Abstract
1. The possibility that the discharge pattern of monkey corticomotoneuronal cells influences the degree to which they facilitate their target hand muscles was tested by compiling spike-triggered averages of EMG recorded from these muscles. 2. Records were made from area 4 corticomotoneuronal cells in three conscious macaque monkeys while they performed a precision grip between index finger and thumb. Simultaneous EMG recordings were made from up to six different intrinsic hand muscles. Twenty cells which produced clear post-spike facilitation of one or more muscles were selected for further analysis. 3. Spikes recorded from these cells were grouped according to the occurrence of a previous spike in the periods 0-10 ms, 10-20 ms, and so on up to 60-70 ms before the trigger spike. The post-spike period in which no additional spikes were allowed to fall was kept at either 12.5 or 25 ms. 4. Spikes selected in this way produced a transient facilitation of their target muscle EMG activity. The peak amplitude of this facilitation was normalized as a percentage of modulation of the background EMG level. The background level was determined from a period in the average to which the cell could not have contributed, because of the post-trigger spike interval. We verified that the percentage of modulation was not influenced by the overall level of EMG activity, since, for a given interval, the modulation was the same whether the relevant spikes were selected during periods of high- or low-level EMG activity. 5. The relative amplitude of the post-spike facilitation (i.e. the percentage of modulation) showed marked variation with interspike interval. A full analysis was completed for seventeen neurones. Spikes with the shortest intervals (less than 10 ms) usually produced the strongest effects, and evidence is presented that this was due to temporal summation and facilitation at the corticomotoneuronal synapse. Mid-range intervals (10-40 ms) were generally far less effective, although they constituted the highest proportion of cell activity. 6. A striking finding was the strong facilitation generated by the longer interspike intervals (40-70 ms). Although the absolute size of this post-spike effect was much smaller than that of the shortest intervals, its percentage of modulation was similar. It is suggested that this enhanced facilitation results from a combination of lower frequency discharge among the active motoneurones, and increased synchrony in the corticomotoneuronal input to them. 7. All of the above results were confirmed by examining cross-correlations between single corticomotoneuronal cells and single motor units in their target muscle.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





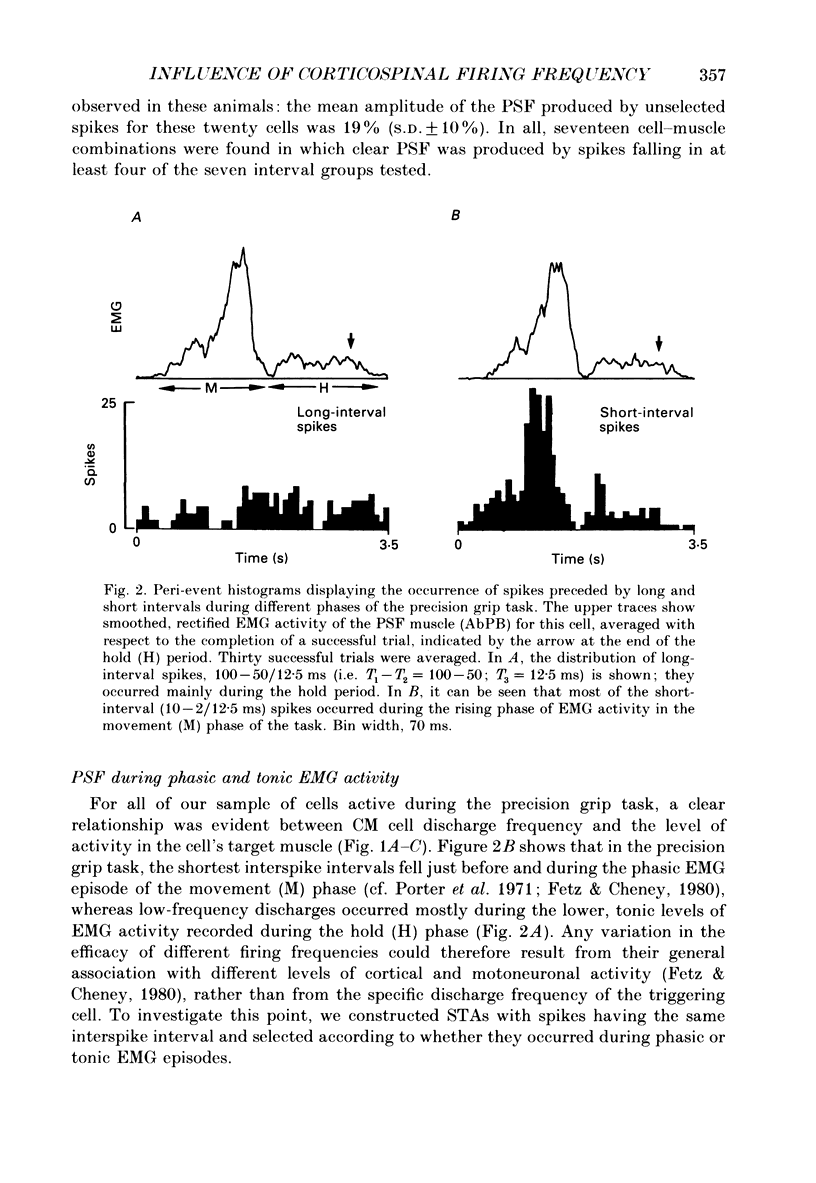




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby P., Zilm D. Relationship between EPSP shape and cross-correlation profile explored by computer simulation for studies on human motoneurons. Exp Brain Res. 1982;47(1):33–40. doi: 10.1007/BF00235883. [DOI] [PubMed] [Google Scholar]
- Buys E. J., Lemon R. N., Mantel G. W., Muir R. B. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986 Dec;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980 Oct;44(4):773–791. doi: 10.1152/jn.1980.44.4.773. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E., Palmer S. S. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. J Neurophysiol. 1985 Mar;53(3):805–820. doi: 10.1152/jn.1985.53.3.805. [DOI] [PubMed] [Google Scholar]
