Abstract
The amniote pallium, a vital component of the forebrain, exhibits considerable evolutionary divergence across species and mediates diverse functions, including sensory processing, memory formation, and learning. However, the relationships among pallial subregions in different species remain poorly characterized, particularly regarding the identification of homologous neurons and their transcriptional signatures. In this study, we utilized single-nucleus RNA sequencing to examine over 130 000 nuclei from the macaque (Macaca fascicularis) neocortex, complemented by datasets from humans (Homo sapiens), mice (Mus musculus), zebra finches (Taeniopygia guttata), turtles (Chrysemys picta bellii), and lizards (Pogona vitticeps), enabling comprehensive cross-species comparison. Results revealed transcriptomic conservation and species-specific distinctions within the amniote pallium. Notable similarities were observed among cell subtypes, particularly within PVALB+ inhibitory neurons, which exhibited species-preferred subtypes. Furthermore, correlations between pallial subregions and several transcription factor candidates were identified, including RARB, DLX2, STAT6, NR3C1, and THRB, with potential regulatory roles in gene expression in mammalian pallial neurons compared to their avian and reptilian counterparts. These results highlight the conserved nature of inhibitory neurons, remarkable regional divergence of excitatory neurons, and species-specific gene expression and regulation in amniote pallial neurons. Collectively, these findings provide valuable insights into the evolutionary dynamics of the amniote pallium.
Keywords: Amniote, Pallium evolution, Cross-species comparison, Comparative transcriptomics, Single-nucleus RNA sequencing
INTRODUCTION
Deciphering the conservation and innovation of the vertebrate brain throughout evolution can offer crucial insights into the origin of cognition. Approximately 320 million years ago, the amniote ancestor diverged into two main lineages: mammals and sauropsids (reptiles and birds) (Benton & Donoghue, 2007; Woych et al., 2022). Despite this extensive period of evolutionary divergence, the fundamental brain architecture of developing amniotes retains strong similarities to that of their shared ancestor, comprising four principal regions: the telencephalon, diencephalon, mesencephalon (or midbrain), and rhombencephalon (or hindbrain) (Pessoa et al., 2019). While this basic structure of the amniote brain is highly conserved, the telencephalon, especially the pallium, has undergone distinct evolutionary expansions in different lineages (Butler, 1994; Kverková et al., 2022; Puelles, 2001). For instance, the mammalian neocortex evolved into a highly expanded, six-layered structure, while the dorsal ventricular ridge (DVR) evolved in the pallium of birds and reptiles, with both serving as neural centers for processing sensory information and supporting advanced cognitive functions (Güntürkün, 2012; Jarvis et al., 2005; Nieder et al., 2020; Roth, 2015). Moreover, the dorsal cortex (DC) of reptiles shares developmental homology with the mammalian neocortex, further underscoring the evolutionary relationships between these pallial structures (Butler et al., 2011; Karten, 2015). Although substantial evidence supports the embryonic homology between the pallium of mammals and sauropsids, the precise relationships among cell types and subregions within the amniote pallium remain to be fully elucidated (Cárdenas & Borrell, 2020; Liao et al., 2024; Montiel & Aboitiz, 2018; Nomura et al., 2014; Puelles et al., 2000). Additionally, the molecular mechanisms underlying the differential expansion of the pallium and the functional convergence observed in amniote brain evolution are not yet well understood (Aboitiz, 2011; Cárdenas & Borrell, 2020; Tosches & Laurent, 2019).
Recent advances in high-resolution cellular technologies, such as single-cell/single-nucleus RNA sequencing (scRNA-seq/snRNA-seq), have significantly enhanced our understanding of the brain across various species (Colquitt et al., 2021; Svensson et al., 2018; Tang et al., 2009; Tosches et al., 2018). These studies have highlighted the conservation of cellular composition in amniote brains and identified species-specific neuronal subtypes (Hain et al., 2022; Han et al., 2022; Hodge et al., 2019; Li et al., 2023). However, most studies have been limited to one or two species, lacking a systematic cellular comparison across a broader range of taxa. Given the distinct evolutionary rates of different cell types (Chen et al., 2023; Jorstad et al., 2023; Tasic et al., 2018), expanding the scope of species included in such analyses is crucial. Furthermore, when analyzing correlations among certain cell types or brain subregions, it is essential to distinguish between the roles of transcription factors (TFs) and non-TFs (effector genes). TFs typically define cell identity, whereas non-TFs directly mediate cellular functions (Arendt et al., 2016; Wagner, 2014). However, previous research has often failed to make this distinction, resulting in inconsistent findings (Colquitt et al., 2021; Liang et al., 2018). Consequently, the homologous signatures of pallial cell types and subregions between mammals and sauropsids remain unresolved.
In this study, we employed snRNA-seq to construct a cellular atlas of the macaque pallium. Integrating these data with human, mouse, zebra finch, turtle, and lizard datasets, we conducted a cross-species comparative analysis to investigate gene expression patterns and regulatory networks at the cellular level. Our approach focused on identifying both conserved and species-specific characteristics within the pallium of amniotes, shedding light on the evolutionary dynamics of this critical brain region.
MATERIALS AND METHODS
Ethics statement and sample collection
This project was carried out in strict compliance with animal research regulations and was approved by the Institutional Animal Care and Use Committee of Yunnan Key Laboratory of Primate Biomedical Research, Institutional Animal Care and Use Committee of Huazhen Bioscience (permit HZ2019027), and Institutional Review Board of the BGI Ethics Committee (permit BGI-IRB A21025-T1).
Tissue samples were isolated from the prefrontal cortex of two 72-month-old female cynomolgus macaques (Macaca fascicularis) and immediately frozen in liquid nitrogen. The dorsal and ventral prefrontal cortex samples were collected in both macaques and subsequently utilized for snRNA-seq analysis.
Isolation and purification of nuclei
Frozen macaque tissue blocks were homogenized in 1 mL of pre-chilled Dounce homogenization buffer using a Dounce homogenizer, applying 10 loose and 10 tight strokes, with the samples immersed in ice throughout. Following homogenization, 2 mL of additional buffer was added, and the homogenate was filtered through a 40 μm cell strainer (Miltenyi Biotec, Germany) into a 15 mL conical tube and centrifuged at 900 ×g for 10 min to pellet the cell nuclei at 4°C. Fluorescence microscopy was used to estimate the quality and quantity of the extracted nuclei.
Library preparation and sequencing for snRNA-seq
The snRNA-seq library was prepared using the DNBelab C Series Single-Cell Library Preparation Kit (MGI, #1000021082, China) for DNBelab C4 RNA-seq analysis based on droplet technology. The single-cell nuclear suspension, obtained from the previously prepared snRNA samples, was washed twice with phosphate-buffered saline (PBS) containing 0.04% bovine serum albumin (BSA). The nuclear suspension was resuspended, filtered through a 40 μm cell strainer, and the cell suspension and nuclear concentrations were measured and recorded. Droplets were prepared using the DNBelab C Series Single-Cell Library Preparation Kit (MGI, #1000021082, China), and cell lysis and mRNA capture were performed with magnetic beads within the droplets. The single-cell magnetic beads were then recovered using a lysis reagent recovery system with a vacuum pump, and the captured mRNA was reverse transcribed into cDNA. Following synthesis, the double-stranded cDNA and Oligo products were amplified and screened. The Oligo products were barcoded using polymerase chain reaction (PCR) for subsequent preparation into Oligo loaded-into-machine libraries. Finally, the cDNA product was fragmented, end repaired, ligated, PCR amplified, denatured, circularized, and digested to prepare a single-stranded DNA library. After library preparation, sequencing was performed using the DIPSEQ T1 sequencing platform at the China National Gene Bank (Shenzhen).
Processing macaque snRNA-seq data
PISA (v.0.7) was employed to filter and demultiplex the raw sequencing reads generated by DNBelab C4 RNA-seq, while STAR (v.2.6.1a) was used to align these reads. For alignment, a modified GTF file of the Macaca_fascicularis_5.0 genome was used, which included both intronic and exonic regions. The aligned sequences were then sorted with Sambamba (v.0.7), and a Unique Molecular Identifier (UMI) counts matrix mapping individual cells to genes was generated.
Quality control, dimensionality reduction, and cell clustering were performed using the R package Seurat (v.4.0.3) (Butler et al., 2018). Low-quality cells were removed by excluding those with fewer than 500 or more than 6 000 detected genes, or a percentage of mitochondrial gene expression exceeding 5%. To identify and eliminate potential doublets within the snRNA-seq data, DoubletFinder (v.2.0.3) (McGinnis et al., 2019) was applied.
Following strict quality control, a total of 135 986 nuclei were retained, with a median of 4 260 UMIs and 1 670 genes per cell. The datasets were then split by individual macaque using the SplitObject function, and each subset was normalized using SCTransform, with the parameter vars.to.regress set to “percent.mt”. Batch correction was subsequently performed by implementing PrepSCTIntegration, FindIntegrationAnchors, and IntegrateData, using 3 000 features identified through SelectIntegrationFeatures. Additionally, RunPCA, RunUMAP, FindNeighbors, and FindClusters were employed for dimensionality reduction and clustering analysis, with 30 principal components (PCs) utilized for principal component analysis (PCA). Differentially expressed genes (DEGs) were identified using FindAllMarkers with the criteria fold-change>1.5 and adjusted P<0.05.
Cell subtype prediction for macaque datasets
Cell subtype annotation for the macaque datasets was performed using Seurat (v.4.0.3) according to previous study (Lei et al., 2022). Macaque cells were mapped to human subtypes by identifying anchors with FindIntegrationAnchors (dimension set to 30) using SCT assays. The TransferData function was then applied to classify macaque cells based on human subtypes, and the predicted labels were used to define macaque cell subtypes. Subsequently, these annotations were verified by assessing the expression of classical marker genes.
