Abstract
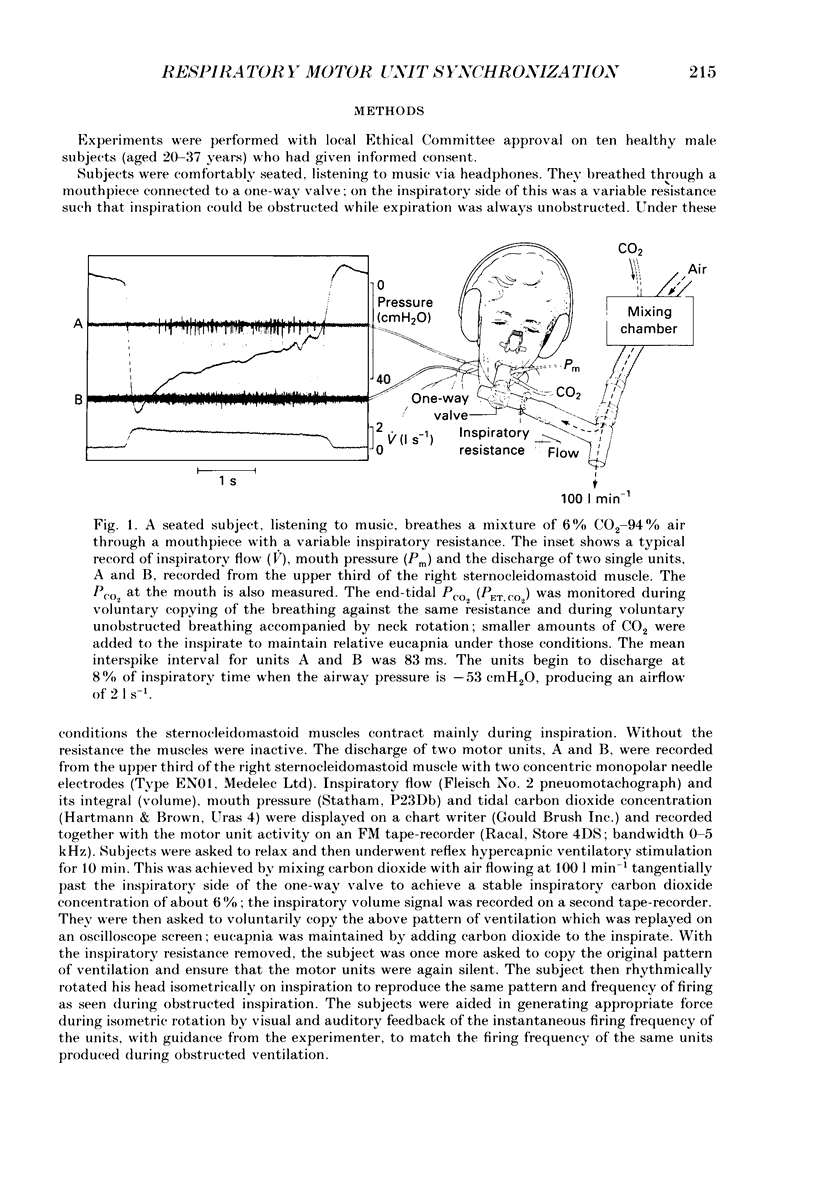
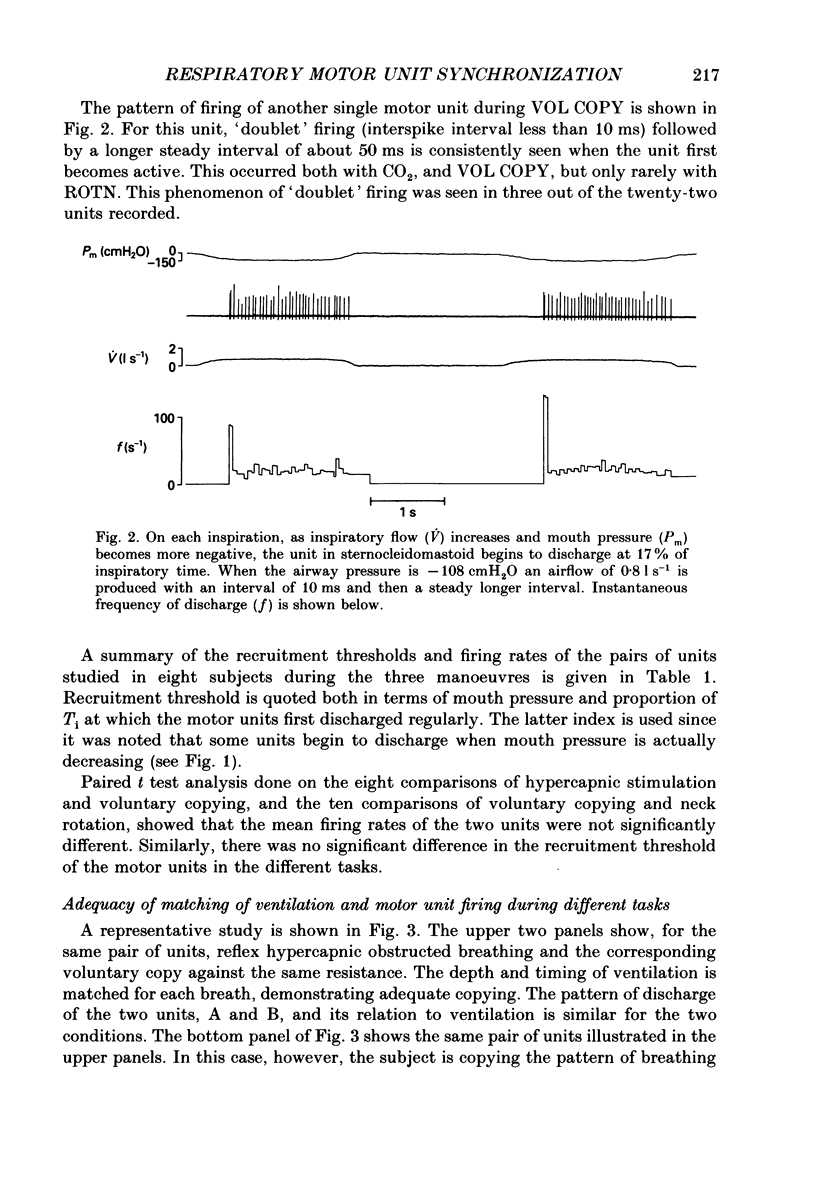
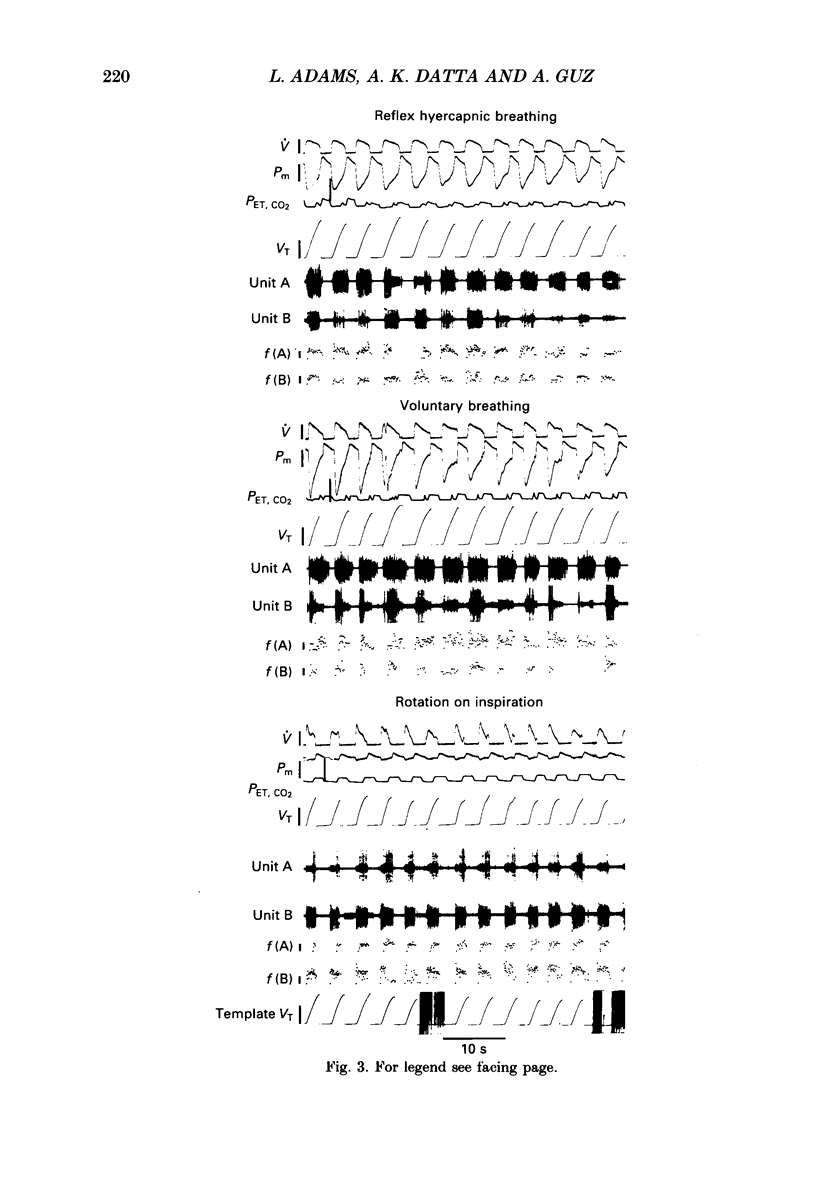
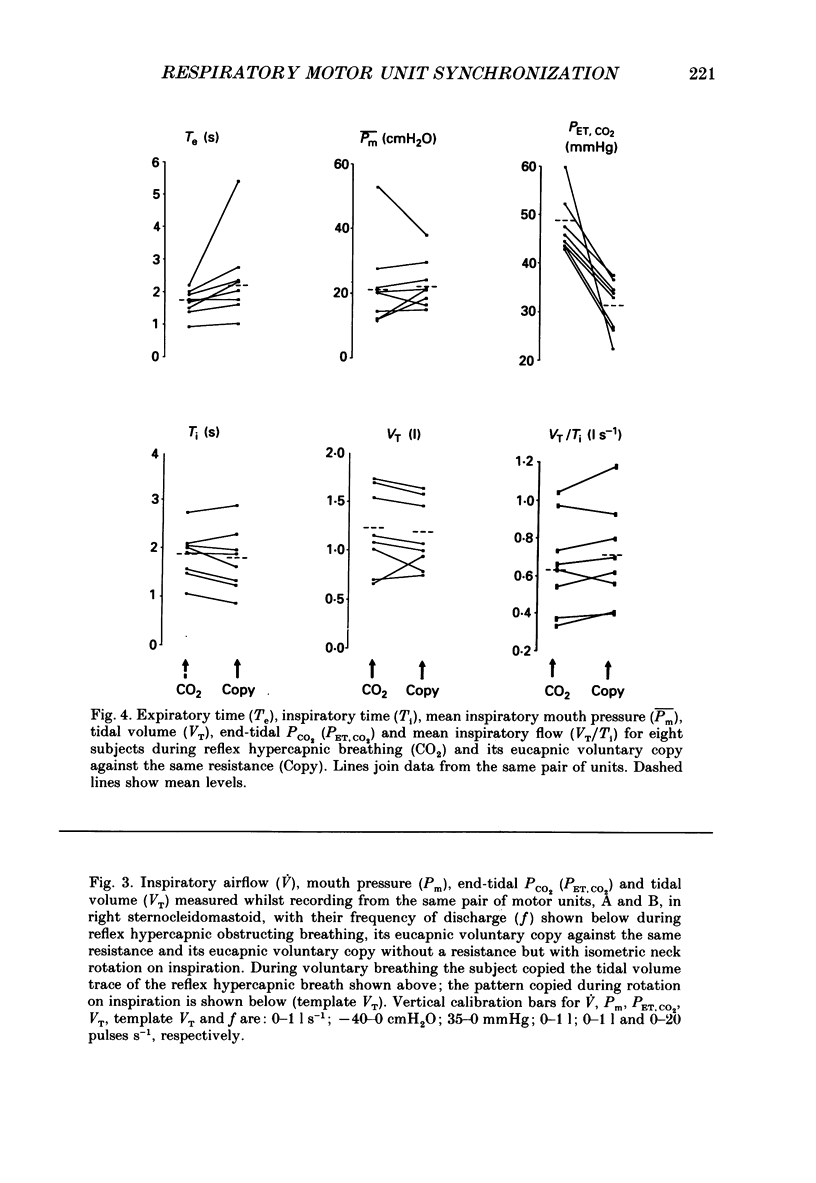
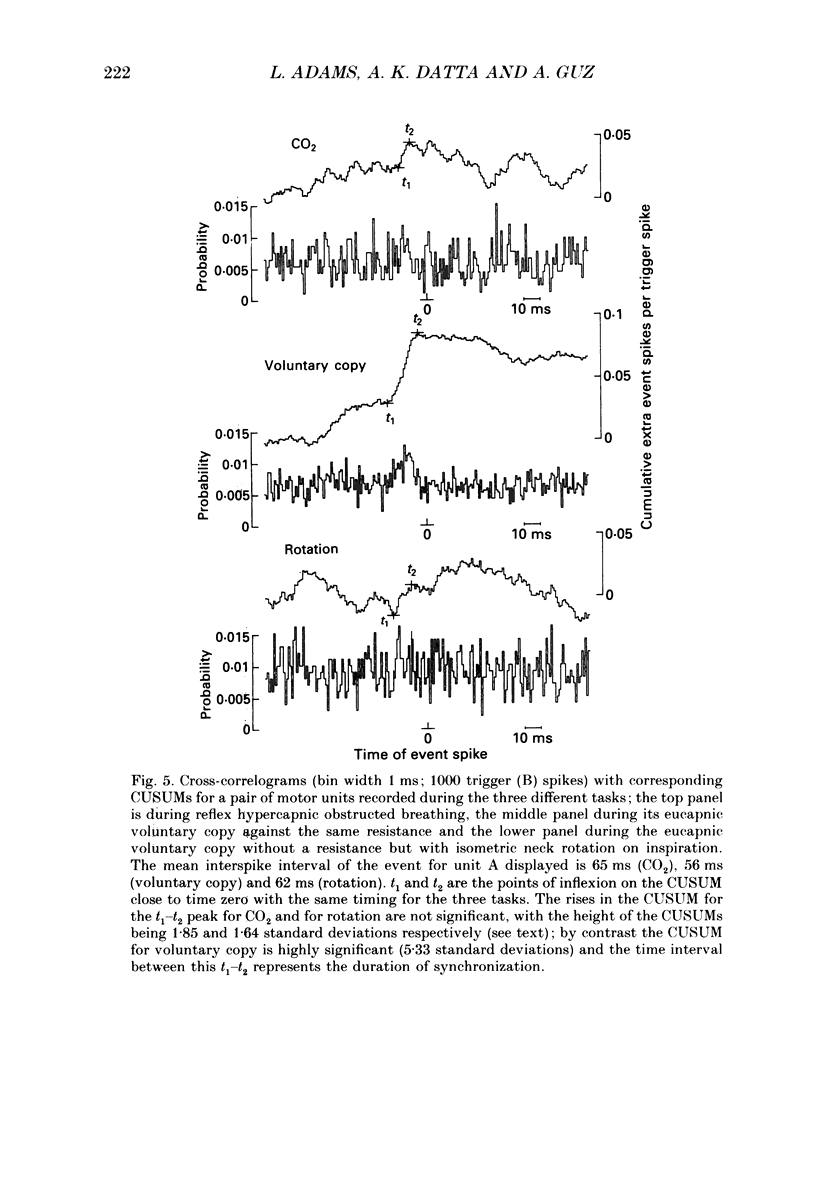
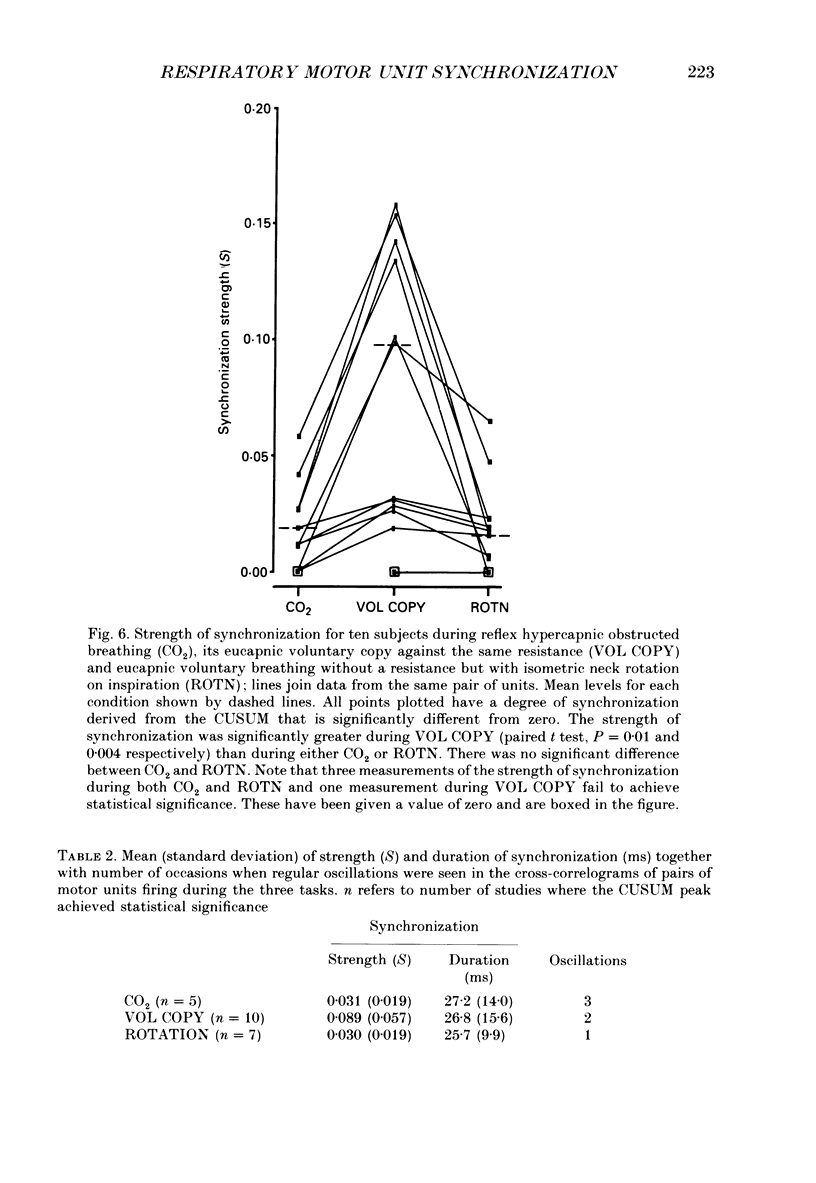
1. Motor unit firing has been studied in human sternocleidomastoid muscle. 2. Two needle electrodes were inserted into the muscle and the activity of pairs of motor units recorded during (a) reflex hypercapnic obstructed breathing, (b) eucapnic voluntary copying of (a) against the same inspiratory resistance and (c) voluntary copying of (a) without any resistance, accompanied by isometric neck rotation. 3. Cross-correlation histograms of the firing of unit pairs showed a clear central peak, indicative of synchronization. The mean duration of the peak during voluntary breathing was 25 ms (range 9-40 ms). There was no difference in duration of synchronization during the different tasks. 