Abstract
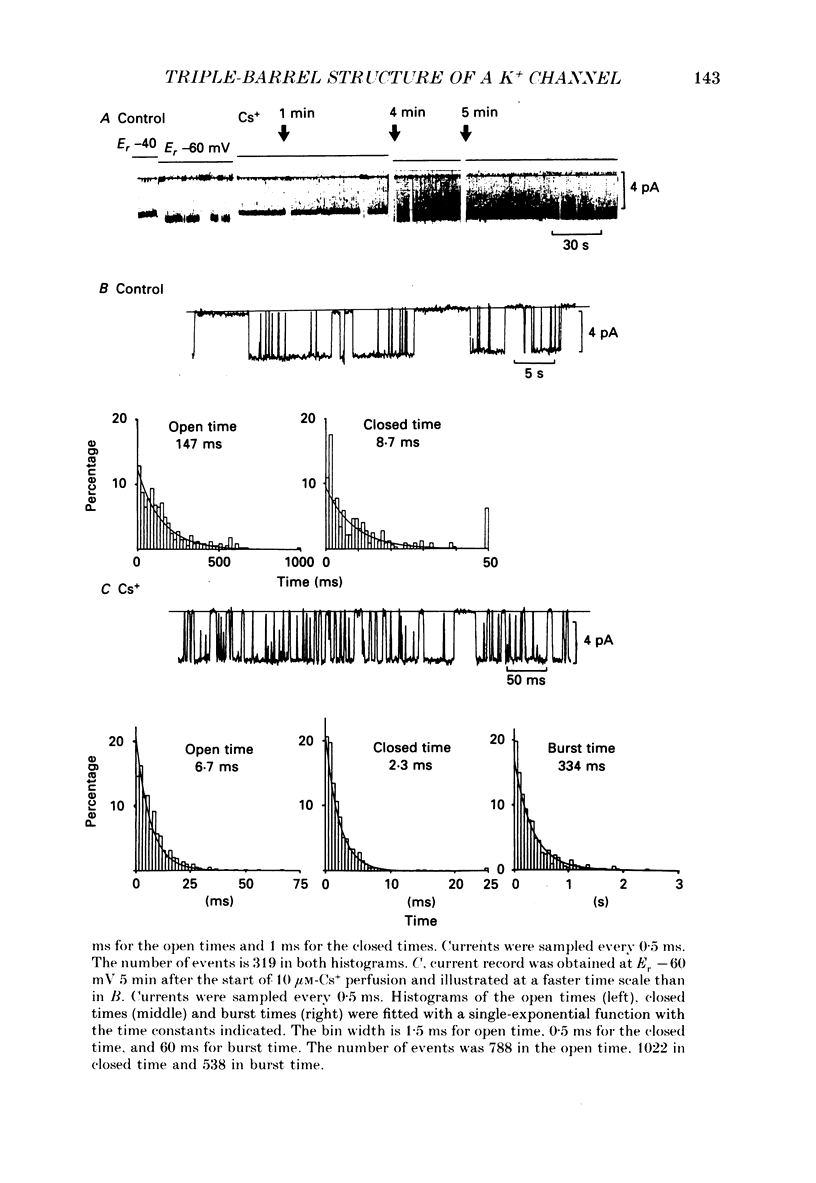
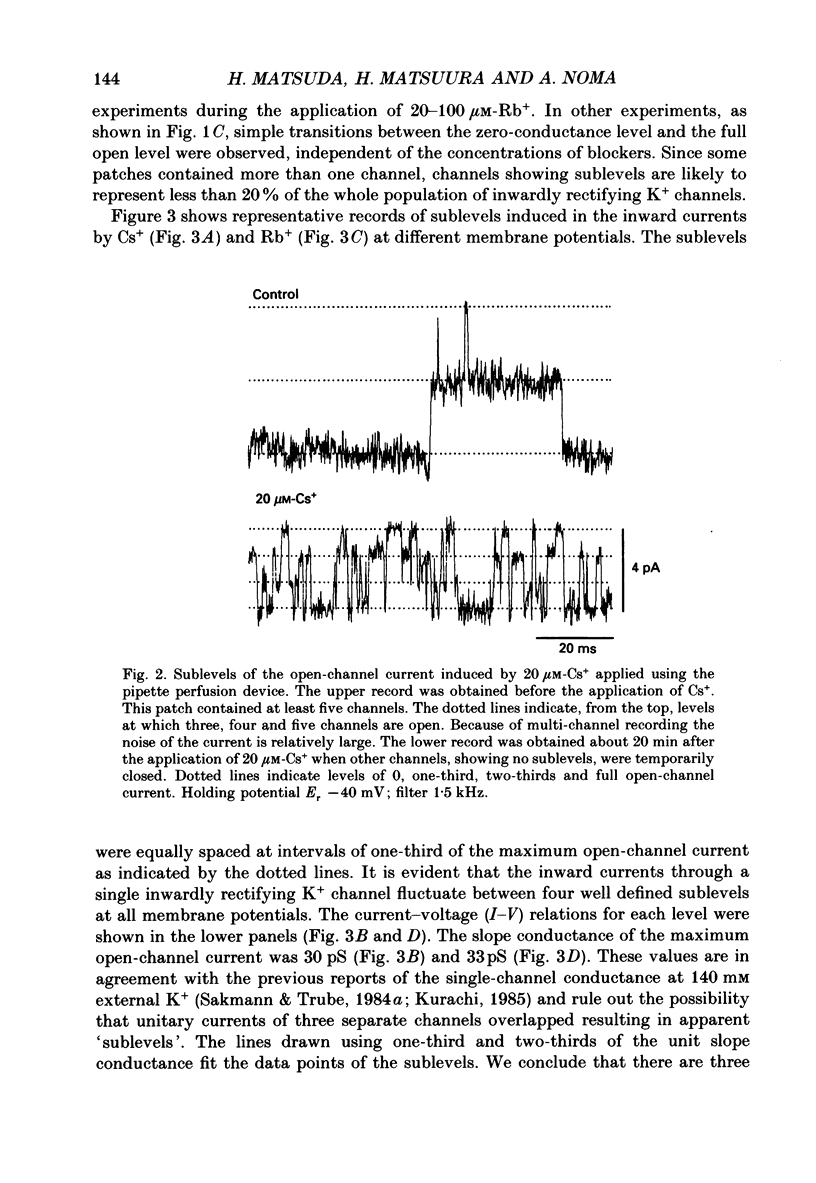
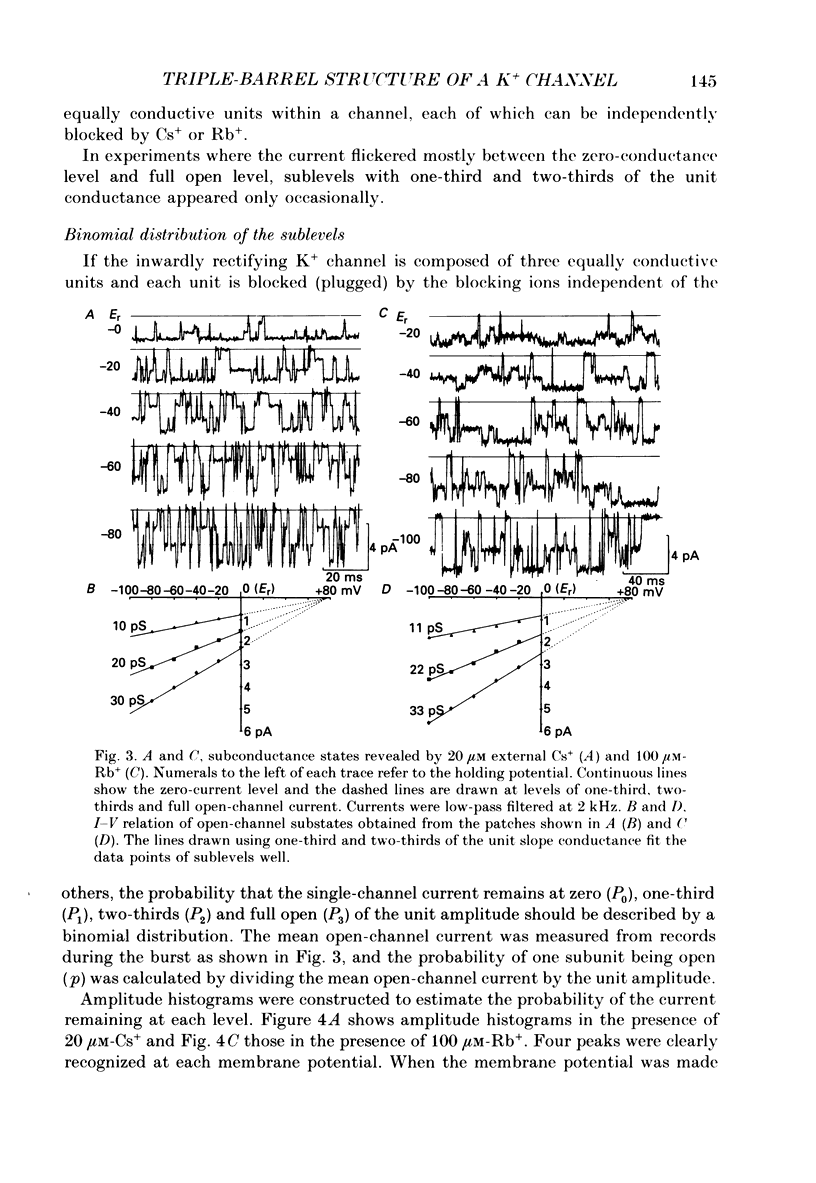
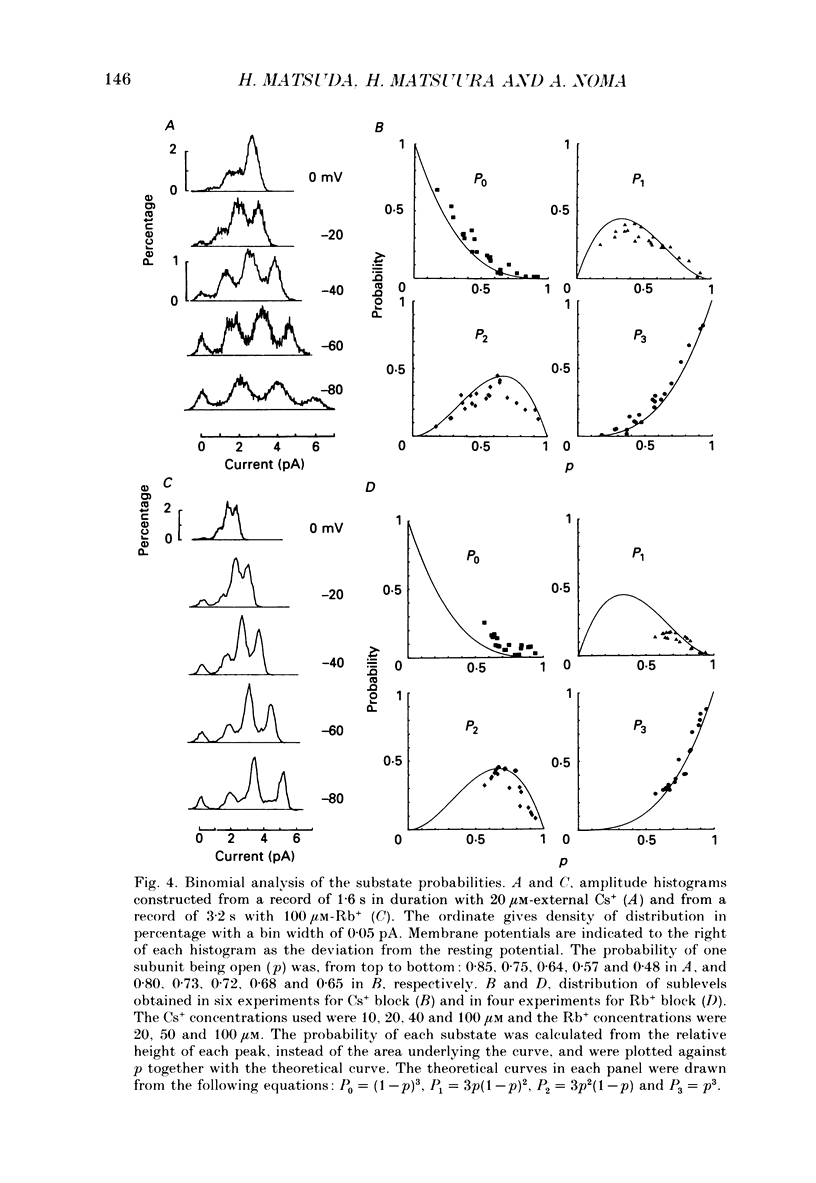
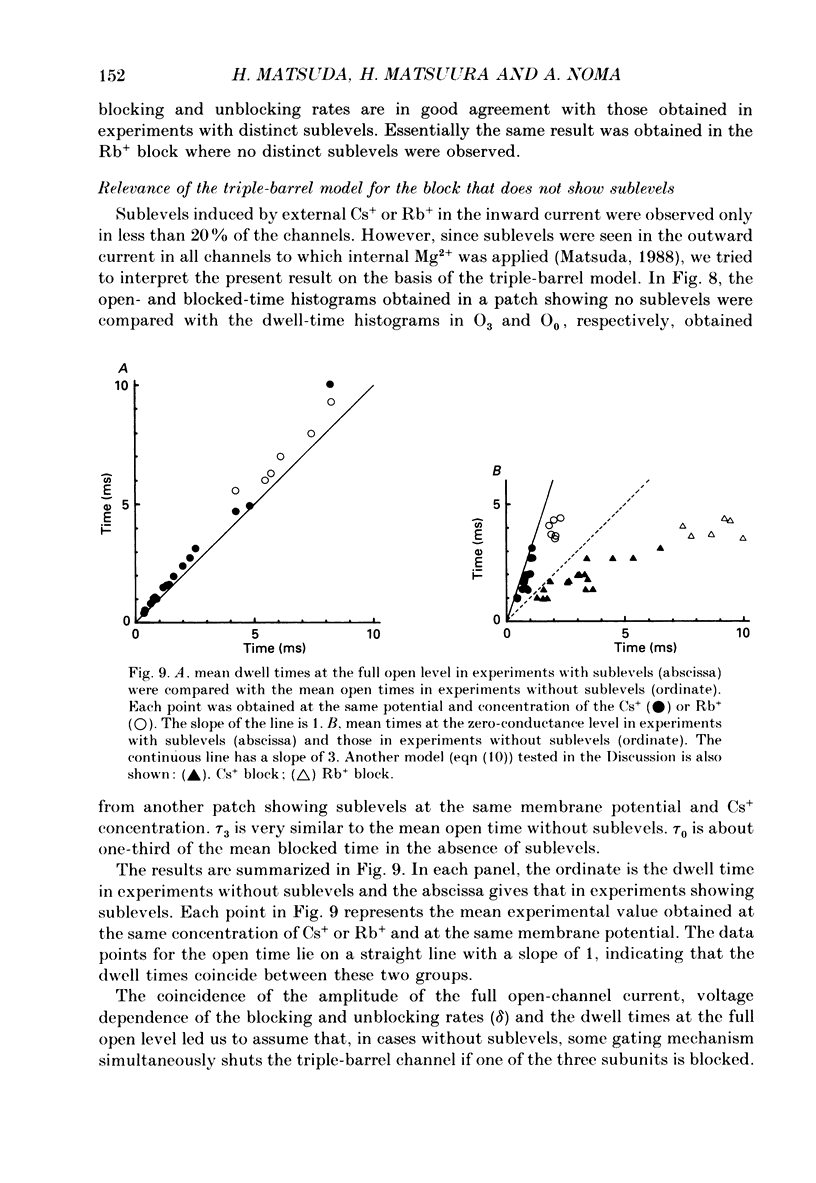
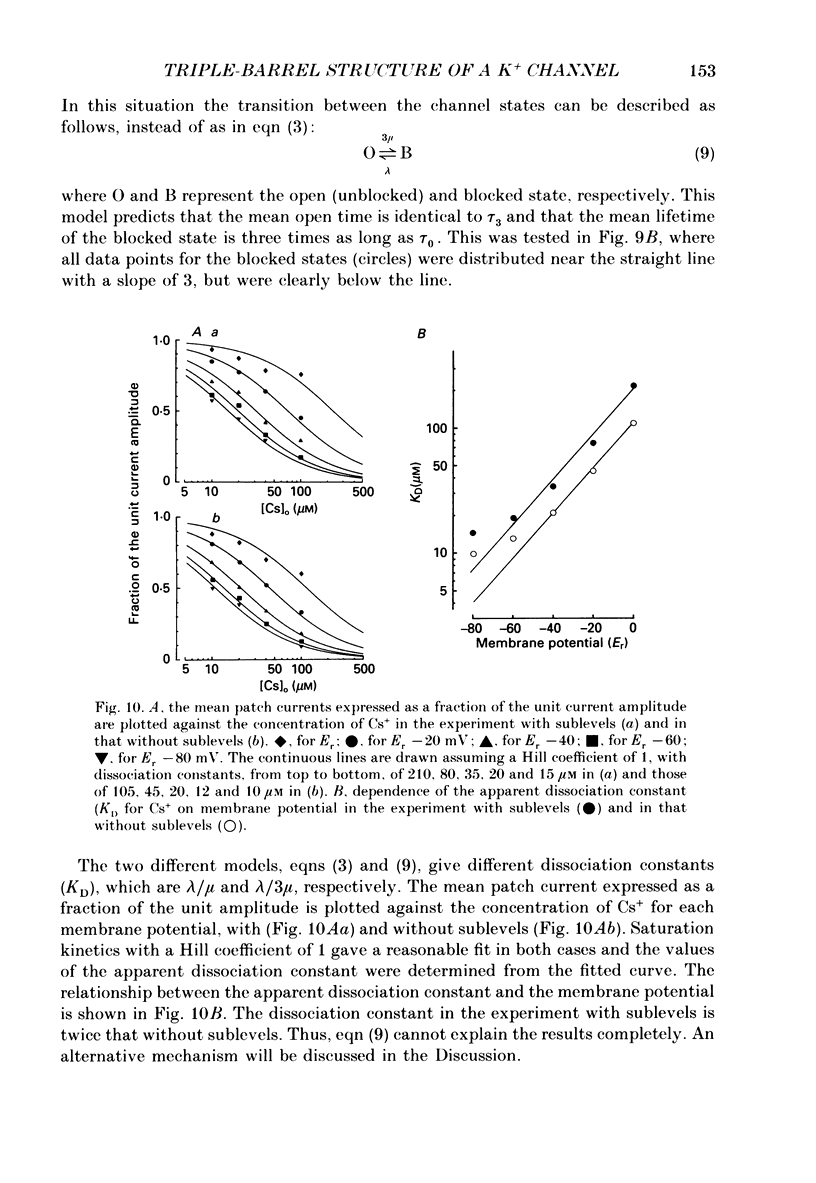
1. The hypothesis that the inwardly rectifying K+ channel consists of a triple-barrel structure was investigated. Inward currents were recorded under the blocking effects of external Cs+ or Rb+ in the cell-attached configuration of the patch-clamp technique using single ventricular cells enzymatically isolated from guinea-pig hearts. 