Hierarchical clustering analysis
Cell group dendrograms were constructed based on hierarchical clustering across cell types, subtypes, and clusters and visualized using ape (v.5.6.2) and ggtree (v.3.4.4) (Yu et al., 2017). Highly variable genes (HVGs) were identified using FindVariableFeatures, and the average expression of 3 000 HVGs was calculated for each cell group. Hierarchical clustering was performed using the dist and hclust functions in R. The dendrogram branches were adjusted manually without changing the tree structure.
DEG expression analysis across species
DEGs were identified for each cell type in each specie using the FindAllMarkers function, retaining only genes with a fold-change>2. Additionally, DEG intersections were calculated using five pseudobulk methods implemented in Libra (v.1.0.0) (Squair et al., 2021). UpSet plots, generated with UpSetR (v.1.4.0), were used to display intersection counts of DEGs for excitatory neurons (EXs), inhibitory neurons (INs), and non-neuronal cells. Conserved genes for cluster C0 were identified using the FindConservedMarkers function with default parameters.
Cross-species pairwise cluster correlations
Correlation analysis among cell subtypes from two species was conducted using one-to-one orthologs (provided in Supplementary Table S1), as previously reported (Tosches et al., 2018). Analysis involved four main steps: (1) Two gene sets were selected for comparison: (i) shared TFs in both datasets and (ii) the union of 3 000 HVGs from both datasets, excluding TFs; (2) Average expression levels of the selected gene sets in each cell group were calculated using the AverageExpression function in Seurat; (3) Resulting “gene set×group type” matrices were transformed into gene specificity matrices; (4) Spearman rank correlations and associated P-values of gene specificities between all pairs of group types across the two datasets were calculated based on scripts provided by Tosches et al. (2018). To enhance the robustness of our findings, additional correlations were calculated using HVGs, excluding TFs, matched in count to the number of TFs (sHVGs). Results were visualized as heatmaps using pheatmap (v.1.0.12) and gene set expression levels were calculated using UCell (v.1.1.0). The accuracy of the macaque data in this study was validated using the macaque dataset from Chen et al. (2023) and human dataset from Nagy et al. (2020).
Gene Ontology (GO) enrichment analysis
GO enrichment analysis was performed using the R package clusterProfiler (v.3.18.0) (Yu et al., 2012) with the org.Hs.eg.db (v.3.12.0) annotation as the background. Results were visualized using the R package enrichplot (v.1.10.1).
Integration of neurons across species
Integration followed the procedures outlined in “Processing macaque snRNA-seq data”, with slight modifications. To address significant differences in the number of EXs and INs across species, down-sampling was applied prior to integration. Approximately 9 000 EXs and 4 000 INs were down-sampled from human, macaque, and mouse datasets. Only one-to-one homologous genes were retained for subsequent analyses. Using the human atlas as a reference for cross-species comparison, one-to-one orthologous gene lists for macaque, mouse, and zebra finch were acquired using biomaRt (v.2.46.0), while those for lizards and turtles were provided by Tosches et al. (2018). Additionally, the FindClusters function was used at a resolution of 0.3 in both EXs and INs groups.
Partition-based graph abstraction (PAGA) trajectory analysis
PAGA analysis was conducted on PVALB+ cell clusters using Scanpy (v.1.9.1) (Wolf et al., 2018). The PAGA graph was computed with the top 5 000 genes, and positions were visualized by setting the parameter threshold to 0.03.
Sauropsid single-cell and mouse in situ hybridization (ISH) comparison
Interspecies correlations were calculated using scripts from Colquitt et al. (2021) and the R package cocoframer (v.0.1.1). For the comparison, shared TFs or 6 000 HVGs excluding TFs were selected. Analysis highlighted the neocortex and other telencephalic compartments, including the pallial amygdala and endopiriform.
Neocortex ISH of mice and humans
ISH images were downloaded from the Allen Brain Atlas and BrainSpan platforms, and images were cropped for optimal visualization. Mouse ISH images were downloaded from various websites (Lein et al., 2007) (https://mouse.brain-map.org/): Bdnf: https://mouse.brain-map.org/experiment/siv?id=79587720&imageId=79593146&initImage=ish&contrast=0.5,0.5,0,255,4; Gfra1: https://mouse.brain-map.org/experiment/siv?id=71064217&imageId=71054294&initImage=ish&contrast=0.5,0.5,0,255,4; Dlx2: https://mouse.brain-map.org/experiment/siv?id=1482&imageId=101313722&initImage=ish&contrast=0.5,0.5,0,255,4; Rarb: https://mouse.brain-map.org/experiment/siv?id=75038442&imageId=74930054&initImage=ish&contrast=0.5,0.5,0,255,4; Stat6: https://mouse.brain-map.org/experiment/siv?id=69290549&imageId=69253420&initImage=ish&contrast=0.5,0.5,0,255,4; Nr3c1: https://mouse.brain-map.org/experiment/siv?id=727&imageId=101309852&initImage=ish&contrast=0.5,0.5,0,255,4; Thrb: https://mouse.brain-map.org/experiment/siv?id=71249069&imageId=71109047&initImage=ish&contrast=0.5,0.5,0,255,4.
Human images were also downloaded from two websites (Miller et al., 2014) (https://www.brainspan.org/): BDNF: https://www.brainspan.org/ish/experiment/dual_view?id=100125098&imageId=101712779&imageType=ish,expression,nissl&initImage=ish&z=-1; GFRA1: https://www.brainspan.org/ish/experiment/dual_view?id=100101516&imageId=101633475&imageType=ish,expression,nissl&initImage=ish&z=-1.
Soft clustering of TF expression
Soft clustering of TF expression in mammalian EXs subtypes was performed using Mfuzz (v.2.50.0). Only gene sets exhibiting layer-biased expression were retained.
Gene regulatory network analysis
SCENIC (v.1.3.1) (Aibar et al., 2017) and pySCENIC (v.0.12.1) were used to analyze activated regulons in each cell group following the official tutorial (https://scenic.aertslab.org/). Initially, differentially activated regulons were identified across all EXs in each species. The top 10 activated regulons for EX subclasses were visualized. Subsequently, a more comprehensive analysis was performed for EX subclasses within each species to generate a complete regulon list. The intersections of key TFs across species-specific subclasses were visualized using Venn diagrams with the R package VennDiagram (v.1.6.20).
Co-expression network analysis
Co-expression analysis was performed using the R package WGCNA (v.1.71). To avoid computational memory issues, pseudo-cells were condensed into groups of 100 cells. Gene modules were calculated separately for each dataset, with soft power thresholds determined using the pickSoftThreshold function. Only representative gene modules were retained.
Protein sequence alignment
Cross-species TF protein sequences were aligned using UniProt (https://www.uniprot.org/) with three iterations. Four mammals (Homo sapiens (human), Macaca fascicularis (cynomolgus monkey), Callithrix jacchus (white-tufted-ear marmoset), and Mus musculus (mouse)), three reptiles (Chrysemys picta bellii (western painted turtle), Pogona vitticeps (central bearded dragon), and Crocodylus porosus (saltwater crocodile)), three birds (Taeniopygia guttata (zebra finch), Columba livia (rock dove), and Gallus gallus (chicken)), Ornithorhynchus anatinus (duckbill platypus), and two amphibians (Xenopus laevis (African clawed frog) and Xenopus tropicalis (western clawed frog)) were used for comparison. Percent identity matrices are provided in Supplementary Table S2.
Validation using spatial transcriptomics
Spatial transcriptomic validation was performed using the macaque and teleost datasets from Chen et al. (2023) and Hegarty et al. (2024), respectively. Selected gene expression levels were visualized using Seurat, and spatial transcriptomic figures for zebra finch, mouse, and Chinese softshell turtle were obtained from the Spatial Transcript Omics Database (https://db.cngb.org/stomics/datasets/STDS0000241).
