Abstract
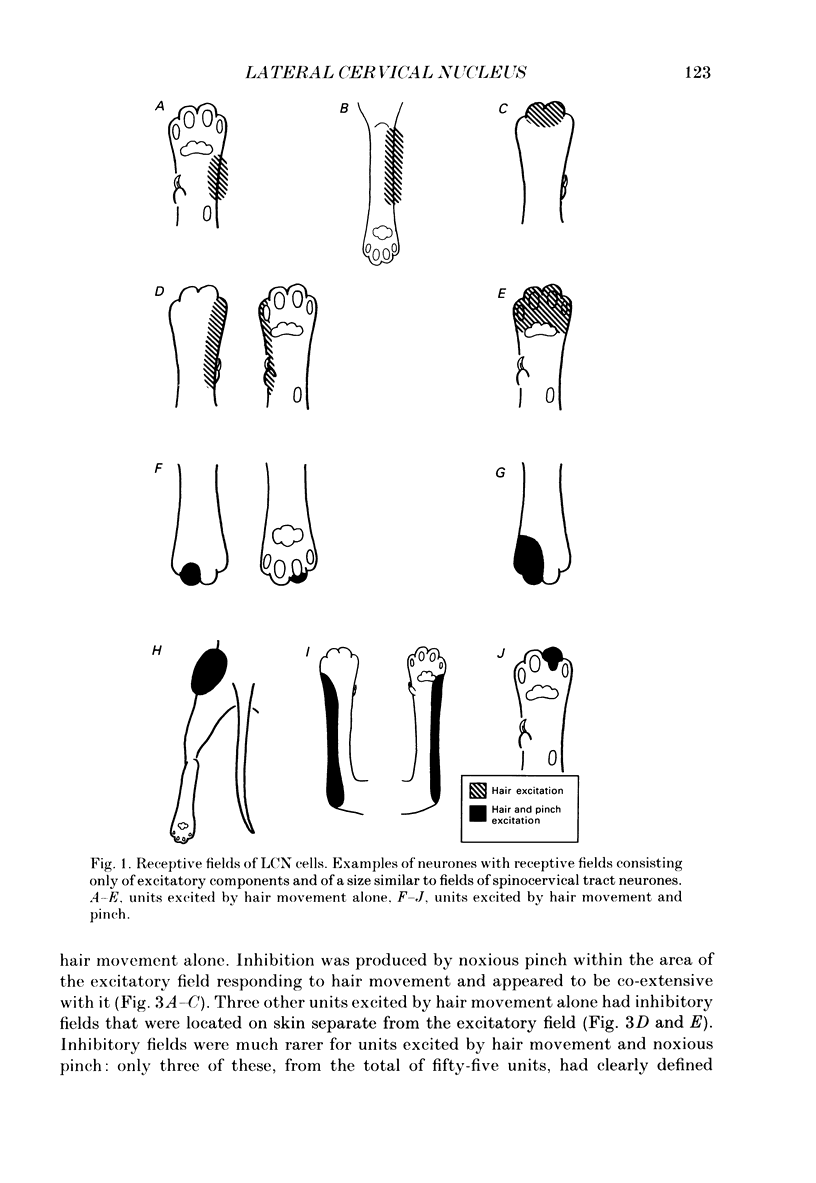
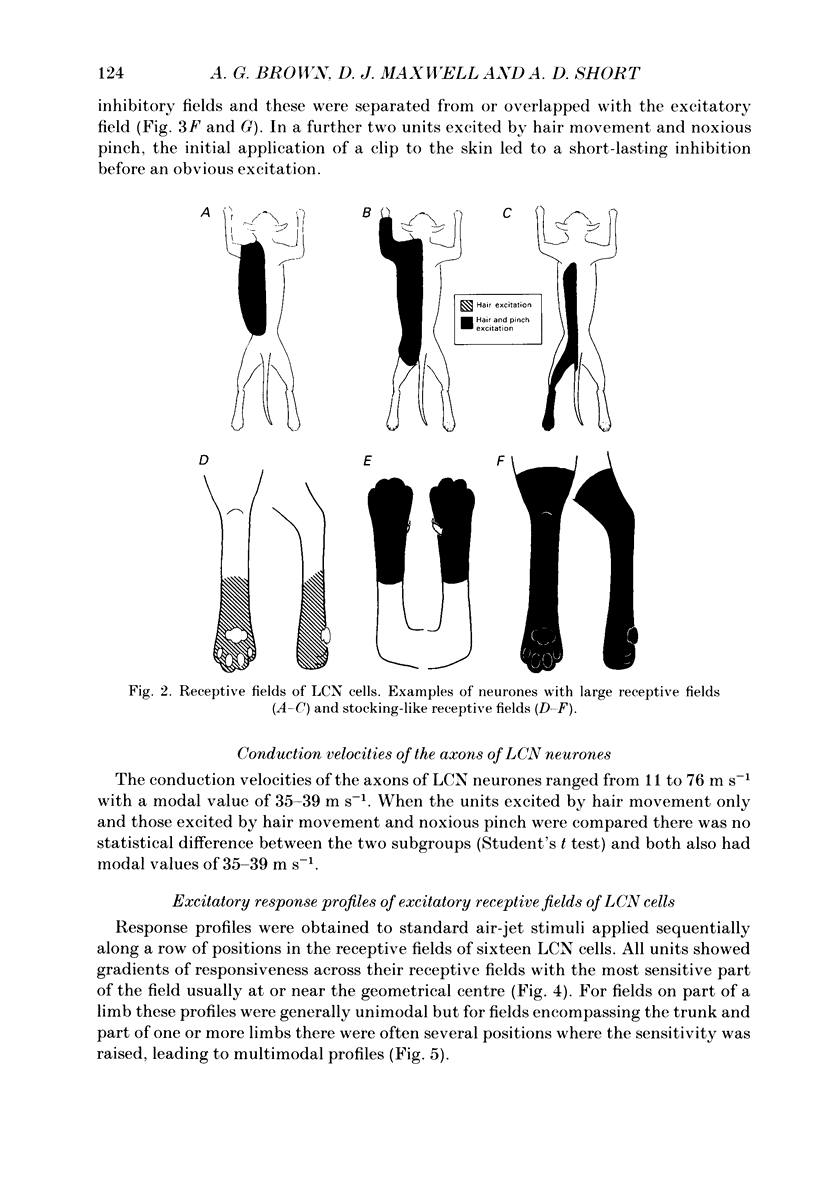
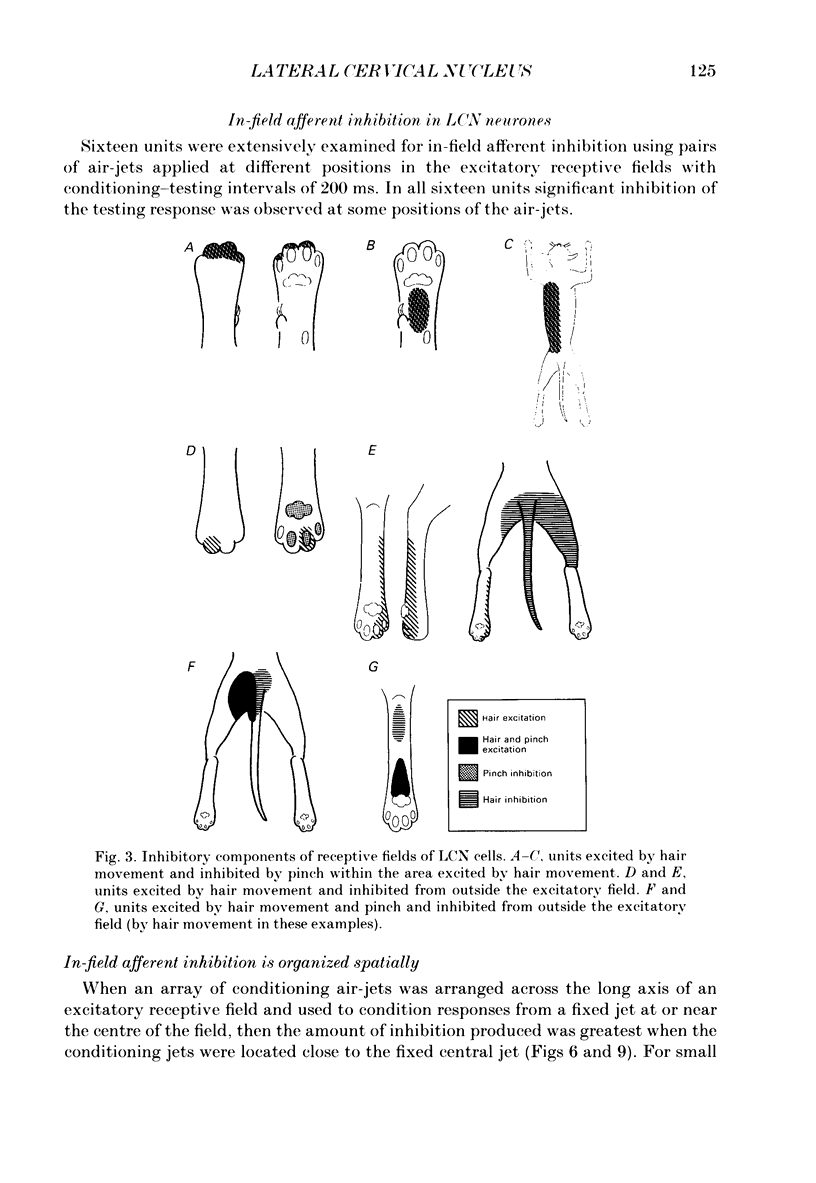
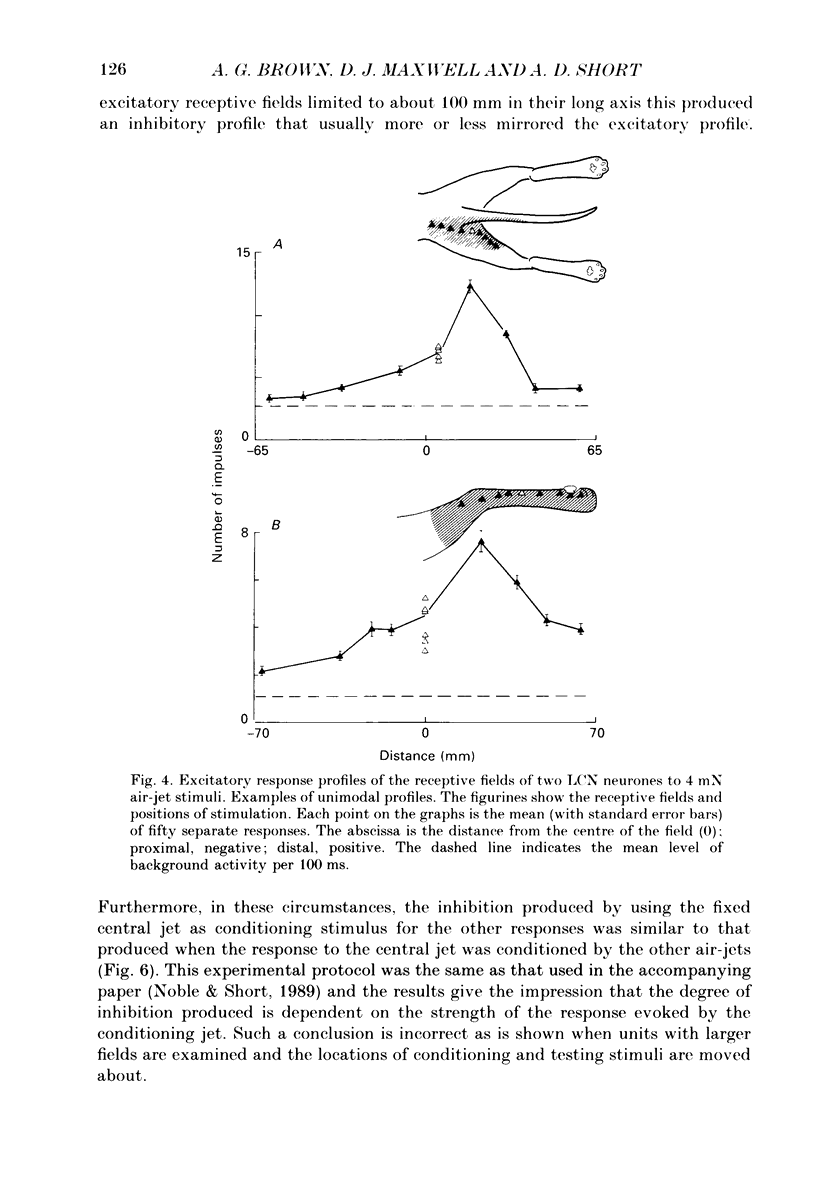
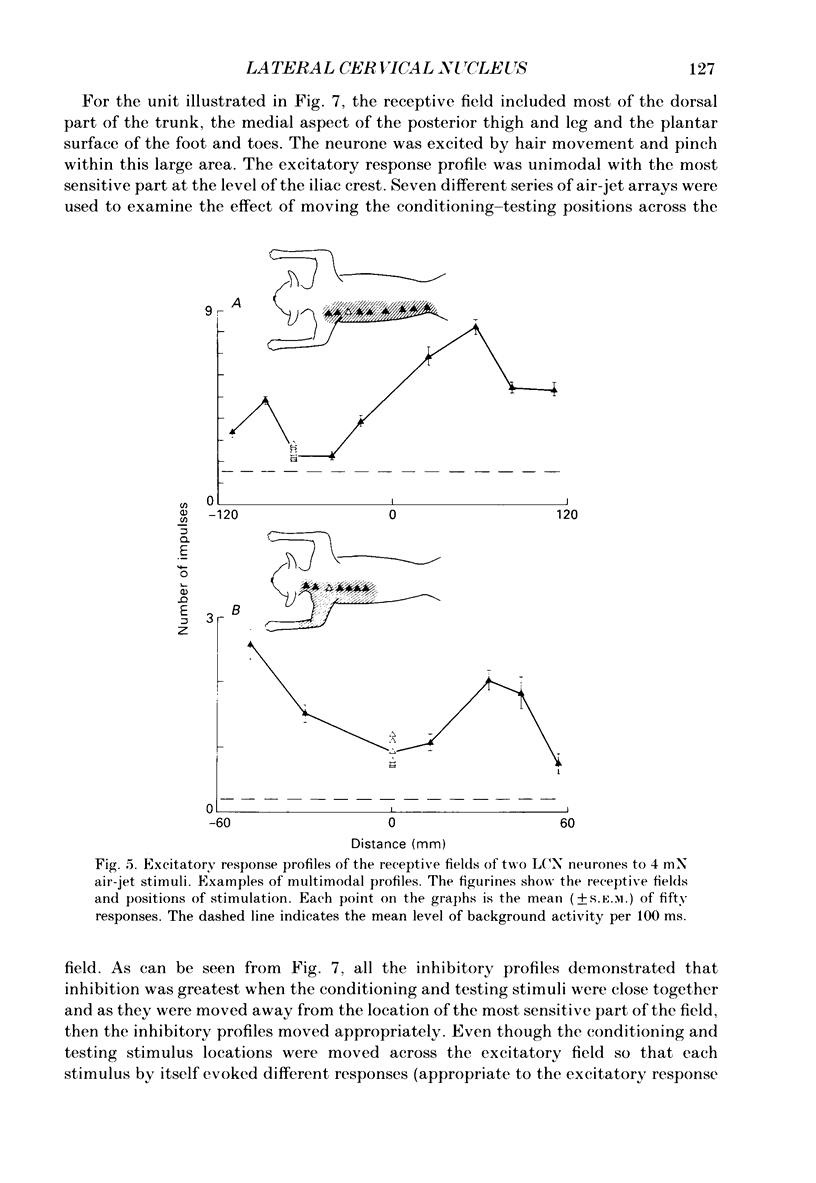
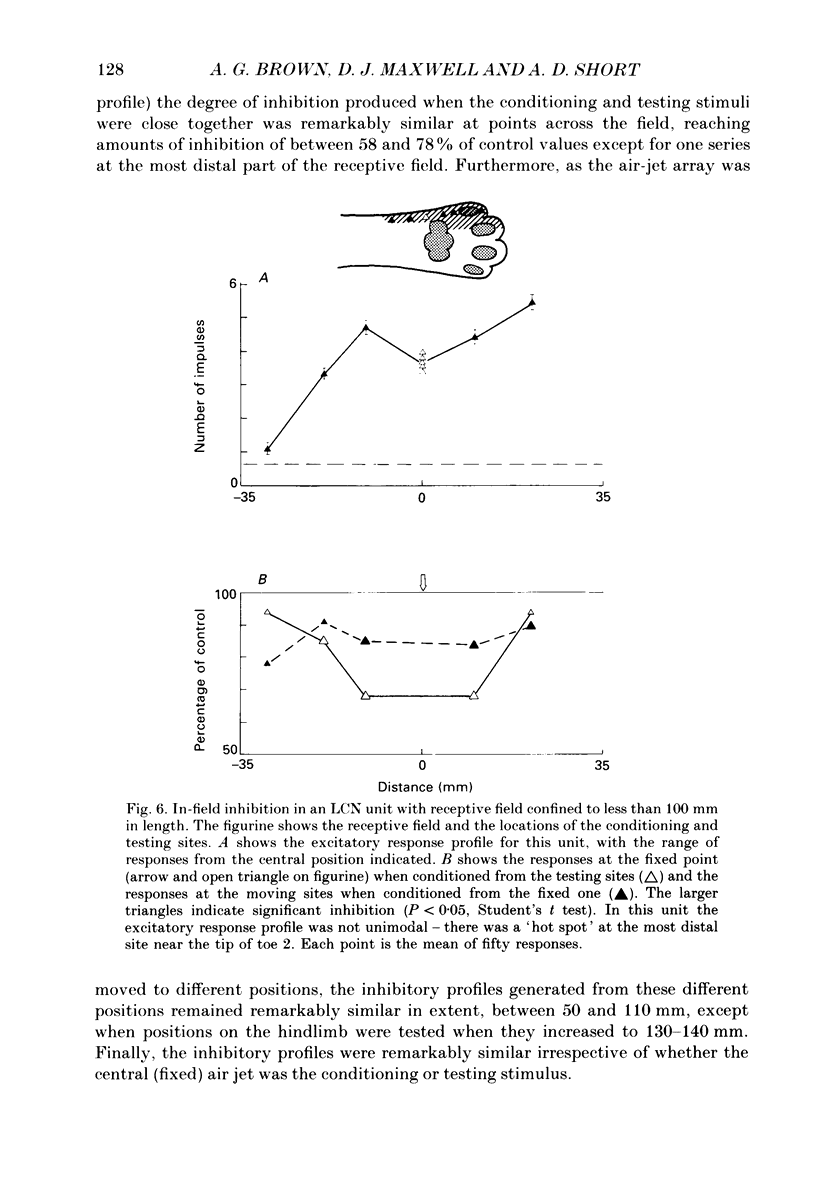
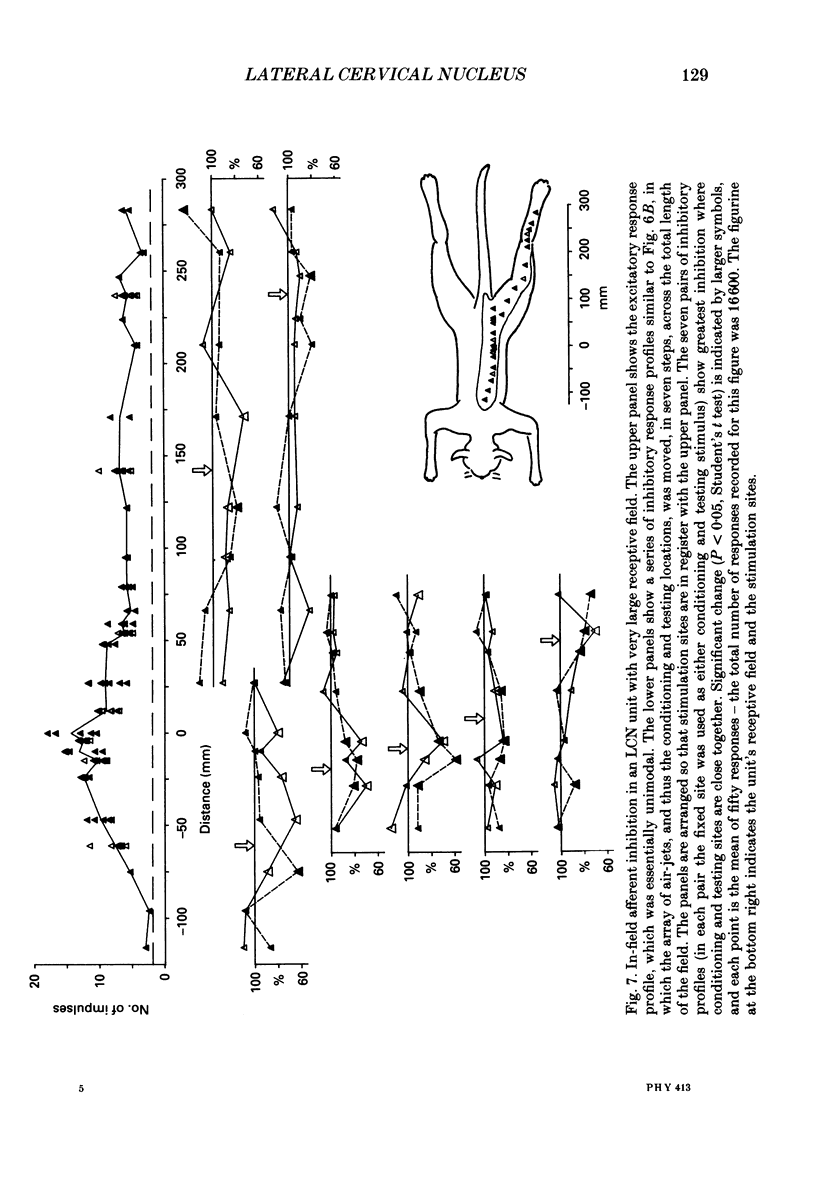
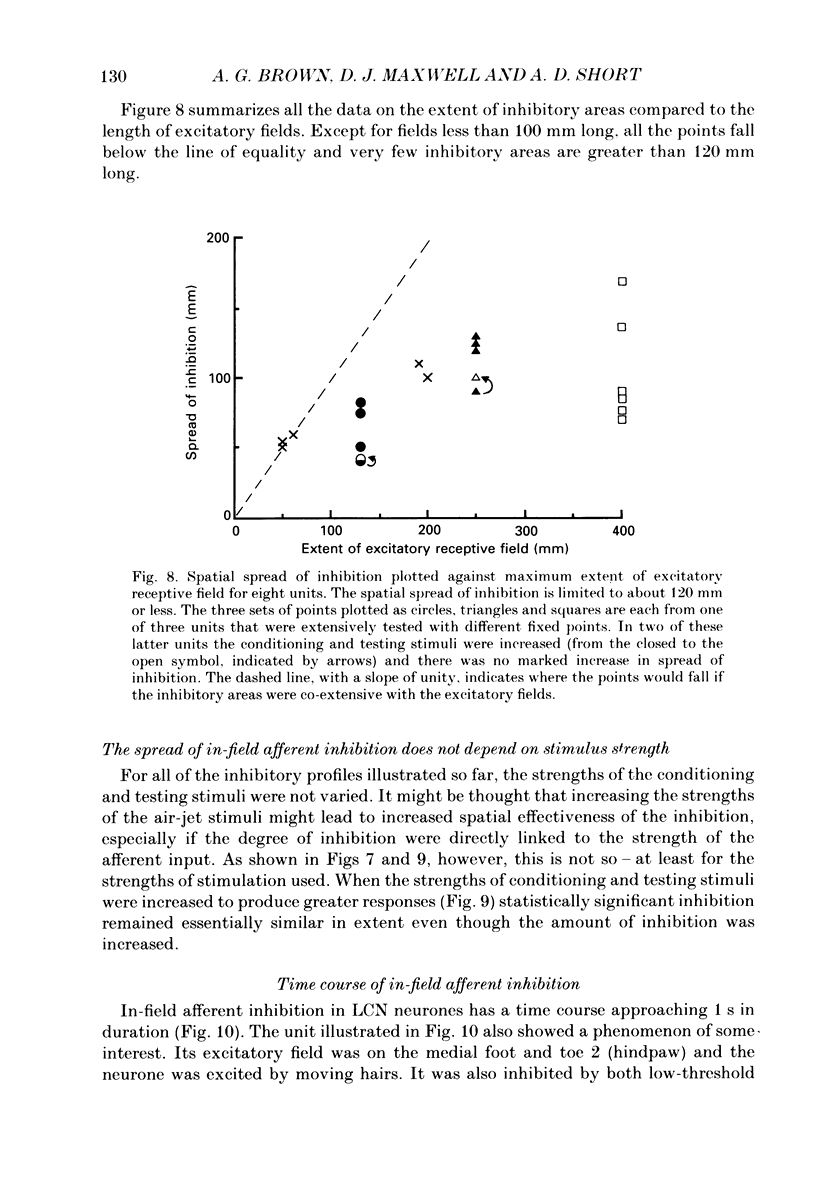
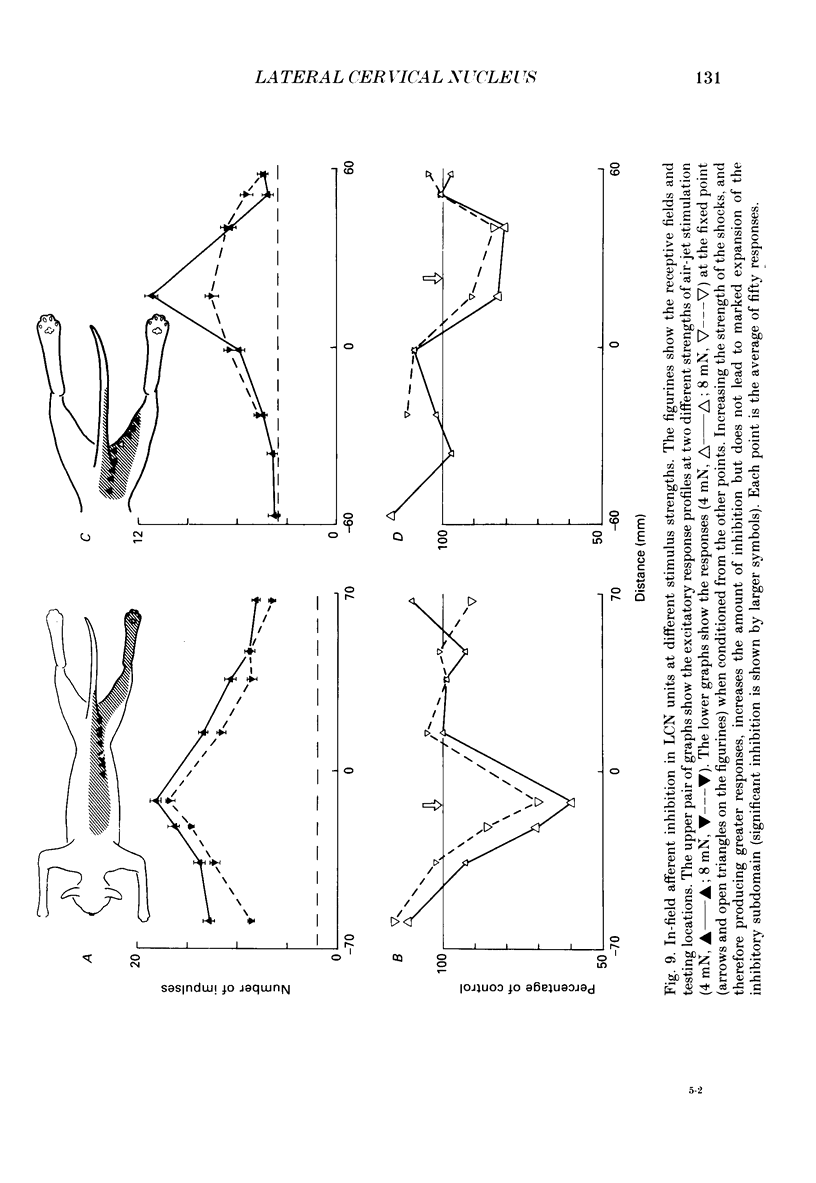
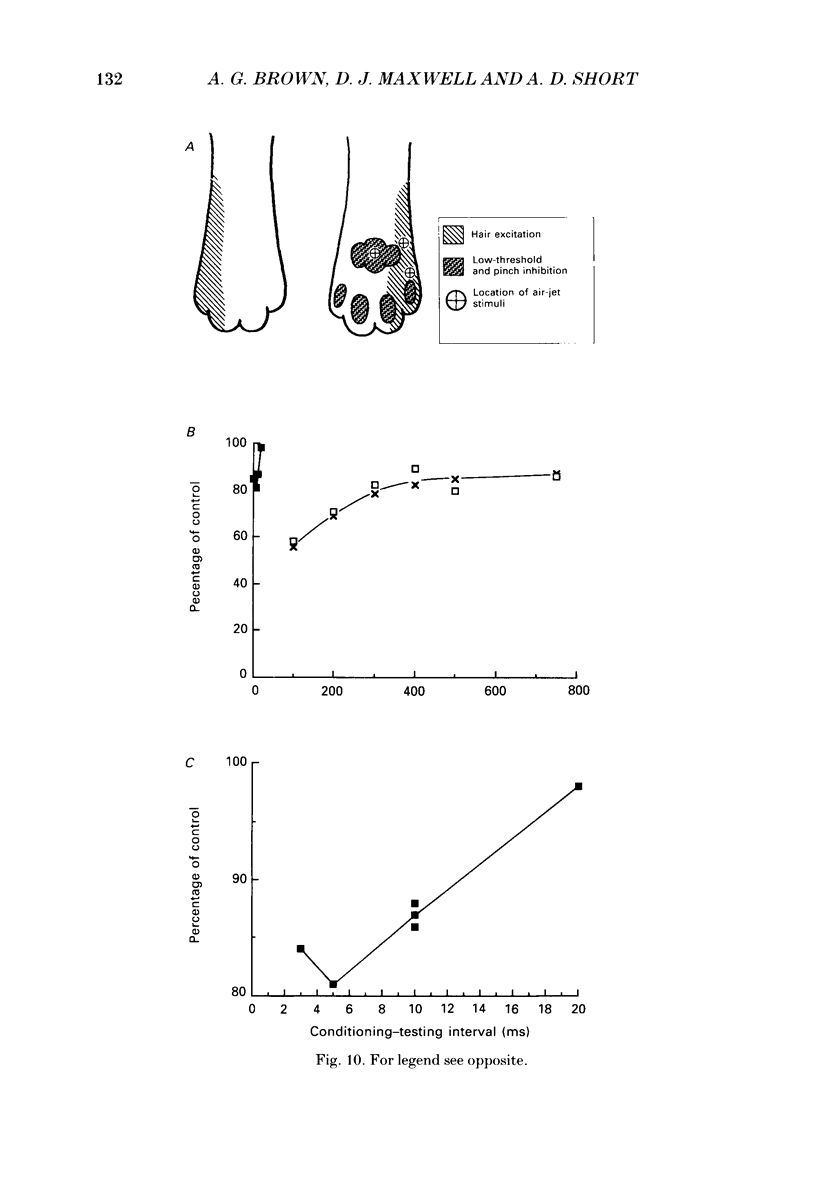
1. Extracellular microelectrode recordings were made from projection neurones of the lateral cervical nucleus (LCN) in cats anaesthetized with chloralose and paralysed with gallamine triethiodide. 2. The receptive fields of eight-five units were analysed. Most units had excitatory receptive fields similar in size and shape to those of spinocervical tract (SCT) cells. A few (14%) had