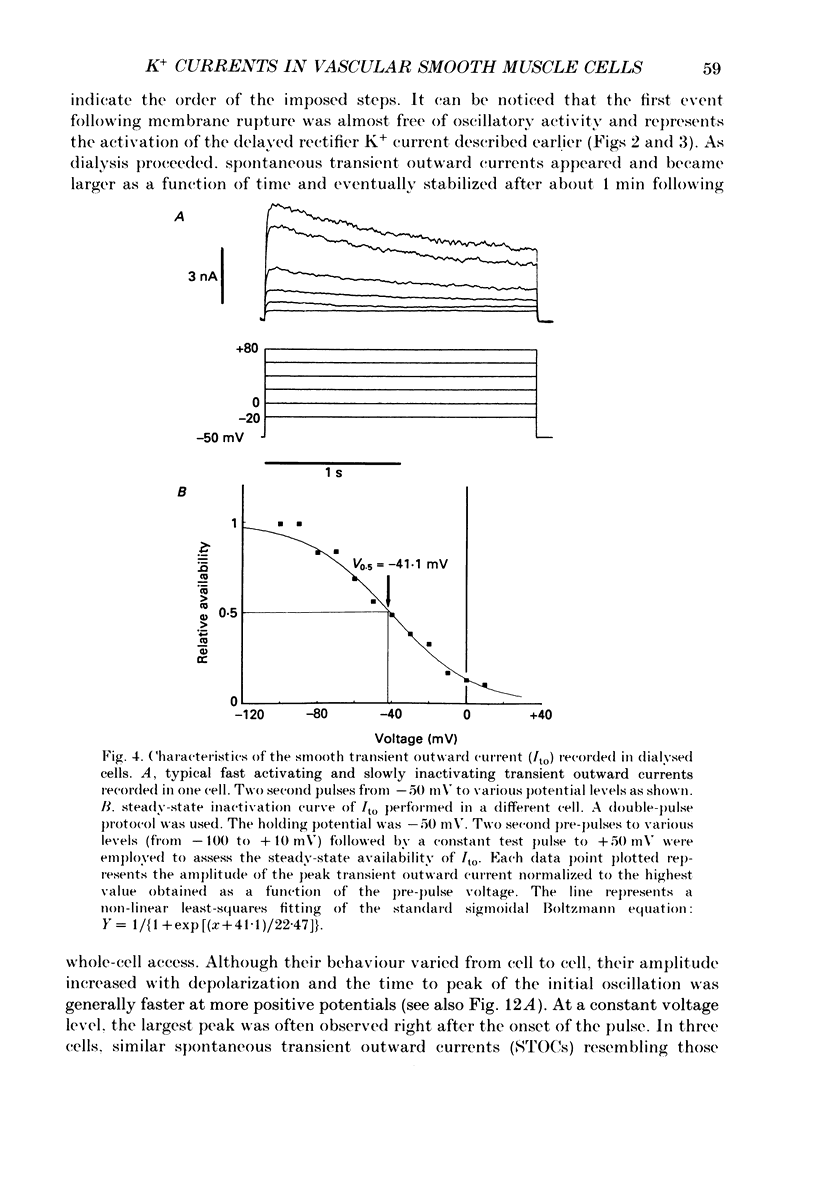
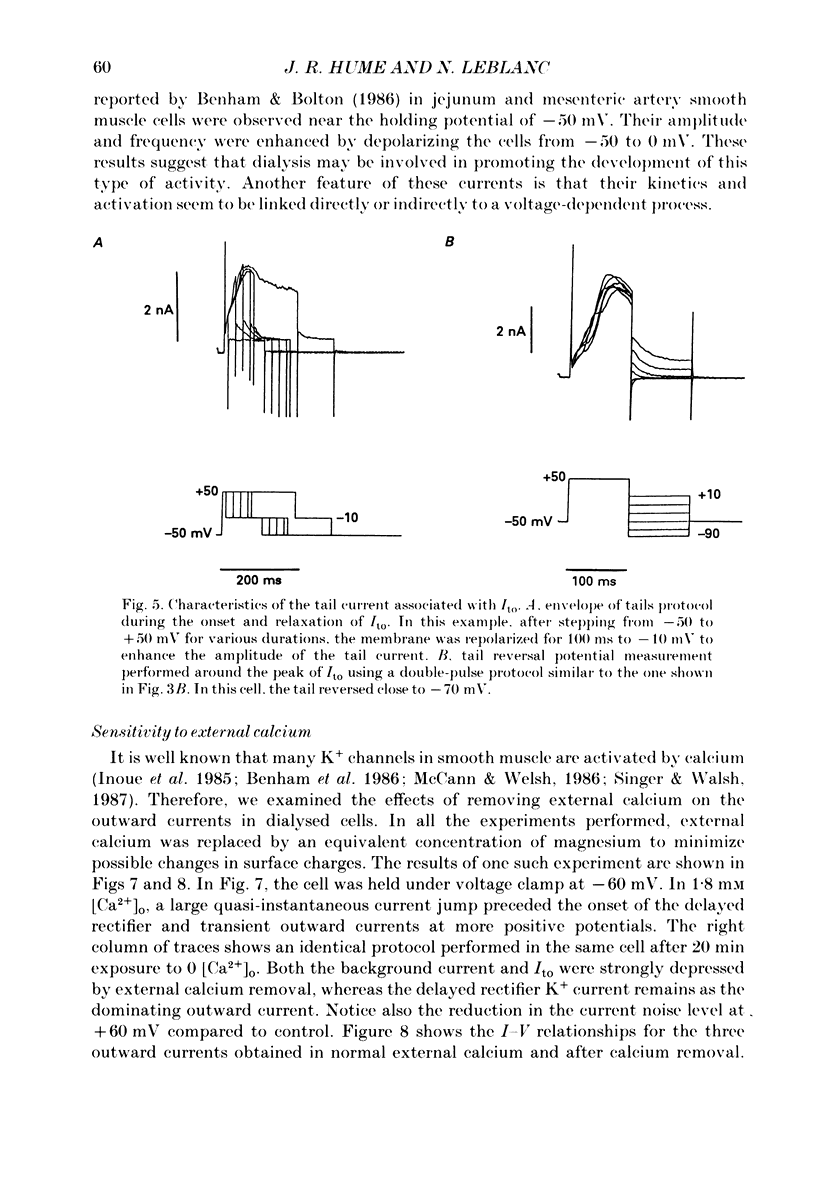
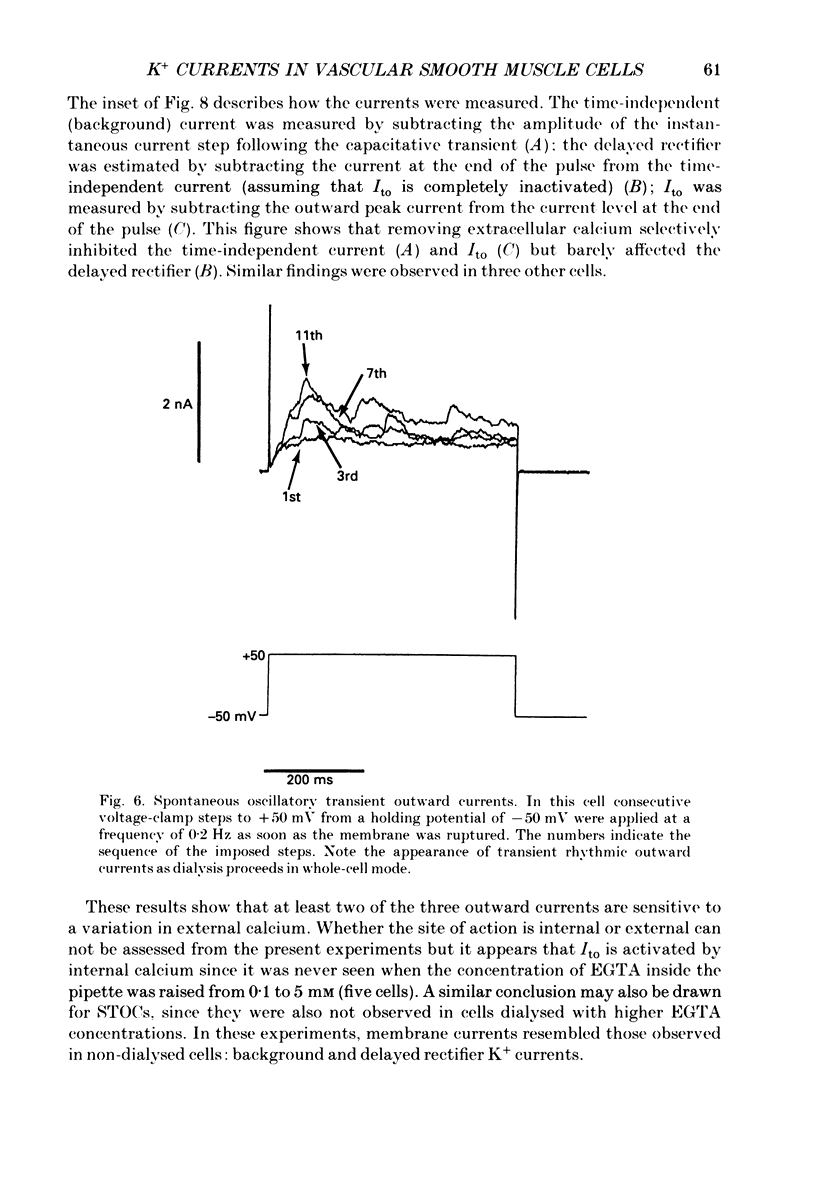
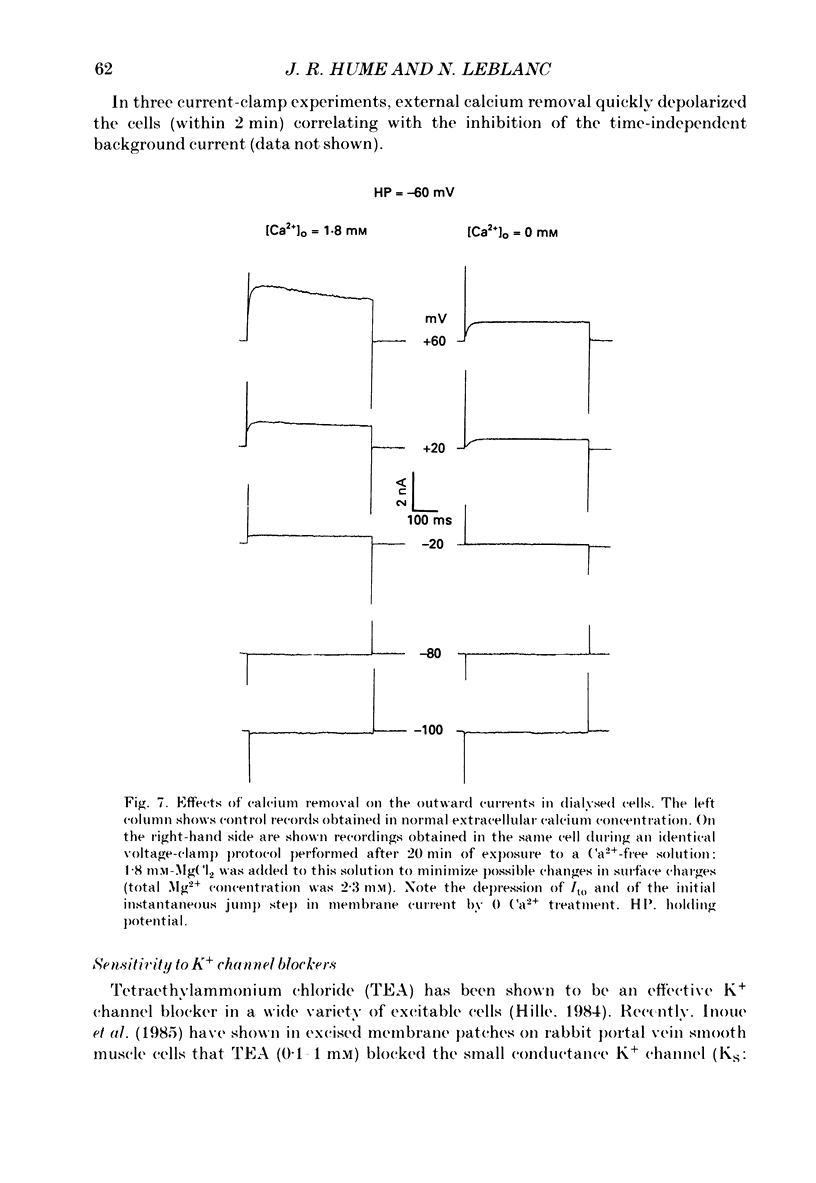






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Patch-clamp studies of slow potential-sensitive potassium channels in longitudinal smooth muscle cells of rabbit jejunum. J Physiol. 1983 Jul;340:469–486. doi: 10.1113/jphysiol.1983.sp014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Grygorcyk R., Schwarz W. Single K+ channels in membrane evaginations of smooth muscle cells. Pflugers Arch. 1984 Sep;402(1):18–23. doi: 10.1007/BF00584826. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintant G. A., Datyner N. B., Cohen I. S. Gating of delayed rectification in acutely isolated canine cardiac Purkinje myocytes. Evidence for a single voltage-gated conductance. Biophys J. 1985 