RESULTS
Cell transcriptomic atlas of the amniote pallium
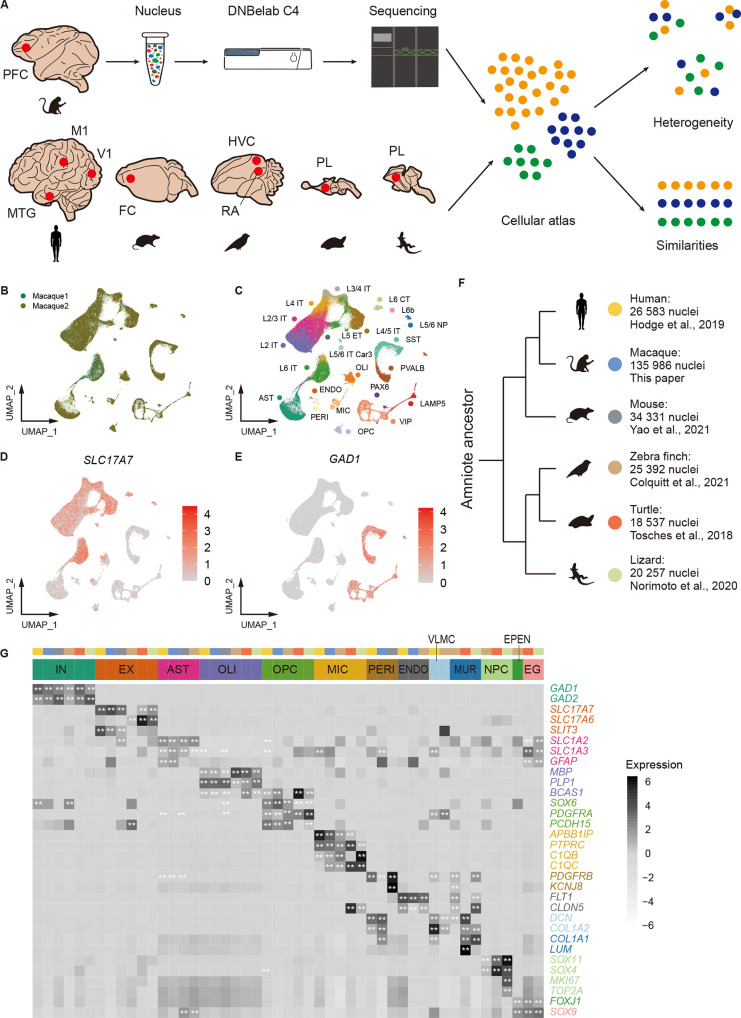
Using snRNA-seq, we generated a high-quality dataset comprising 135 986 nuclei from the prefrontal cortex (PFC), a region of the neocortex, dissected from two female adult macaques (Figure 1A, B). To identify cell subtypes, nuclei from the macaque dataset were matched against human cell subtype expression patterns using a reference transcriptomic atlas of human. These annotations were validated based on the expression of classical marker genes (Figure 1C–E; Supplementary Figure S1A) and correlations with reliable external datasets (Supplementary Figure S2A, B), and included 85 868 excitatory neurons (EXs: SLC17A7, SLC17A6), 27 397 inhibitory neurons (INs: GAD1, GAD2), and 22 721 non-neuronal cells (astrocytes (ASTs): SLC1A2, GFAP; oligodendrocytes (OLIs): MOG, OPALIN; oligodendrocyte progenitor cells (OPCs): PDGFRA, PCDH15; microglial cells (MICs): APBB1IP, CTSS; pericytes (PERIs): PDGFRB; and endothelial cells (ENDOs): FLT1). Additionally, we downloaded five amniote datasets (Figure 1F; Supplementary Figure S1C), including 26 583 nuclei from the human neocortical regions (primary motor cortex, M1; primary visual cortex, V1; middle temporal gyrus, MTG), 34 331 nuclei from the mouse neocortex (frontal cortex, FC), 25 392 nuclei from the zebra finch DVR (HVC proper name (HVC), robust nucleus of the arcopallium (RA)), 18 537 nuclei from the turtle pallium (PL) (including DC and DVR), and 20 257 nuclei from the lizard PL (including DC and DVR).
Figure 1.
Single-cell atlas of amniote pallium
A: Schematic exhibiting overall study design. PFC, prefrontal cortex; M1, primary motor cortex; V1, primary visual cortex; MTG, middle temporal gyrus; FC, frontal cortex; HVC, HVC proper name; RA, robust nucleus of the arcopallium; PL, pallium. B, C: UMAP representation of 135 986 macaque (Macaca fascicularis) nuclei colored by sample (B) and cell type (C). AST, astrocyte; ENDO, endothelial cell; IT, intra-telencephalic-projecting neuron; ET, extra-telencephalic-projecting neuron; NP, near-projecting neuron; CT, corticothalamic neuron; L1–L6, layer 1 to layer 6; MIC, microglia; OLI, oligodendrocyte; OPC, oligodendrocyte precursor cell; EPEN, ependymal cell; PERI, pericyte; LAMP5, PAX6, PVALB, SST and VIP are inhibitory neuron subtypes; IN, inhibitory neuron; EX, excitatory neuron. D, E: Expression of EX marker gene SLC17A7 (D) and IN marker gene GAD1 (E) in macaque dataset. F: Phylogenetic tree of six species. G: Heatmap of classical marker genes in all cell types across species. MUR, mural cell; EG, ependymoglial cell; VLMC, vascular and leptomeningeal cell; NPC, neuronal progenitor cell. **: P<0.01.
Marker gene expression patterns were compared across the six species. Consistent with previous reports (Colquitt et al., 2021; Tosches et al., 2018), INs exhibited conserved expression of GAD1 and GAD2 across all species (Figure 1G). In contrast, EXs and certain non-neuronal cells, such as oligodendrocytes and astrocytes, displayed species-specific expression patterns, reflecting potential divergence. However, other non-neuronal cell types, such as microglial and endothelial cells, showed conserved expression of classical marker genes. Cell type proportions across species are presented in Supplementary Figure S1B; however, these proportions were not comparable due to experimental bias. For example, the mouse dataset was experimentally enriched for neuronal populations, resulting in a reduced representation of non-neuronal cells (Yao et al., 2021).
Conservation and divergence of cell types across species
To investigate the conservation and divergence of cell types across species, we conducted a series of comparative analyses using six datasets. Initially, hierarchical clustering was performed on neurons and glial cells. INs from different species were clustered together (Figure 2A), while other cell types were more dispersed. Next, we identified DEGs for each cell type using six methods across the species (Figure 2B) and analyzed their intersections. Most DEGs were species-specific, with only a small number shared among the six species across the three cell types, including four in EXs, one in INs, and several in non-neuronal cells. This pattern suggests that divergence among cell types across species may be driven by species-specific DEGs, while shared DEGs likely preserve fundamental cell type identities. Notably, INs displayed fewer species-specific DEGs, highlighting their conserved nature, whereas non-neuronal cells demonstrated a higher proportion of species-specific DEGs (Supplementary Table S3). Nonetheless, non-neuronal cells also shared several genes, indicating a balance between conservation and divergence. These findings reflect the conservation of INs, while supporting the hypothesis that non-neuronal cells are more divergent across the amniote pallium (Hodge et al., 2019; Ma et al., 2022).
Figure 2.
Comparison across cell types in amniote pallium
A: Hierarchical clustering of cell types using average expression profiles. B: UpSet plots exhibiting DEG counts of EXs, INs, and non-neuronal cells across species. OLI lineage includes OLI and OPC; AST-like lineage includes AST, EG, and EPEN; vascular cell lineage includes ENDO, PERI, VLMC, and MUR. C, D: Correlation heatmap between humans and macaques using TF profiles (C) and HVG profiles (D). Asterisks represent significance, ns: Not significant; *: P<0.05; **: P<0.01; ***: P<0.001; ****: P<0.0001. E: Bubble plot showing expression levels of differentially expressed markers for IN1 and IN2 in sauropsids. F: Box plots comparing correlation coefficients between IN1 and IN2. G: Section of correlation heatmap for INs between macaques and turtles using TF profiles (left) and HVG profiles (right).
Gene regulatory networks governed by TFs are typically conserved, while downstream effector genes exhibit greater flexibility (Wagner, 2014). Mutations or deletions in TFs can disrupt entire regulatory networks, potentially altering cell types (Arendt et al., 2016). In contrast, changes in effector genes are less likely to cause significant disruptions as they are often functionally replaceable. Consequently, the expression patterns of TFs and effector genes convey different biological significance. TFs reflect regulatory programs that preserve regional or cell type identity, whereas non-TFs/effector genes are more flexible and directly linked to neuronal functions (Colquitt et al., 2021; Wagner, 2014). To compare cell subtype signatures across species, we calculated pairwise correlations between cell groups using TF expression profiles and HVGs excluding TFs (representing non-TFs/effector genes). In mammals, nearly all macaque cell subtypes exhibited one-to-one orthologous relationships with human or mouse subtypes based on both TF and HVG profiles (Figure 2C, D; Supplementary Figure S3A, B). However, when compared to sauropsids, macaque EX and IN subtypes showed broad similarities with most neuronal subtypes and even some non-neuronal subtypes (Supplementary Figure S3C–H), consistent with previously reported homologies (Colquitt et al., 2021; Hodge et al., 2019; Norimoto et al., 2020; Tosches et al., 2018). These results reveal conserved correlations among pallial cell subtypes from closely related mammals and broad similarities among major cell types in distantly related amniotes.
When analyzing datasets from closely related species or the same species, the correlation coefficients derived from TF expression profiles were comparable to those obtained using HVGs, with the latter often being higher. This indicates that non-TFs may contribute more significantly to pallial similarities among closely related species than TFs (Figure 2C, D; Supplementary Figure S2A–C). Conversely, for distantly related species, correlation coefficients based on TF expression profiles were consistently higher than those obtained using HVGs (Supplementary Figure S3A–H), supporting the conserved nature of TF expression, as previously reported (Colquitt et al., 2021; Tosches et al., 2018). Additional analyses using HVGs matched in number to TFs (sHVGs) confirmed that these observations were not influenced by unequal gene set sizes or varying expression levels (Supplementary Figure S4A–E).
Intriguingly, we identified two IN subtypes in sauropsids, distinguished by their correlation patterns: IN1, marked by lateral ganglionic eminence (LGE)-derived IN markers (MEIS2+, TSHZ1+), exhibited weaker correlations with macaque INs; IN2, characterized by medical/caudal ganglionic eminence (MGE/CGE)-derived IN markers (LHX6+, SATB1+) (Colquitt et al., 2021), exhibited stronger correlation with macaque INs (Figure 2E–G; Supplementary Figures S3K, S2F). Notably, mammalian cerebral cortex INs are predominantly composed of MGE/CGE neurons (Su-Feher et al., 2022; Torigoe et al., 2016; Tosches & Laurent, 2019). Based on these markers, we defined IN1 MEIS2 TSHZ1 as LGE-derived INs and IN2 LHX6 SATB1 as MGE/CGE-derived INs. Analysis of DEGs revealed that IN2 shared more DEGs across the three species than IN1 ( Supplementary Figure S3I). These findings imply that IN2 is more conserved and involved in pathways related to pallium formation (Supplementary Figure S3J and Table S4), indicating functional similarities between mammalian INs and sauropsid IN2.