2. Cs+ (10-100 microM) or Rb+ (20-100 microM) added to the 150 mM-K+ pipette solution induced rapid open-blocked transitions in the inward open-channel currents. In about 20% of experiments the inward current showed two intermediate current levels equally spaced between the unit amplitude and the zero-conductance level. The current fluctuated between these four levels. In the remaining experiments no obvious sublevels were observed except spontaneous ones, whose amplitudes were not always equal to one-third or two-thirds of the unit amplitude. 3. In experiments showing sublevels, the probability that the open-channel current stayed at each level was measured at various concentrations of blockers and membrane potentials. In both Cs+ and Rb+ block, the distribution of the current levels showed reasonable agreement with the binomial theorem. This finding suggests that the inwardly rectifying K+ channel is composed of three equally conductive subunits and each subunit is independently blocked by Cs+ or Rb+. 4. The dwell-time histogram in each substate was well fitted with a single-exponential function. On the assumption of the binomial model, the blocking (mu) and unblocking (lambda) rate for Cs+ and Rb+ were calculated. The value of mu was linearly proportional to the concentration of the blocking ion at a given membrane potential and increased with hyperpolarization (e-fold increase with a change of -43.5 mV in the Cs+ block). lambda was almost independent of the concentration of the blocking ion and less dependent on the membrane potential than mu. 5. The open and blocked times were calculated in experiments showing no clear sublevels. The mean open time was almost equal to the mean dwell time at the full open level in experiments showing sublevels under the same conditions. On the other hand, the mean blocked time was about two or three times longer than the mean dwell time at the zero-conductance level measured in experiments with sublevels. These results may suggest that the instant one of the three subunits is plugged by blocking ions, the remaining two subunits are closed by unknown mechanisms. 6. Our results support the hypothesis that the cardiac inwardly rectifying K+ channel is composed of three equally conductive subunits.