either very large fields or 'stocking-like' fields. The majority of the LCN neurones (fifty-five, 65%) were excited by hair movement and, in addition, by noxious mechanical stimulation within the skin area responding to hair movement. Twenty-five units (29%) were excited by hair movement alone. For seven of these twenty-five neurones, noxious mechanical stimulation within the excitatory receptive field produced inhibition of the background discharge. One unit was excited by noxious mechanical stimulation and for the remaining four units no receptive field could be found. In six units inhibitory receptive fields outside the excitatory field were found. 3. Air-jet stimuli were used to define the excitatory profiles of the units' receptive fields to hair movement. In general, receptive fields had single regions of greatest sensitivity usually at or near the centre of the field, where that was oval in shape, with the sensitivity declining towards the field's circumference. In some units with very large fields that included parts of one or two limbs and the trunk there could be more than one highly sensitive region. 4. Pairs of air-jet stimuli were used to investigate in-field afferent inhibition in LCN cells. One jet was used to condition the responses to another jet located at a different position within the excitatory receptive field and occurring 200 ms later. Sixteen units were tested and significant in-field inhibition was observed in all sixteen. 5. The in-field afferent inhibition was organized spatially in the sense that inhibition was generally strongest when the conditioning and testing stimuli were close together and became weaker as they were moved apart. The afferent inhibition was not simply a function of the response produced by the conditioning stimulus. Furthermore, increasing the strength of the stimuli did not in general lead to larger areas from which the inhibition could be produced. The inhibitory areas defined in these experiments were generally less than 120 mm in length in units with receptive fields much longer than 100 mm.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Actions of trains and pairs of impulses from single primary afferent fibres on single spinocervical tract cells in cat. J Physiol. 1987 Jan;382:313–329. doi: 10.1113/jphysiol.1987.sp016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987 Jan;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R. Connexions