Our comparative analysis across six amniote species highlighted transcriptomic conservation of INs and heterogeneity among major cell types. Cell subtype homology became ambiguous with increasing phylogenetic distance between species. Notably, INs displayed fewer species-specific DEGs, while non-neuronal cells exhibited a higher degree of species-specific divergence. Correlation coefficients from TF expression profiles were higher than those from non-TFs in distantly related amniotes, whereas the reverse was true for closely related species, highlighting the conserved nature of TF expression. Additionally, the identification of two IN subtypes in sauropsids, based on their correlations with macaque INs, implies that MGE/CGE-derived INs are more conserved than LGE-derived INs within the amniote pallium.
Shared and distinct signatures of INs in amniote pallium
Building on our findings regarding marker gene expression and subtype correlations of INs across the amniote pallium, we further investigated their defining signatures. By integrating INs from six species and performing unsupervised clustering, 10 distinct cell clusters were identified (Figure 3A, B). These clusters were identified based on marker expression: C5 PROX1 NR2E1 and C6 VIP PROX1 as CGE-derived INs (VIP+, PROX1+, NR2E1+), C0 VIP MEIS2 as a CGE/LGE-derived mixed INs (VIP+, MEIS2+, TSHZ1+), and the remaining INs as MGE-derived INs (SOX6+, LHX6+, SATB1+) (Figure 3B; Supplementary Figure S5A, B).
Figure 3.
Inhibitory neurons (INs) in amniote pallium
A: UMAP plots of sampled INs, colored by species (left) and unsupervised clustering cell clusters (right). B: Heatmap of DEG expression across IN cell clusters. C: Sankey diagram showing cellular compositional makeup of species and cell subtypes in species-biased cell clusters. D: Bubble plot showing expression of differentially expressed markers for IN subtypes and cell clusters. E: Network of key genes and pathways in species-biased clusters. F: PAGA analysis of PVALB clusters. Left, PAGA positions of three clusters; right, expression of PVALB in PAGA positions. Edge weights represent confidence in the presence of connections. G: Bar plot showing representative GO enrichment pathways of C0. H: Network exhibiting key genes and pathways in (G).
Most clusters were evenly distributed across the six datasets, except for C7 RELN NR2E1, C8 PVALB NR2E1, and C9 PVALB LHX6 (Figure 3C; Supplementary Figure S5C, D). Notably, C7 and C9 predominantly consisted of mammalian INs, while C8 was primarily composed of sauropsid INs, suggesting species-specific IN subtypes in the mammalian and sauropsid pallium. C7 RELN NR2E1 was inferred to represent an MGE-derived IN subtype with DEGs enriched in telencephalon formation pathways (Figure 3D, E; Supplementary Figure S5E). C8 PVALB NR2E1 and C9 PVALB LHX6 were identified as PVALB+ IN subtypes involved in neuronal signal transmission in the sauropsid and mammalian pallium, respectively. Additionally, PVALB+ cluster C2 (PVALB, SOX6) was analyzed alongside these subtypes. Trajectory analysis revealed that C2 extended into two distinct branches connecting to C8 and C9, with numerous genes such as PVALB, DAAM2, and NR2E1 showing dynamic expression along the trajectory (Figure 3F; Supplementary Figure S5F). These results indicate that C2 represents a conserved PVALB+ IN subtype, whereas C8 and C9 may be evolutionarily novel subtypes with significant changes in gene expression. Prior research has reported high PVALB expression in specific layers of the mammalian neocortex and the song motor pathway of birds (Glezer et al., 1993; Hara et al., 2012; Sherwood et al., 2010), hinting at potential divergence in PVALB+ IN subtypes across the amniote pallium. Further experimental verification is required to confirm these evolutionary patterns and functional implications.
Previous studies have reported that LGE-derived INs share greater transcriptomic similarity with CGE-derived INs than MGE-derived INs (Su-Feher et al., 2022), and that VIP+ INs are absent from the reptilian pallium (Briscoe et al., 2018; Tosches et al., 2018). Consistent with these findings, our data showed that C0 VIP MEIS2 was highly expressed in both LGE-derived and CGE-derived IN marker genes and was predominantly composed of mammalian VIP+ INs and sauropsid LGE-derived INs (Figure 3D; Supplementary Figure S5C, D). Based on its composition and gene expression patterns, C0 was deduced to represent a mixture of INs and was enriched in pathways related to axonogenesis (Figure 3G–H). Further analysis revealed that C0 could be divided into high and low VIP+ C0 subclusters, similar to the CGE-derived clusters C5 PROX1 NR2E1 and C6 VIP PROX1, respectively (Supplementary Figure S6A–D), highlighting the close relationship between LGE- and CGE-derived INs (Su-Feher et al., 2022). These findings suggest that C0 may represent an intermediate population reflecting shared features between LGE- and CGE-derived INs within the amniote pallium. Furthermore, some reptilian LGE-derived INs may share similar functions with mammalian VIP+ INs, given their high expression of conserved genes within C0 (Supplementary Figure S5G).
In conclusion, we observed homologous IN subtypes across amniotes and notable interspecies variability. For example, we identified PVALB+ IN subtypes with evident differences between mammals and sauropsids, as well as a mixed cell cluster of LGE- and CGE-derived INs (C0 VIP MEIS2). These findings provide evidence for divergent evolutionary pathways in neuronal diversification and offer insights into the absence of VIP+ INs in the reptilian pallium.
Transcriptomic heterogeneity among EXs in the mammalian neocortex and avian DVR
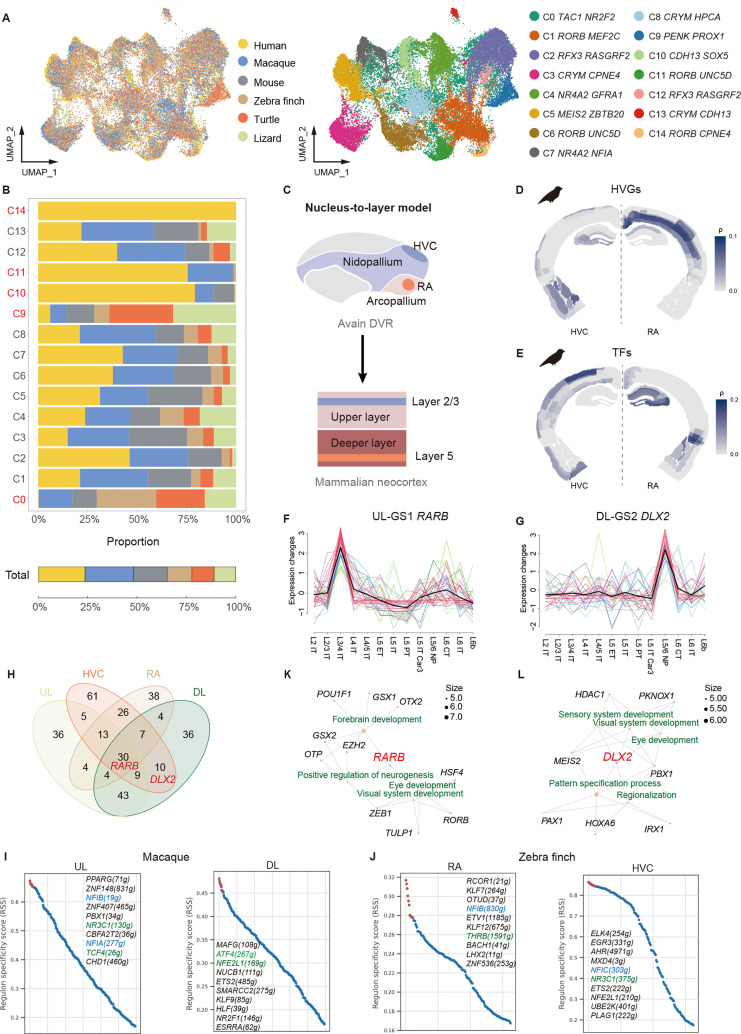
EXs exhibit diversity and abundance within the mammalian pallium (Hodge et al., 2019; Ma et al., 2022; Yao et al., 2021). To explore whether homologous EX subtypes exist across amniotes, we co-clustered EX subtypes from six species, resulting in 15 distinct clusters. These clusters were then classified as resembling upper layer (UL)-like EXs (RFX3+, RORB+, UNC5D+), deeper layer (DL)-like EXs (CRYM+, CDH13+, NR4A2+), mixture populations, or species-specific subtypes (Figure 4A, B; Supplementary Figure S7A, B). However, consistent with prior correlation analyses, identifying one-to-one homologous EX subtypes was challenging due to widespread co-expression of UL and DL marker genes across clusters. Five species-biased clusters were distinguished, with C0 TAC1 NR2F2 cells being present across all amniotes except humans, while the C9 PENK PROX1 cluster was primarily found in sauropsids, the C10 CDH13 SOX5 cluster predominantly in mammals, the C11 RORB UNC5D cluster mostly in primates, and the C14 RORB CPNE4 cluster uniquely in humans. Further analysis indicated that C0 likely represented a low-quality cluster (Supplementary Figure S6E), while C14 was validated using other integration methods (Supplementary Figure S6F, G). These findings imply that while transcriptomic homology exists among amniote EXs, the evolution of more specialized cell subtypes in higher vertebrates, such as primates and humans, has contributed to significant diversification (Lamanna et al., 2023; Musser et al., 2021; Naumann & Laurent, 2020; Norimoto et al., 2020). This evolutionary divergence likely explains the difficulty in identifying homologous EX subtypes across all amniotes.
Figure 4.
Heterogeneity among mammalian neocortex and avian DVR
A: UMAP plots of sampled EXs colored by species (left) and unsupervised clustering cell clusters (right). B: Bar plots showing distribution of cell clusters across species. C: Schematic illustrating nucleus-to-layer model (Colquitt et al., 2021; Karten, 1991). D, E: Spearman correlations between zebra finch EXs (HVC and RA) and mouse pallium, mapped onto coronal sections from the Adult Mouse Atlas (ABA) (Lein et al., 2007). F, G: Soft clustering gene sets of TFs in UL and DL EXs. H: Venn diagram showing intersections of active TFs between mammalian and avian EXs. I, J: Top 10 active regulons in EXs in macaques (UL and DL) and zebra finches (RA and HVC). Conserved TFs are labeled in green, while NFI gene family is labeled in blue. K, L: Network showing representative GO enrichment pathways of groups in F (UL-GS1 RARB) and G (DL-GS2 DLX2).