4. For the duration of the synchronization peak, the mean strength of synchronization expressed as the number of concomitant discharges of the two units as a proportion of the total number of discharges was 0.026 (range 0.011-0.058) for reflex hypercapnic obstructed breathing. For the same unit pairs the strength of synchronization for isometric neck rotation was the same as that during reflex hypercapnic breathing but for voluntary obstructed breathing it was, on average, threefold greater. 5. In three out of twenty-two motor units studied, 'discharge' occurred with an interval of less than 10 ms ('doublet' firing) at the onset of each inspiration during both types of obstructed breathing; this was rarely observed during neck rotation. 6. The results are interpreted in terms of different synaptic drives to the motor units during the three different tasks.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff M. J., Sears T. A. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971 Jun;215(2):557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassal M., Bianchi A. L. Inspiratory onset or termination induced by electrical stimulation of the brain. Respir Physiol. 1982 Oct;50(1):23–40. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Buys E. J., Lemon R. N., Mantel G. W., Muir R. B. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986 Dec;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL E. J. The role of the scalene and sternomastoid muscles in breathing in normal subjects; an electromyographic study. J Anat. 1955 Jul;89(3):378–386. [PMC free article] [PubMed] [Google Scholar]
- COLLE J., MASSION J. Effet de la stimulation du cortex moteur sur l'activité électrique des nerfs phréniques et médians. Arch Int Physiol Biochim. 1958 Nov;66(4):496–514. doi: 10.3109/13813455809084226. [DOI] [PubMed] [Google Scholar]
- Calvin W. H. Generation of spike trains in CNS neurons. Brain Res. 1975 Jan 24;84(1):1–22. doi: 10.1016/0006-8993(75)90796-9. [DOI] [PubMed] [Google Scholar]
- Cheema S. S., Rustioni A., Whitsel B. L. Light and electron microscopic evidence for a direct corticospinal projection to superficial laminae of the dorsal horn in cats and monkeys. J Comp Neurol. 1984 May 10;225(2):276–290. doi: 10.1002/cne.902250211. [DOI] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H., Stein R. B. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986 Aug;17(2-3):153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Plum F. Separation of descending spinal pathways to respiratory motoneurons. Exp Neurol. 1972 Jan;34(1):78–94. doi: 10.1016/0014-4886(72)90189-6. [DOI] [PubMed] [Google Scholar]
- Dietz V., Bischofberger E., Wita C., Freund H. J. Correlation between the dischanges of two simultaneously recorded motor units and physiological tremor. Electroencephalogr Clin Neurophysiol. 1976 Jan;40(1):97–105. doi: 10.1016/0013-4694(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Duffin J., Lipski J. Monosynaptic excitation of thoracic motoneurones by inspiratory neurones of the nucleus tractus solitarius in the cat. J Physiol. 1987 Sep;390:415–431. doi: 10.1113/jphysiol.1987.sp016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger H. H., Feldman J. L. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988 Mar 1;269(1):47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Freund H. J., Büdingen H. J., Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol. 1975 Jul;38(4):933–946. doi: 10.1152/jn.1975.38.4.933. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Rothwell J. C. Activation of the human diaphragm from the motor cortex. J Physiol. 1987 Mar;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R., Kernell D., Lamarre Y. Synaptic stimulation superimposed on motoneurones firing in the 'secondary range' to injected current. J Physiol. 1966 Nov;187(2):401–415. doi: 10.1113/jphysiol.1966.sp008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Facteurs déterminant l'order de recrutement des motoneurones phréniques. J Physiol (Paris) 1979;75(7):765–781. [PubMed] [Google Scholar]
- Hoffer J. A., Sugano N., Loeb G. E., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol. 1987 Feb;57(2):530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. The spatial distribution of synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J., Bektas A., Porter R. Short latency inputs to phrenic motoneurones from the sensorimotor cortex in the cat. Exp Brain Res. 1986;61(2):280–290. doi: 10.1007/BF00239518. [DOI] [PubMed] [Google Scholar]
- Muir R. B., Lemon R. N. Corticospinal neurons with a special role in precision grip. Brain Res. 1983 Feb 21;261(2):312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Orem J., Netick A. Behavioral control of breathing in the cat. Brain Res. 1986 Feb 26;366(1-2):238–253. doi: 10.1016/0006-8993(86)91301-6. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell G. C., Bystrzycka E. K., Nail B. S. The identification of brainstem neurones projecting to thoracic respiratory motoneurones in the cat as demonstrated by retrograde transport of HRP. Brain Res Bull. 1985 Jan;14(1):25–37. doi: 10.1016/0361-9230(85)90174-1. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell G. C., Törk I., Bystrzycka E. K. Distribution of corticospinal motor fibres within the cervical spinal cord with special reference to the phrenic nucleus: a WGA-HRP anterograde transport study in the cat. Brain Res. 1986 Jul 30;379(1):75–83. doi: 10.1016/0006-8993(86)90257-x. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. D., Weaver L. C. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. J Physiol. 1988 Feb;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]