between hair follicle afferent fibres and spinocervical tract neurones in the cat: the synthesis of receptive fields. J Physiol. 1982 Feb;323:77–91. doi: 10.1113/jphysiol.1982.sp014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R., Rowe M. J. Receptive field profiles and integrative properties of spinocervical tract cells in the cat. J Physiol. 1986 May;374:335–348. doi: 10.1113/jphysiol.1986.sp016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr, Tapper D. N. Lateral cervical nucleus in the cat: functional organization and characteristics. J Neurophysiol. 1978 Nov;41(6):1511–1534. doi: 10.1152/jn.1978.41.6.1511. [DOI] [PubMed] [Google Scholar]
- Fedina L., Gordon G., Lundberg A. The source and mechanisms of inhibition in the lateral cervical nucleus of the cat. Brain Res. 1968 Dec;11(3):694–696. doi: 10.1016/0006-8993(68)90160-1. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler G. J., Jr, Urca G., Cannon J. T., Liebeskind J. C. Response properties of neurons of the lateral cervical nucleus in the rat. J Comp Neurol. 1979 Jul 1;186(1):65–77. doi: 10.1002/cne.901860105. [DOI] [PubMed] [Google Scholar]
- Horrobin D. F. The lateral cervical nucleus of the cat; an electrophysiological study. Q J Exp Physiol Cogn Med Sci. 1966 Oct;51(4):351–371. doi: 10.1113/expphysiol.1966.sp001869. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schoultz T., Spencer W. A. Temporal and spatial parameters of excitation and afferent inhibition in cuneothalamic relay neurons. J Neurophysiol. 1977 Jul;40(4):822–835. doi: 10.1152/jn.1977.40.4.822. [DOI] [PubMed] [Google Scholar]