The mammalian neocortex and reptilian DC are derived from the dorsal pallium, while the sauropsid DVR originates from the ventral pallium (Güntürkün et al., 2020; Naumann & Laurent, 2020; Puelles et al., 2017). Earlier research noted significant correlations among these regions, yet their complex interrelationships remain poorly understood (Norimoto et al., 2020; Tosches et al., 2018). To address this, we first performed correlation analysis of mammalian and avian EX subtypes to identify distinctions and homologies. According to the “nucleus-to-layer” model l(Dugas-Ford et al., 2012; Jarvis et al., 2005; Karten, 1991), HVC was homologous to the neocortical layer 2/3, and RA was homologous to layer 5 (L5) (Figure 4C). In our macaque data, RA EXs exhibited strong similarities to L5 neurons as well as correlations with other DL neurons (Supplementary Figure S7C, D), consistent with earlier studies (Colquitt et al., 2021). Similarly, HVC EXs resembled macaque DL neurons, mirroring previous research comparing mouse DL profiles (Colquitt et al., 2021). However, no correlations were found between HVC EXs and UL neurons, potentially due to divergence between primate and rodent UL neurons (Fame et al., 2017; Huang et al., 2023; Loomba et al., 2022). Further insights were obtained by mapping zebra finch EX data onto ISH profiles from the mouse pallium. RA EXs exhibited stronger correlations with entorhinal DL neurons using TF expression profiles, whereas HVG-based analysis revealed greater similarity to non-entorhinal DLs (Figure 4D, E), consistent with prior studies (Colquitt et al., 2021; Wagner, 2014). HVC EXs, in contrast, demonstrated significant similarities to both UL and DL neurons based on HVGs and to DL neurons using TF profiles. Verification with ISH data for BDNF and GFRA1 in mouse and human pallia showed high expression of these HVC marker genes in DLs (Supplementary Figure S7J, S8A, B). Overall, these results indicate that both avian RA and HVC EXs may be more akin to mammalian DL neurons than to UL neurons, with TF profiles providing stronger correlations. While the "nucleus-to-layer" model offers valuable insights, our findings indicate that this framework may oversimplify these relationships and requires further refinement and additional confirmatory evidence.
We speculated that the observed similarities between the avian DVR and mammalian DLs may be driven by key TFs. To explore this, we first conducted soft clustering of all TFs using the primate expression matrix and identified four distinct gene sets: GS1, GS3, and GS4 associated with ULs and GS2 associated with DLs). These gene sets were enriched in pattern formation and regionalization of the amniote pallium (Figure 4F, G; Supplementary Figure S7E–G and Table S2). Next, we performed gene regulatory network analysis on EXs from both mammals and zebra finch (Figure 4H–J; Supplementary Figure S7H, I). This analysis identified two TFs, RARB and DLX2, as pivotal regulators. Both TFs are vital for avian brain regionalization (Koszinowski et al., 2015; Marcucio et al., 2005) and were extensively expressed in the macaque pallium (Supplementary Figure S6H, S8C). RARB, a member of GS1, emerged as a key TF in the regulon intersections across four EX classes. This TF is involved in eye development, cognitive function, and synapse formation (Srour et al., 2016), aligning with GS1 enrichment analysis (Figure 4K, L). In contrast, DLX2 was associated with GS2 and identified as a key TF in the regulon intersections of most EX classes, except for mammalian ULs. This TF is critical for forebrain morphogenesis, retina formation, and ganglion cell development (Tan & Testa, 2021), consistent with the enrichment results. In addition, the nuclear factor I gene family, implicated in brain development and intelligence (Zenker et al., 2019), was active across the four EX classes, with NFIX, NFIA, and NFIB known to play vital roles in the regulatory network of salamander cortical neurons (Lust et al., 2022; Wei et al., 2022). These findings underscore the pivotal role of TFs in amniote pallial formation and regulation, potentially driving the similarities between the avian DVR and mammalian neocortex. Such regulatory mechanisms provide further evidence supporting the convergent evolution of these brain regions in avians and mammals (Hintermann et al., 2022; Yokoyama & Pollock, 2012).
This study highlighted both the heterogeneity and shared characteristics of EXs across mammals and sauropsids. We distinguished species-specific EX subtypes, including human-absent C0, sauropsid-preferred C9, mammal-preferred C10, primate-preferred C11, and human-exclusive C14, suggesting the emergence of novel subtypes as species complexity increases. Our findings showed that avian RA EXs exhibit strong similarity to macaque DL EXs, while HVC EXs resemble both DLs and ULs, challenging the “nucleus-to-layer” model (Colquitt et al., 2021) and reflecting the likeness of HVCs to primate DLs. Furthermore, we identified key TFs, especially RARB and DLX2, as crucial to these similarities, potentially reflecting convergent evolution in the avian and mammalian pallium (Hintermann et al., 2022; Yokoyama & Pollock, 2012). These findings indicate that while ancestral TFs are conserved across amniotes, their regulatory networks may have undergone distinct evolutionary adaptations, a hypothesis requiring further verification.
Transcriptomic heterogeneity among EXs in the mammalian neocortex and reptilian DVR and DC
To elucidate the relationship between mammalian and reptilian pallial EXs, we analyzed their gene expression signatures. Turtle DC EXs exhibited stronger similarities to mouse neocortex ISH data when TF profiles were used, whereas DVR EXs aligned more closely with the neocortex when HVG profiles were used (Figure 5A, B). Comparable trends were observed when using macaque and lizard datasets, although the results were less pronounced due to fewer cells and poorer quality (Supplementary Figure S9A–D). Likewise, turtle DVR EXs were correlated with macaque L6b neurons based on HVGs and with L4 IT based on TFs, while DC EXs were only correlated with L5/6 NP neurons based on TFs (Figure 5C). DC EXs showed no significant similarity to human neocortex EXs using HVGs but exhibited similarities to DL EXs using TFs (Supplementary Figure S9A). Comparatively, lizard DVR EXs were correlated with both DL and UL neurons based on HVGs but only with DL neurons when using TFs, while DC EXs were similar to DL neurons for both gene sets (Supplementary Figure S9B). These findings highlight that TFs and non-TFs convey distinct biological insights. The reptilian DC appears to resemble the neocortex in cellular or regional identity, likely due to a common developmental origin (Güntürkün et al., 2020; Naumann & Laurent, 2020; Puelles et al., 2017), whereas the DVR may parallel neocortical functionality, reflecting evolutionary adaptations inherited from shared ancestors. Our results further underscore significant differences in TF- and HVG-based profiles in comparative analyses across amniote pallial EXs (Figure 5D). These observations support the hypothesis that reptilian DC may be homologous to mammalian DL neurons (Briscoe & Ragsdale, 2018; Güntürkün et al., 2020; Luzzati, 2015), while the DVR may perform functions analogous to the neocortex (Briscoe et al., 2018; Yamashita & Nomura, 2017).
Figure 5.
Heterogeneity among mammalian neocortex and reptilian DVR and DC
A, B: Spearman correlations between zebra finch EXs (DC and DVR) and mouse pallium, mapped onto coronal sections from ABA (Lein et al., 2007). C: Heatmap reflecting correlations between macaque EX subtypes and turtle DVR EX subtypes using TFs or HVGs (excluding TFs). D: Overview of correlations among amniote regional EXs. E, F: Venn diagram showing intersections of active TFs between mammalian neocortex, reptilian DC (E) and DVR (F) EXs. G, H: Top 10 active regulons in EXs in turtles (G) and lizards (H). I, J: Heatmap of STAT6 (I) and NR3C1 (J) protein sequence percent identity among amniotes and amphibians. K, L: Heatmap of gene module expression levels in amniote pallial EXs.
Despite distinct signatures between the reptilian DC and DVR, their EXs shared numerous active TFs with those in the mammalian neocortex (Figure 5E–H). Among these, three representative TFs (STAT6, THRB, and NR3C1) were identified as key regulators due to their essential roles in neural pathways. Notably, STAT6 was inactive in zebra finch EXs, while THRB and NR3C1 were members of the intersection of active TFs across all six species, alongside BACH2, BCLAF1, EP300, GABPA, NFE2L1, and SREBF2. The conservation of these TFs was validated by their widespread expression in both the macaque and teleost pallium (Supplementary Figure S8D, S9H, I). STAT6 is involved in the neuroimmunological response, including maintaining homeostatic microglia and regulating inflammatory mediators (olde Heuvel et al., 2019). Protein sequence alignment of STAT6 across 12 species revealed high conservation among mammals, with the platypus exhibiting greater sequence similarity to other mammals than to avians, lizards, crocodiles, turtles, and frogs (internally conservative) (Figure 5I). These findings suggest that while amniotes share conserved TFs regulating neuroimmunological responses, different evolutionary branches may employ orthologous TFs in divergent neuroimmune pathways, which warrants additional verification.
In contrast, NR3C1 and THRB protein sequences demonstrated high percent identity across amniotes (Figure 5J), with THRB also showing high scores in frogs (Supplementary Figure S9F). Both TFs are crucial for maintaining nervous system homeostasis. For example, NR3C1 expression is reduced in individuals with histories of child abuse, schizophrenia, depression, and suicide, as well as in alcohol-dependent animal models (Gatta et al., 2021; Holmes et al., 2019). THRB plays critical physiological roles in the biological activity of thyroid hormones, development, growth, and metabolism (Liu et al., 2021; Liu & Brent, 2018). In brief, our findings imply that NR3C1 function may be conserved in amniotes, while THRB function may be conserved in both amniotes and amphibians, indicating that homologous TFs may show varied transcriptomic conservation due to differences in protein sequences or regulatory networks.