Dec;48(6):1059–1064. doi: 10.1016/S0006-3495(85)83869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W., Robinson K., Shibata E. F., Nathan R. D., Kanai K., Rasmusson R. A time- and voltage-dependent K+ current in single cardiac cells from bullfrog atrium. J Gen Physiol. 1986 Dec;88(6):777–798. doi: 10.1085/jgp.88.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Leblanc R. N. A whole-cell patch clamp technique which minimizes cell dialysis. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):49–57. doi: 10.1007/BF00231003. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Inoue R., Okabe K., Kitamura K., Kuriyama H. A newly identified Ca2+ dependent K+ channel in the smooth muscle membrane of single cells dispersed from the rabbit portal vein. Pflugers Arch. 1986 Feb;406(2):138–143. doi: 10.1007/BF00586674. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. Effects of caffeine and procaine on the membrane and mechanical properties of the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(4):467–481. doi: 10.2170/jjphysiol.27.467. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N., Shigenobu K., Kasuya Y. Contractile response and electrophysiological properties in enzymatically dispersed smooth muscle cells of rat vas deferens. Pflugers Arch. 1987 Feb;408(2):112–119. doi: 10.1007/BF00581338. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Modulation of ionic currents in smooth muscle balls of the rabbit intestine by intracellularly perfused ATP and cyclic AMP. Pflugers Arch. 1987 May;408(5):465–473. doi: 10.1007/BF00585070. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Henderson J. S., Meissner G. Single channel and 45Ca2+ flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys J. 1986 Nov;50(5):1009–1014. doi: 10.1016/S0006-3495(86)83543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. A., Creazzo T., Hartzell H. C. A time-dependent and voltage-sensitive K+ current in single cells from frog atrium. J Gen Physiol. 1986 Dec;88(6):739–755. doi: 10.1085/jgp.88.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Toro L., Stefani E. Ca2+ and K+ current in cultured vascular smooth muscle cells from rat aorta. Pflugers Arch. 1987 Apr;408(4):417–419. doi: 10.1007/BF00581139. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Identification and characterization of major ionic currents in isolated smooth muscle cells using the voltage-clamp technique. Pflugers Arch. 1987 Feb;408(2):83–97. doi: 10.1007/BF00581336. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981 May;390(2):207–210. doi: 10.1007/BF00590209. [DOI] [PubMed] [Google Scholar]