- Jänig W., Spencer W. A., Younkin S. G. Spatial and temporal features of afferent inhibition of thalamocortical relay cells. J Neurophysiol. 1979 Sep;42(5):1450–1460. doi: 10.1152/jn.1979.42.5.1450. [DOI] [PubMed] [Google Scholar]
- KITAI S. T., HA H., MORIN F. LATERAL CERVICAL NUCLEUS OF THE DOG: ANATOMICAL AND MICROELECTRODE STUDIES. Am J Physiol. 1965 Aug;209:307–311. doi: 10.1152/ajplegacy.1965.209.2.307. [DOI] [PubMed] [Google Scholar]
- Kajander K. C., Giesler G. J., Jr Responses of neurons in the lateral cervical nucleus of the cat to noxious cutaneous stimulation. J Neurophysiol. 1987 Jun;57(6):1686–1704. doi: 10.1152/jn.1987.57.6.1686. [DOI] [PubMed] [Google Scholar]
- Laskin S. E., Spencer W. A. Cutaneous masking. II. Geometry of excitatory andinhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol. 1979 Jul;42(4):1061–1082. doi: 10.1152/jn.1979.42.4.1061. [DOI] [PubMed] [Google Scholar]
- Metherate R. S., da Costa D. C., Herron P., Dykes R. W. A thalamic terminus of the lateral cervical nucleus: the lateral division of the posterior nuclear group. J Neurophysiol. 1986 Dec;56(6):1498–1520. doi: 10.1152/jn.1986.56.6.1498. [DOI] [PubMed] [Google Scholar]
- Noble R., Short A. D. Spatial spread of in-field afferent inhibition in the cat's spinocervical tract. J Physiol. 1989 Jun;413:107–118. doi: 10.1113/jphysiol.1989.sp017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSWALDO-CRUZ E., KIDD C. FUNCTIONAL PROPERTIES OF NEURONS IN THE LATERAL CERVICAL NUCLEUS OF THE CAT. J Neurophysiol. 1964 Jan;27:1–14. doi: 10.1152/jn.1964.27.1.1. [DOI] [PubMed] [Google Scholar]