To further investigate the role of TFs in shaping gene regulatory networks across amniotes, we analyzed gene modules containing at least one of the identified TFs in EXs from distinct cell classes (Figure 5K, L). Gene modules obtained from mammalian neocortex EXs, such as UL_STAT6_THRB and DL_STAT6_NR3C1, exhibited higher expression levels in mammalian and zebra finch cells compared to reptilian cells, whereas gene modules obtained from reptilian DC EXs, such as DC_STAT6, DC_THRB and DC_NR3C1, displayed similar expression across species. These findings signify that novel gene regulatory networks involving TFs have evolved in the mammalian and avian pallium, while ancestral regulatory networks existing in reptiles have been retained in other modern amniotes.
Further analysis of active regulons in reptilian pallial EXs and sauropsid DVR EXs identified 25 shared key TFs (Supplementary Figure S9E). From these, we discerned three gene modules, each containing at least one of these TFs. The HVC_GFRA1 gene module was highly expressed in both zebra finch DVR and mammalian neocortex EXs, RA_ELK4 was exclusively expressed in zebra finch EXs, and DVR_BCLAF1 was solely expressed in reptilian EXs (Supplementary Figure S9G). These results indicate that orthologous TFs may regulate different gene networks in pallial EXs across amniotes (Berto & Nowick, 2018; Wang et al., 2016), potentially contributing to the observed heterogeneity.
Building on earlier research (Colquitt et al., 2021; Norimoto et al., 2020; Tosches et al., 2018), our findings further elucidated the divergent and conserved signatures of EXs across the mammalian and reptilian pallium. TF expression patterns of reptilian DC EXs displayed greater similarity to those of mammalian neocortical EXs, while DVR EXs were significantly correlated with mammalian EXs when non-TFs were analyzed. These observations suggest developmental homology between the reptilian DC and mammalian neocortex, alongside an ancestral evolutionary connection between the DVR and neocortex. Additionally, three TF candidates (STAT6, NR3C1, and THRB) were identified as shared between reptilian and mammalian pallial EXs. These TFs demonstrated varying levels of protein sequence conservation and were associated with distinct gene co-expression networks, indicating significant evolutionary divergence in their regulatory mechanisms. Notably, reptilian pallial EXs exhibited closer homology to mammalian DL neurons than to UL neurons, supporting the hypothesis that ULs may have originated from DLs (Naumann & Laurent, 2020).
DISCUSSION
The amniote pallium, essential for a range of functions, such as sensation, sleep, movement, and learning, has undergone remarkable structural and functional diversification throughout evolution (Güntürkün et al., 2020; Naumann & Laurent, 2020; Puelles et al., 2017; Roth, 2015), leading to the emergence of unique structures such as the mammalian neocortex and sauropsid DVR. In this study, we constructed a single-cell transcriptomic atlas of the macaque pallium and incorporated datasets from humans, mice, zebra finches, turtles, and lizards for cross-species comparison. Our analysis revealed both conserved and divergent gene expression and regulatory patterns among pallial neurons across amniotes.
Our findings highlighted conservation of INs across species, as evidenced by fewer species-specific DEGs in these populations, but evident divergence in species-preferred PVALB+ IN subtypes. Among INs, MGE/CGE-derived INs exhibited greater conservation than LGE-derived INs, although LGE-derived INs showed notable similarities to CGE-derived INs. In contrast, EXs exhibited greater transcriptomic heterogeneity, with distinct species-preferred subtypes correlating with increasing organismal complexity (Lamanna et al., 2023; Musser et al., 2021; Naumann & Laurent, 2020; Norimoto et al., 2020). Mammalian and sauropsid EXs shared many transcriptomic commonalities, but also distinct differences, pointing to shared developmental origins and potential convergent or parallel evolution (Colquitt et al., 2021; Güntürkün, 2012; Hintermann et al., 2022; Roth, 2015; Yokoyama & Pollock, 2012). In addition, TFs, such as DLX2, RARB, STAT6, NR3C1, and THRB, emerged as key conserved regulators among pallial neurons across distantly related amniotes. Conversely, non-TFs appeared to play a pivotal role in maintaining homology among pallial neurons of closely related species. These findings enhance our understanding of the evolutionary signatures of the amniote pallium and provide the foundation for future explorations into the molecular features of brain complexity.
This study explored the conserved and divergent aspects of gene expression and regulation in pallial neurons, shedding light on the evolutionary characteristics and trajectories of the amniote pallium. The evolution of neuronal cell types has become a focal area of research in recent years (Briscoe et al., 2018; Dugas-Ford et al., 2012; Hodge et al., 2019; Musser et al., 2021; Tosches, 2021), presenting challenges in defining homologous cell types—a task that demands consideration of developmental origins, function, multi-omics features, and morphology. Advances in single-cell technologies have alleviated some difficulties, allowing for more precise classification of homologous cell types across species (Colquitt et al., 2021; Ma et al., 2022; Norimoto et al., 2020; Tosches et al., 2018). For example, while the conserved expression patterns of INs allow for their classification into homologous subtypes across species, rapidly evolving cell types, like EXs, pose greater complexity due to their divergent transcriptomic profiles and phenotypic diversity. TFs have emerged as relatively conserved regulators across amniotes (Colquitt et al., 2021; Tosches, 2021; Tosches et al., 2018), providing a foundation for understanding homology. However, significant evolutionary modifications in their sequences or structures may lead to shifts in regulatory networks, cellular morphology, and function. This poses a conundrum: should cells with pronounced phenotypic divergence but shared ancestry be considered homologous, or do they represent entirely novel cell types? Moreover, heavy reliance on model species for gene function studies introduces uncertainties about the applicability of findings to non-model species. Variability in the availability and quality of spatial and molecular data across species, particularly between well-studied mammals and less-studied groups like birds and reptiles, further complicates the accurate identification of certain subpopulations. For example, both prior studies and our results reveal substantial differences between mammalian UL EXs and those in sauropsids, indicating that UL EXs may represent an entirely new subtype (Naumann & Laurent, 2020; Tosches & Laurent, 2019). Future research should aim to standardize criteria for identifying homologous cell types and expand foundational studies to non-model organisms at pivotal evolutionary junctures to clarify the evolutionary paths of the brain, pallium, and neurons. Key approaches could include fine mapping of brain regions in non-model organisms through ISH, exploration of neural pathways via connectomics, application of spatial transcriptomics to elucidate gene expression characteristics in different brain regions, and integration of behavioral and electrophysiological techniques to validate the biological functions of various brain regions.
Mammals, reptiles, and avians share a common ancestor and possess pallial frameworks that largely adhere to the tetrapartite model (Cárdenas & Borrell, 2020; Puelles et al., 2017). Despite this shared blueprint, these taxa exhibit significant interspecies variations in structural organization, cellular composition, and molecular profiles. In mammals, the neocortex dominates the pallium and serves as the primary center for advanced cognitive functions, while in birds, the DVR occupies a similar role, functioning as a hub for complex behaviors and intelligence (Güntürkün, 2012; Hara et al., 2012; Roth, 2015). In contrast, the reptilian pallium, containing regions developmentally homologous to both the mammalian neocortex and avian DVR, is often regarded as the “original version” of the amniote pallium (Güntürkün et al., 2020; Karten, 2015; Naumann & Laurent, 2020; Puelles et al., 2017). Our results indicated that EXs in the reptilian DC resembled DL neurons in the mammalian neocortex, while INs in both reptilian and zebra finch DVRs shared lineage markers characteristic of LGE-derived INs. However, significant differences were also observed, consistent with previous studies (Colquitt et al., 2021; Tosches et al., 2018). For instance, species-specific cell clusters were identified in the mammalian neocortex, while the gene expression profiles of EXs in the avian DVR exhibited significant divergence from their reptilian counterparts. Additionally, similarities between the mammalian neocortex and avian DVR, such as divergent PVALB+ IN subtypes and analogous gene expression patterns observed in zebra finch RA and HVC nuclei with mammalian cortical layers, may signify convergent evolution (Hintermann et al., 2022; Yokoyama & Pollock, 2012). The evolutionary trajectories of amniote pallial structures may be shaped by variations in TFs (Arendt et al., 2016), as we distinguished key TFs with varying degrees of conservation that exhibited species-restricted similarities in protein sequences and co-expression networks. Although development and evolution are distinct processes—development representing an orderly progression based on the genomic blueprint, and evolution reflecting the long-term accumulation of adaptive traits—development often mirrors certain aspects of evolutionary history (Kuratani et al., 2022). This interplay is especially important when defining homologous brain regions or cell types. Future investigations should prioritize studies on pivotal species, such as reptiles and oviparous mammals, as well as the role of TFs in shaping regional and cellular identities. Integrating developmental studies with evolutionary research will be crucial for clarifying the complex evolutionary history of the amniote pallium.