Full text
PDF




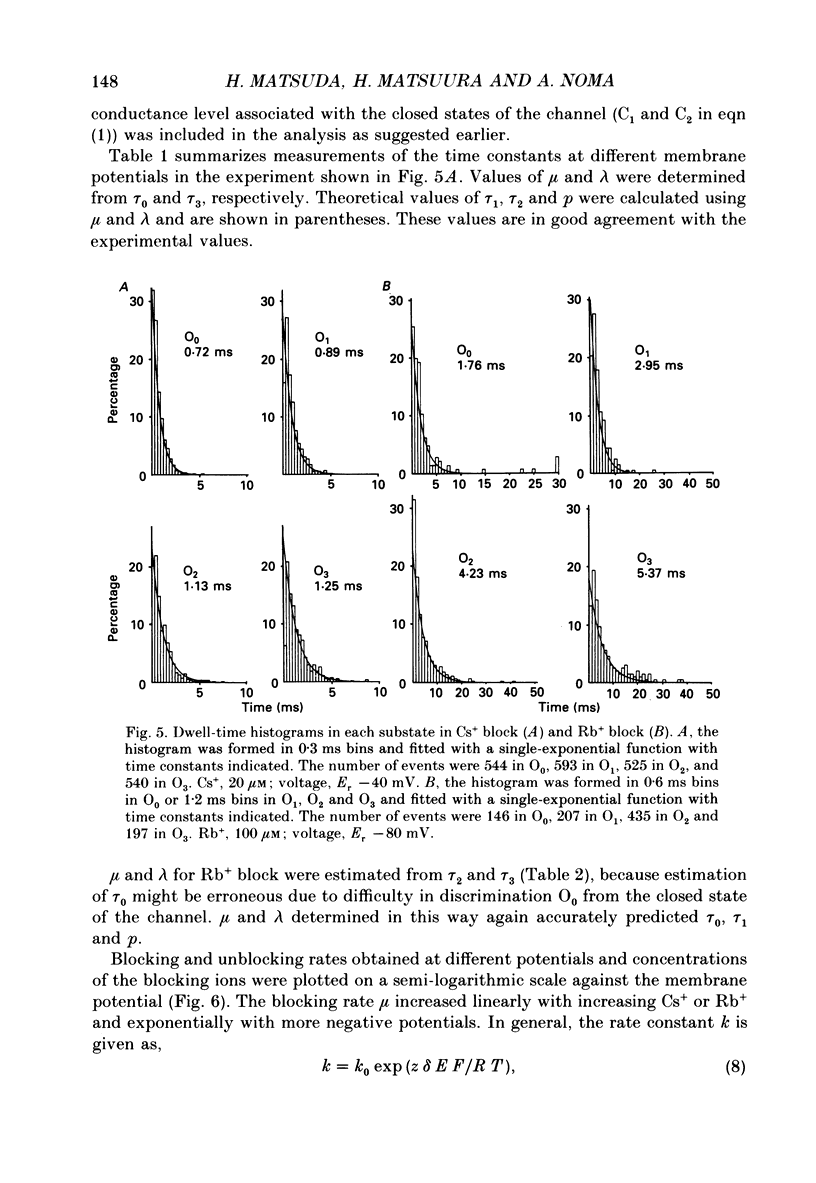
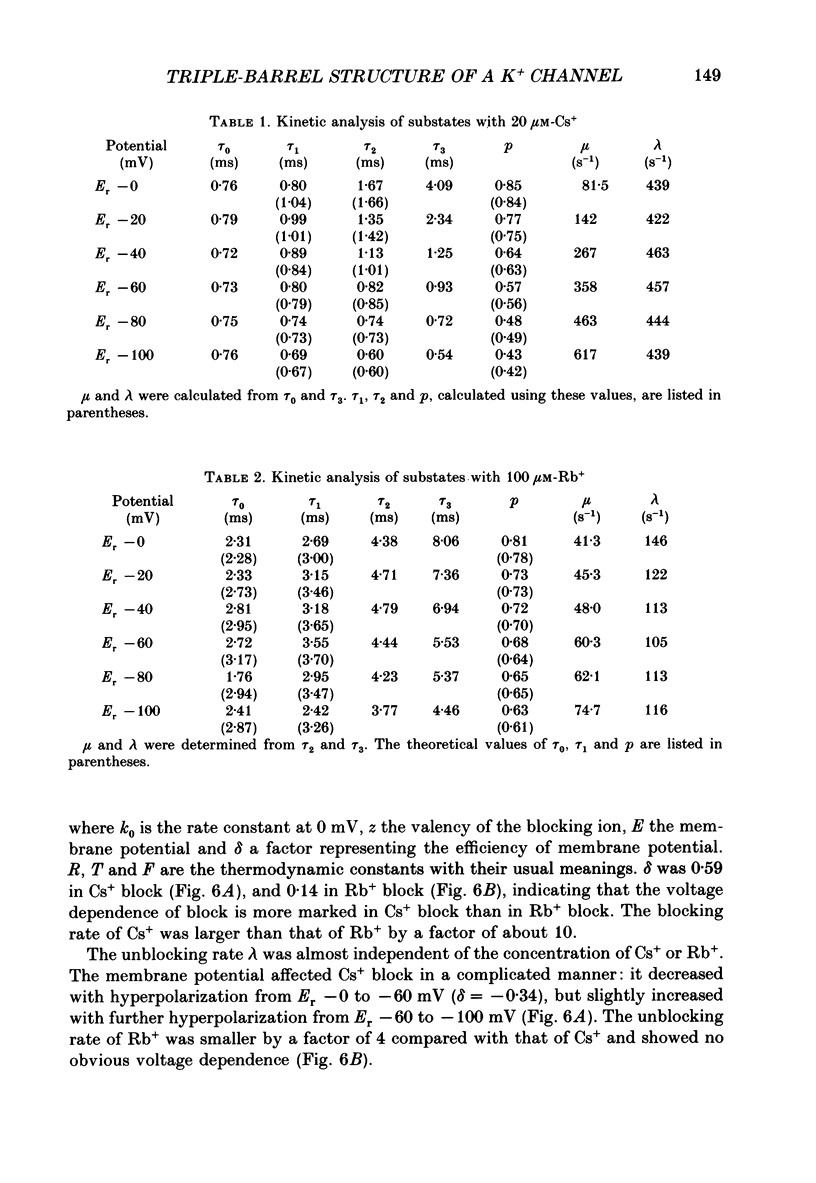
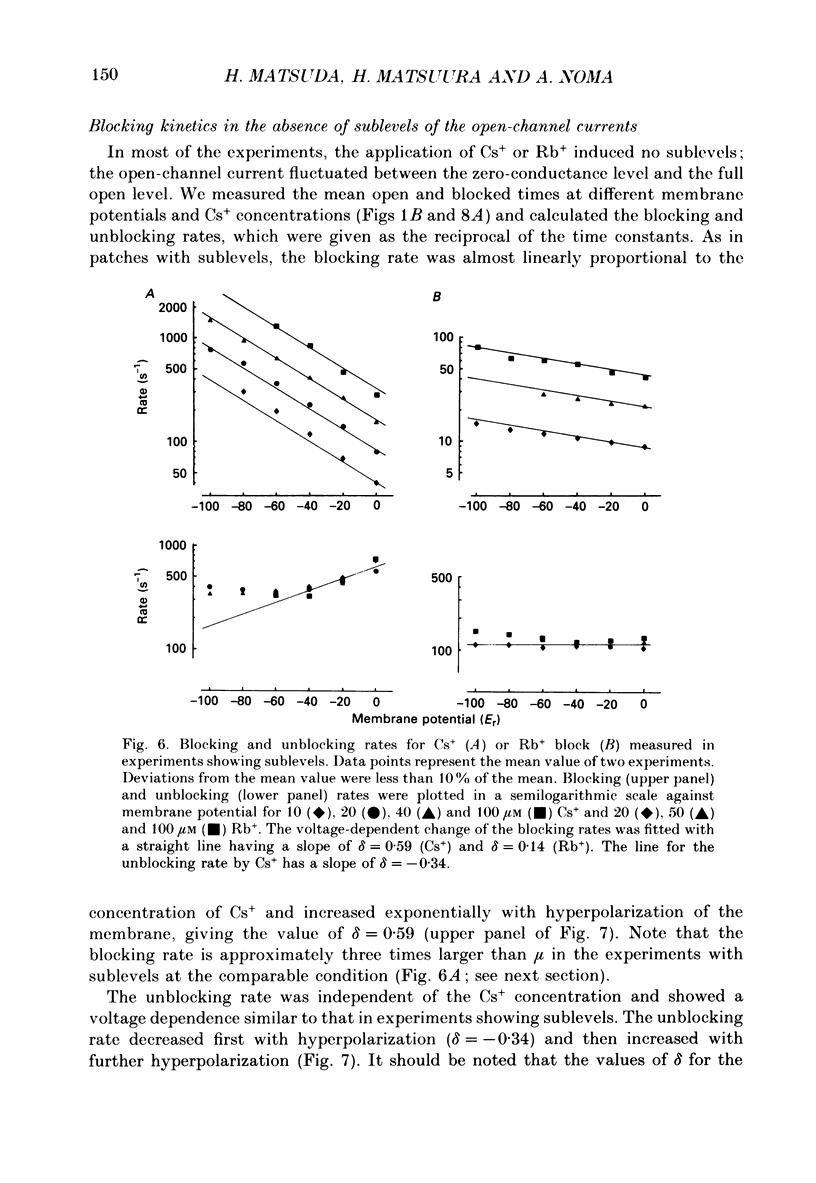
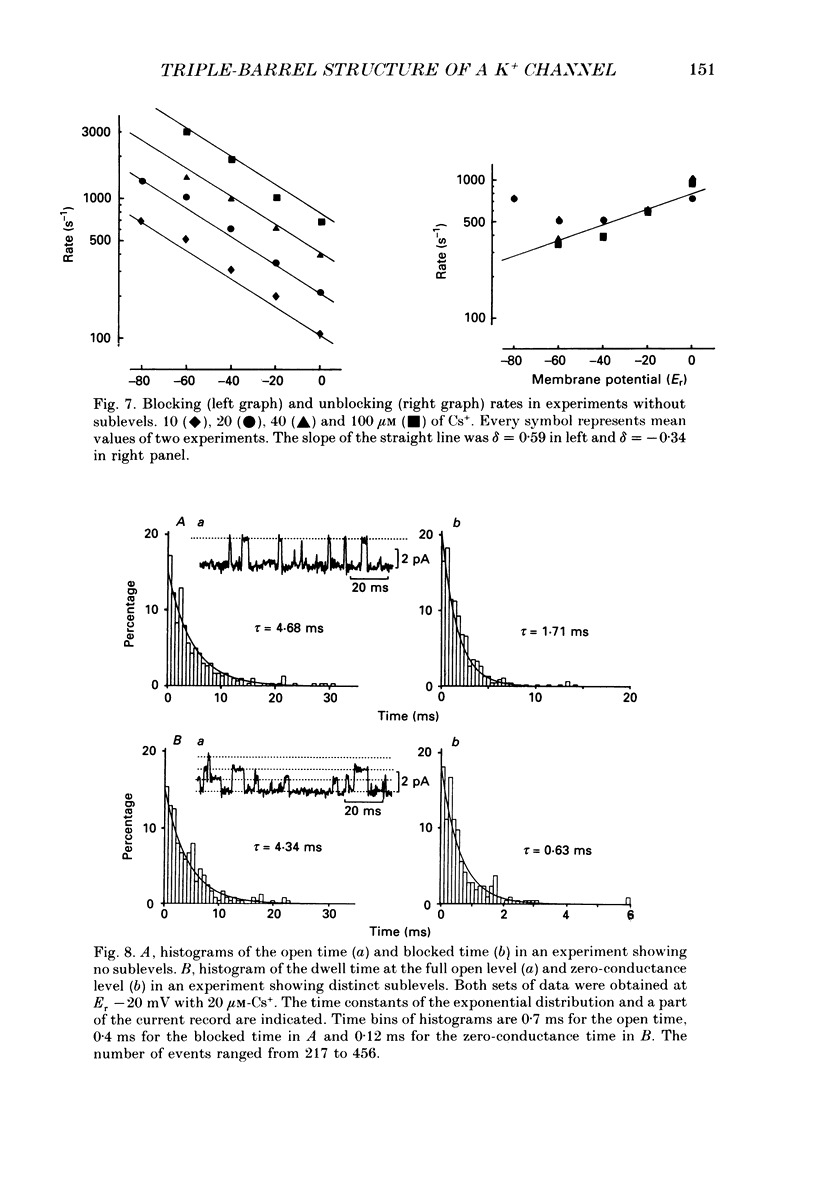




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Hunter M., Giebisch G. Multi-barrelled K channels in renal tubules. Nature. 1987 Jun 11;327(6122):522–524. doi: 10.1038/327522a0. [DOI] [PubMed] [Google Scholar]
- Imoto Y., Ehara T., Matsuura H. Voltage- and time-dependent block of iK1 underlying Ba2+-induced ventricular automaticity. Am J Physiol. 1987 Feb;252(2 Pt 2):H325–H333. doi: 10.1152/ajpheart.1987.252.2.H325. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Kazachenko V. N., Geletyuk V. I. The potential-dependent K+ channel in molluscan neurones is organized in a cluster of elementary channels. Biochim Biophys Acta. 1984 Jun 13;773(1):132–142. doi: 10.1016/0005-2736(84)90558-3. [DOI] [PubMed] [Google Scholar]
- Krouse M. E., Schneider G. T., Gage P. W. A large anion-selective channel has seven conductance levels. Nature. 1986 Jan 2;319(6048):58–60. doi: 10.1038/319058a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. J Physiol. 1980 Jul;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]