Our study has several areas that could be enhanced and refined. While the pallium encompasses a vast region, our analysis were restricted to specific regions within each species. This limitation raises the possibility that some observed differences may arise from variability across pallial regions rather than true interspecies divergence. Nevertheless, we believe that interspecies differences are more significant than intraspecies regional differences within the pallium. The patterns of conservation and divergence identified in our study are supported by previous research, providing confidence in the robustness of these findings. Comparative transcriptomic analyses offer valuable insights into the molecular underpinnings of evolutionary processes, such as the development of regulatory networks and evolution of cell type-specific characteristics. However, to achieve a more comprehensive understanding of these processes, it is essential to integrate transcriptomic data with other omics datasets. For example, combining transcriptomics with genomics, proteomics, and phenotype-related studies could elucidate the fundamental drivers of transcriptomic changes during evolution and their impact on related pathways and phenotypes. Additionally, combining transcriptomic and phenotypic data through comparative morphology, physiology, behavior, and functional genomics would provide a deeper understanding of the biological significance of observed transcriptomic patterns. A persistent challenge in evolutionary studies, including our own, is the limited number of species available for comparison. While our study included a broader range of species and provided a more comprehensive analysis than many prior efforts, this remains a critical area for improvement. Expanding the scope of future studies to include a wider array of species and a more detailed examination of diverse pallial regions will likely yield more robust and reliable results, further advancing our understanding of the evolutionary dynamics of the amniote pallium.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.K.Z., S.P.L., and F.B.Q.H. conceived and designed the study. F.B.Q.H. and K.L. wrote the manuscript and Z.K.Z., Y.N.L., D.Y.C., S.P.L., K.L., and F.B.Q.H. contributed to the discussion and revision of the manuscript. Y.L., S.Y.J., Z.H.L., and. Y.N.S contributed to sample collection. Z.K.Z, S.P.L., Y.N.L., and F.B.Q.H. participated in guiding and providing suggestions for the study. F.B.Q.H., K.L., P.F.L., Y.R.Z., and Z.Y.Z. provided technical support and conducted data analysis. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank China National GeneBank for providing technical support.
Funding Statement
This work was supported by the National Key Research and Development Program (2022YEF0203200), National Science and Technology Innovation 2030 Major Program (STI2030-2021ZD0200100), and National Key Research and Development Program (2018YFA0801400, 2021YFA0805100)
Contributor Information
You-Ning Lin, Email: linyouning@genomics.cn.
Zhen-Kun Zhuang, Email: zhuangzhenkun@genomics.cn.
DATA AVAILABILITY
The raw sequencing data generated in this study were deposited in the CNGB Nucleotide Sequence Archive (CNSA: https://db.cngb.org/cnsa) under accession code CNP0003026. Sample numbers include MK-002-1L-10, MK-002-2L-44, MK-002-2L-45, MK-003-1L-10, MK-003-1L-9, MK-003-2L-44, and MK-003-2L-45. The macaque expression matrix is available under accession code CSE0000427. The code can be found at: https://github.com/FupaulHuang/AmniotePalliumTranscriptome. Datasets generated in this study were also deposited in the Genome Sequence Archive (GSA) database (https://ngdc.cncb.ac.cn/gsa/) (accession number PRJCA031422), Science Data Bank (doi: 10.57760/sciencedb.17214), and NCBI database (BioProjectID PRJNA1175883).
References
- Aboitiz F Genetic and developmental homology in amniote brains. Toward conciliating radical views of brain evolution. Brain Research Bulletin. 2011;84(2):125–136. doi: 10.1016/j.brainresbull.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Aibar S, González-Blas CB, Moerman T, et al SCENIC: single-cell regulatory network inference and clustering. Nature Methods. 2017;14(11):1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Musser JM, Baker CVH, et al The origin and evolution of cell types. Nature Reviews Genetics. 2016;17(12):744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- Benton M J, Donoghue P C Paleontological evidence to date the tree of life. Molecular Biology and Evolution. 2007;24(1):26–53. doi: 10.1093/molbev/msl150. [DOI] [PubMed] [Google Scholar]
- Berto S, Nowick K Species-specific changes in a primate transcription factor network provide insights into the molecular evolution of the primate prefrontal cortex. Genome Biology and Evolution. 2018;10(8):2023–2036. doi: 10.1093/gbe/evy149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe SD, Albertin CB, Rowell JJ, et al. 2018. Neocortical association cell types in the forebrain of birds and alligators. Current Biology, 28 (5): 686–696. e6.
- Briscoe SD, Ragsdale CW Molecular anatomy of the alligator dorsal telencephalon. Journal of Comparative Neurology. 2018;526(10):1613–1646. doi: 10.1002/cne.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, et al Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology. 2018;36(5):411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AB The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis. Brain Research Reviews. 1994;19(1):66–101. doi: 10.1016/0165-0173(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Butler AB, Reiner A, Karten HJ Evolution of the amniote pallium and the origins of mammalian neocortex. Annals of the New York Academy of Sciences. 2011;1225:14–27. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas A, Borrell V Molecular and cellular evolution of corticogenesis in amniotes. Cellular and Molecular Life Sciences. 2020;77(8):1435–1460. doi: 10.1007/s00018-019-03315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Sun YD, Lei Y, et al. 2023. Single-cell spatial transcriptome reveals cell-type organization in the macaque cortex. Cell, 186 (17): 3726–3743. e24.
- Colquitt BM, Merullo DP, Konopka G, et al Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits. Science. 2021;371(6530):eabd9704. doi: 10.1126/science.abd9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW Cell-type homologies and the origins of the neocortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fame RM, Dehay C, Kennedy H, et al Subtype-specific genes that characterize subpopulations of callosal projection neurons in mouse identify molecularly homologous populations in macaque cortex. Cerebral Cortex. 2017;27(3):1817–1830. doi: 10.1093/cercor/bhw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Grayson DR, Auta J, et al Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1) Molecular Psychiatry. 2021;26(3):1029–1041. doi: 10.1038/s41380-019-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer II, Hof PR, Leranth C, et al Calcium-binding protein-containing neuronal populations in mammalian visual cortex: a comparative study in whales, insectivores, bats, rodents, and primates. Cerebral Cortex. 1993;3(3):249–272. doi: 10.1093/cercor/3.3.249. [DOI] [PubMed] [Google Scholar]
- Güntürkün O The convergent evolution of neural substrates for cognition. Psychological Research. 2012;76(2):212–219. doi: 10.1007/s00426-011-0377-9. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Stacho M, Ströckens F. 2020. The brains of reptiles and birds. In: Kaas JK. Evolutionary Neuroscience. 2nd ed. London: Academic Press, 159–212.
- Hain D, Gallego-Flores T, Klinkmann M, et al Molecular diversity and evolution of neuron types in the amniote brain. Science. 2022;377(6610):eabp8202. doi: 10.1126/science.abp8202. [DOI] [PubMed] [Google Scholar]
- Han L, Wei XY, Liu CY, et al Cell transcriptomic atlas of the non-human primate Macaca fascicularis. Nature. 2022;604(7907):723–731. doi: 10.1038/s41586-022-04587-3. [DOI] [PubMed] [Google Scholar]
- Hara E, Rivas MV, Ward JM, et al Convergent differential regulation of parvalbumin in the brains of vocal learners. PLoS One. 2012;7(1):e29457. doi: 10.1371/journal.pone.0029457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty BE, Gruenhagen GW, Johnson ZV, et al Spatially resolved cell atlas of the teleost telencephalon and deep homology of the vertebrate forebrain. Communications Biology. 2024;7(1):612. doi: 10.1038/s42003-024-06315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann A, Guerreiro I, Lopez-Delisle L, et al Developmental and evolutionary comparative analysis of a regulatory landscape in mouse and chicken. Development. 2022;149(12):dev200594. doi: 10.1242/dev.200594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Bakken TE, Miller JA, et al Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573(7772):61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Jr L, Shutman E, Chinaka C, et al Aberrant epigenomic modulation of glucocorticoid receptor gene (NR3C1) in early life stress and major depressive disorder correlation: systematic review and quantitative evidence synthesis. International Journal of Environmental Research and Public Health. 2019;16(21):4280. doi: 10.3390/ijerph16214280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LW, Ma ZY, Yu LT, et al Deep spiking neural networks with high representation similarity model visual pathways of macaque and mouse. Proceedings of the AAAI Conference on Artificial Intelligence. 2023;37(1):31–39. doi: 10.1609/aaai.v37i1.25073. [DOI] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, et al Avian brains and a new understanding of vertebrate brain evolution. Nature Reviews Neuroscience. 2005;6(2):151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad NL, Song JHT, Exposito-Alonso D, et al Comparative transcriptomics reveals human-specific cortical features. Science. 2023;382(6667):eade9516. doi: 10.1126/science.ade9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ. 1991. Homology and evolutionary origins of the 'neocortex'. Brain, Behavior and Evolution, 38 (4–5): 264–272.
- Karten HJ Vertebrate brains and evolutionary connectomics: on the origins of the mammalian 'neocortex'. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1684):20150060. doi: 10.1098/rstb.2015.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski S, Boerries M, Busch H, et al RARβ regulates neuronal cell death and differentiation in the avian ciliary ganglion. Developmental Neurobiology. 2015;75(11):1204–1218. doi: 10.1002/dneu.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Uesaka M, Irie N How can recapitulation be reconciled with modern concepts of evolution? Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2022;338(1–2):28–35. doi: 10.1002/jez.b.23020. [DOI] [PubMed] [Google Scholar]
- Kverková K, Marhounová L, Polonyiová A, et al The evolution of brain neuron numbers in amniotes. Proceedings of the National Academy of Sciences of the United States of America. 2022;119(11):e2121624119. doi: 10.1073/pnas.2121624119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna F, Hervas-Sotomayor F, Oel AP, et al A lamprey neural cell type atlas illuminates the origins of the vertebrate brain. Nature Ecology & Evolution. 2023;7(10):1714–1728. doi: 10.1038/s41559-023-02170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Cheng MN, Li ZH, et al Spatially resolved gene regulatory and disease-related vulnerability map of the adult Macaque cortex. Nature Communications. 2022;13(1):6747. doi: 10.1038/s41467-022-34413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, et al Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li Z, Sun Y, Ding L, et al Deciphering the distinct transcriptomic and gene regulatory map in adult macaque basal ganglia cells. GigaScience. 2023;12:giad095. doi: 10.1093/gigascience/giad095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Musser JM, Cloutier A, et al Pervasive correlated evolution in gene expression shapes cell and tissue type transcriptomes. Genome Biology and Evolution. 2018;10(2):538–552. doi: 10.1093/gbe/evy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K, Xiang Y, Huang FBQ, et al Spatial and single-nucleus transcriptomics decoding the molecular landscape and cellular organization of avian optic tectum. Iscience. 2024;27(2):109009. doi: 10.1016/j.isci.2024.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang XD, Wang Y Curcumin alleviates Aβ42-induced neuronal metabolic dysfunction via the Thrb/SIRT3 Axis and improves cognition in APPTG mice. Neurochemical Research. 2021;46(12):3166–3178. doi: 10.1007/s11064-021-03414-x. [DOI] [PubMed] [Google Scholar]
- Liu YY, Brent GA Thyroid hormone and the brain: mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacology & Therapeutics. 2018;186:176–185. doi: 10.1016/j.pharmthera.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba S, Straehle J, Gangadharan V, et al Connectomic comparison of mouse and human cortex. Science. 2022;377(6602):eabo0924. doi: 10.1126/science.abo0924. [DOI] [PubMed] [Google Scholar]
- Lust K, Maynard A, Gomes T, et al Single-cell analyses of axolotl telencephalon organization, neurogenesis, and regeneration. Science. 2022;377(6610):eabp9262. doi: 10.1126/science.abp9262. [DOI] [PubMed] [Google Scholar]
- Luzzati F A hypothesis for the evolution of the upper layers of the neocortex through co-option of the olfactory cortex developmental program. Frontiers in Neuroscience. 2015;9:162. doi: 10.3389/fnins.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SJ, Skarica M, Li Q, et al Molecular and cellular evolution of the primate dorsolateral prefrontal cortex. Science. 2022;377(6614):eabo7257. doi: 10.1126/science.abo7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu DN, et al Molecular interactions coordinating the development of the forebrain and face. Developmental Biology. 2005;284(1):48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- McGinnis CS, Murrow LM, Gartner ZJ. 2019. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Systems, 8 (4): 329–337. e4.
- Miller JA, Ding SL, Sunkin SM, et al Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel JF, Aboitiz F Homology in amniote brain evolution: the rise of molecular evidence. Brain Behavior and Evolution. 2018;91(2):59–64. doi: 10.1159/000489116. [DOI] [PubMed] [Google Scholar]
- Musser JM, Schippers KJ, Nickel M, et al Profiling cellular diversity in sponges informs animal cell type and nervous system evolution. Science. 2021;374(6568):717–723. doi: 10.1126/science.abj2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Maitra M, Tanti A, et al Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nature Neuroscience. 2020;23(6):771–781. doi: 10.1038/s41593-020-0621-y. [DOI] [PubMed] [Google Scholar]
- Naumann R, Laurent G. 2020. Function and evolution of the reptilian cerebral cortex. In: Kaas JK. Evolutionary Neuroscience. 2nd ed. London: Academic Press, 213–245.
- Nieder A, Wagener L, Rinnert P A neural correlate of sensory consciousness in a corvid bird. Science. 2020;369(6511):1626–1629. doi: 10.1126/science.abb1447. [DOI] [PubMed] [Google Scholar]
- Nomura T, Murakami Y, Gotoh H, et al Reconstruction of ancestral brains: exploring the evolutionary process of encephalization in amniotes. Neuroscience Research. 2014;86:25–36. doi: 10.1016/j.neures.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Norimoto H, Fenk LA, Li HH, et al A claustrum in reptiles and its role in slow-wave sleep. Nature. 2020;578(7795):413–418. doi: 10.1038/s41586-020-1993-6. [DOI] [PubMed] [Google Scholar]
- olde Heuvel F, Holl S, Chandrasekar A, et al. 2019. STAT6 mediates the effect of ethanol on neuroinflammatory response in TBI. Brain, Behavior, and Immunity, 81 : 228–246.
- Pessoa L, Medina L, Hof PR, et al Neural architecture of the vertebrate brain: implications for the interaction between emotion and cognition. Neuroscience & Biobehavioral Reviews. 2019;107:296–312. doi: 10.1016/j.neubiorev.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L Brain segmentation and forebrain development in amniotes. Brain Research Bulletin. 2001;55(6):695–710. doi: 10.1016/S0361-9230(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, et al Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx‐2, Emx‐1, Nkx‐2.1, Pax‐6, and Tbr‐1. Journal of Comparative Neurology. 2000;424(3):409–438. doi: 10.1002/1096-9861(20000828)424:3<409::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Sandoval JE, Ayad A, et al. 2017. The pallium in reptiles and birds in the light of the updated tetrapartite pallium model. In: Kaas J. Evolution of Nervous Systems. 2nd ed. Oxford: Amsterdam, Boston: Academic Press, 519–555.
- Roth G Convergent evolution of complex brains and high intelligence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1684):20150049. doi: 10.1098/rstb.2015.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, et al Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1684):1011–1020. doi: 10.1098/rspb.2009.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squair JW, Gautier M, Kathe C, et al Confronting false discoveries in single-cell differential expression. Nature Communications. 2021;12(1):5692. doi: 10.1038/s41467-021-25960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M, Caron V, Pearson T, et al Gain‐of‐function mutations in RARB cause intellectual disability with progressive motor impairment. Human Mutation. 2016;37(8):786–793. doi: 10.1002/humu.23004. [DOI] [PubMed] [Google Scholar]
- Su-Feher L, Rubin AN, Silberberg SN, et al Single cell enhancer activity distinguishes GABAergic and cholinergic lineages in embryonic mouse basal ganglia. Proceedings of the National Academy of Sciences of the United States of America. 2022;119(15):e2108760119. doi: 10.1073/pnas.2108760119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson V, Vento-Tormo R, Teichmann SA Exponential scaling of single-cell RNA-seq in the past decade. Nature Protocols. 2018;13(4):599–604. doi: 10.1038/nprot.2017.149. [DOI] [PubMed] [Google Scholar]
- Tan YF, Testa JR DLX genes: roles in development and cancer. Cancers. 2021;13(12):3005. doi: 10.3390/cancers13123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FC, Barbacioru C, Wang YZ, et al mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6(5):377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tasic B, Yao ZZ, Graybuck LT, et al Shared and distinct transcriptomic cell types across neocortical areas. Nature. 2018;563(7729):72–78. doi: 10.1038/s41586-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe M, Yamauchi K, Kimura T, et al Evidence that the laminar fate of LGE/CGE-derived neocortical interneurons is dependent on their progenitor domains. Journal of Neuroscience. 2016;36(6):2044–2056. doi: 10.1523/JNEUROSCI.3550-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosches MA From cell types to an integrated understanding of brain evolution: the case of the cerebral cortex. Annual Review of Cell and Developmental Biology. 2021;37:495–517. doi: 10.1146/annurev-cellbio-120319-112654. [DOI] [PubMed] [Google Scholar]
- Tosches MA, Laurent G Evolution of neuronal identity in the cerebral cortex. Current Opinion in Neurobiology. 2019;56:199–208. doi: 10.1016/j.conb.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Tosches MA, Yamawaki TM, Naumann RK, et al Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360(6391):881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- Wagner GP. 2014. Homology, Genes, and Evolutionary Innovation. Princeton, Oxford: Princeton University Press.
- Wang P, Zhao DJ, Rockowitz S, et al Divergence and rewiring of regulatory networks for neural development between human and other species. Neurogenesis. 2016;3(1):e1231495. doi: 10.1080/23262133.2016.1231495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XY, Fu SL, Li HB, et al Single-cell Stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science. 2022;377(6610):eabp9444. doi: 10.1126/science.abp9444. [DOI] [PubMed] [Google Scholar]
- Wolf FA, Angerer P, Theis FJ SCANPY: large-scale single-cell gene expression data analysis. Genome Biology. 2018;19(1):15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woych J, Ortega Gurrola A, Deryckere A, et al Cell-type profiling in salamanders identifies innovations in vertebrate forebrain evolution. Science. 2022;377(6610):eabp9186. doi: 10.1126/science.abp9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita W, Nomura T. 2017. The neocortex and dorsal ventricular ridge: functional convergence and underlying developmental mechanisms. In: Shigeno S, Murakami Y, Nomura T. Brain Evolution by Design: from Neural Origin to Cognitive Architecture. Tokyo: Springer, 291–309.
- Yao ZZ, van Velthoven CTJ, Nguyen TN, et al. 2021. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell, 184 (12): 3222–3241. e26.
- Yokoyama KD, Pollock DD SP transcription factor paralogs and DNA-binding sites coevolve and adaptively converge in mammals and birds. Genome Biology and Evolution. 2012;4(11):1102–1117. doi: 10.1093/gbe/evs085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GC, Smith DK, Zhu HC, et al GGTREE: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution. 2017;8(1):28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- Yu GC, Wang LG, Han YY, et al clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A Journal of Integrative Biology. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker M, Bunt J, Schanze I, et al Variants in nuclear factor I genes influence growth and development. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2019;181(4):611–626. doi: 10.1002/ajmg.c.31747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The raw sequencing data generated in this study were deposited in the CNGB Nucleotide Sequence Archive (CNSA: https://db.cngb.org/cnsa) under accession code CNP0003026. Sample numbers include MK-002-1L-10, MK-002-2L-44, MK-002-2L-45, MK-003-1L-10, MK-003-1L-9, MK-003-2L-44, and MK-003-2L-45. The macaque expression matrix is available under accession code CSE0000427. The code can be found at: https://github.com/FupaulHuang/AmniotePalliumTranscriptome. Datasets generated in this study were also deposited in the Genome Sequence Archive (GSA) database (https://ngdc.cncb.ac.cn/gsa/) (accession number PRJCA031422), Science Data Bank (doi: 10.57760/sciencedb.17214), and NCBI database (BioProjectID PRJNA1175883).