- Cope T. C., Fetz E. E., Matsumura M. Cross-correlation assessment of synaptic strength of single Ia fibre connections with triceps surae motoneurones in cats. J Physiol. 1987 Sep;390:161–188. doi: 10.1113/jphysiol.1987.sp016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murthy K. S. The origins and characteristics of cross-correlated activity between gamma-motoneurones in the cat. Q J Exp Physiol. 1985 Apr;70(2):219–232. doi: 10.1113/expphysiol.1985.sp002905. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Fromm C., Kröller J., Jennings V. A. Motor Cortex control of finely graded forces. J Neurophysiol. 1983 May;49(5):1199–1215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968 Jan;31(1):14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980 Oct;44(4):751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P., Kalaska J. F., Caminiti R., Massey J. T. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982 Nov;2(11):1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey D. R. Relating motor cortex spike trains to measures of motor performance. Brain Res. 1972 May 12;40(1):7–18. doi: 10.1016/0006-8993(72)90099-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976 Jun;258(2):467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser R. J., Cheney P. D. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol. 1985 Apr;53(4):959–978. doi: 10.1152/jn.1985.53.4.959. [DOI] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y., Bioulac B., Jacks B. Activity of pre-entral neurones in conscious monkeys: effects of deafferentation and cerebellar ablation. J Physiol (Paris) 1978;74(3):253–264. [PubMed] [Google Scholar]
- Lemon R. N., Mantel G. W., Muir R. B. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol. 1986 Dec;381:497–527. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N., Muir R. B., Mantel G. W. The effects upon the activity of hand and forearm muscles of intracortical stimulation in the vicinity of corticomotor neurones in the conscious monkey. Exp Brain Res. 1987;66(3):621–637. doi: 10.1007/BF00270695. [DOI] [PubMed] [Google Scholar]
- Mantel G. W., Lemon R. N. Cross-correlation reveals facilitation of single motor units in thenar muscles by single corticospinal neurones in the conscious monkey. Neurosci Lett. 1987 Jun 1;77(1):113–118. doi: 10.1016/0304-3940(87)90617-3. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986 May;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir R. B., Porter R. The effect of a preceding stimulus on temporal facilitation at corticomotoneuronal synapses. J Physiol. 1973 Feb;228(3):749–763. doi: 10.1113/jphysiol.1973.sp010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S. S., Fetz E. E. Effects of single intracortical microstimuli in motor cortex on activity of identified forearm motor units in behaving monkeys. J Neurophysiol. 1985 Nov;54(5):1194–1212. doi: 10.1152/jn.1985.54.5.1194. [DOI] [PubMed] [Google Scholar]
- Porter R. Early facilitation at corticomotoneuronal synapses. J Physiol. 1970 May;207(3):733–745. doi: 10.1113/jphysiol.1970.sp009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R., Lewis M. M., Horne M. Analysis of patterns of natural activity of neurones in the precentral gyrus of conscious monkeys. Brain Res. 1971 Nov;34(1):99–113. doi: 10.1016/0006-8993(71)90353-2. [DOI] [PubMed] [Google Scholar]
- Porter R., Muir R. B. The meaning for motoneurones of the temporal pattern of natural activity in pyramidal tract neurones of conscious monkeys. Brain Res. 1971 Nov;34(1):127–142. doi: 10.1016/0006-8993(71)90355-6. [DOI] [PubMed] [Google Scholar]
- Porter R. Relationship of the discharges of cortical neurones to movement in free-to-move monkeys. Brain Res. 1972 May 12;40(1):39–43. doi: 10.1016/0006-8993(72)90103-5. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I. Neuronal organization and synaptic mechanisms of supraspinal motor control in vertebrates. Rev Physiol Biochem Pharmacol. 1975;72:1–54. doi: 10.1007/BFb0031545. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Yokota J., Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981 Apr 9;23(1):7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Smith A. M., Hepp-Reymond M. C., Wyss U. R. Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp Brain Res. 1975 Sep 29;23(3):315–332. doi: 10.1007/BF00239743. [DOI] [PubMed] [Google Scholar]
- Smith W. S., Fetz E. E. Effects of synchrony between primate corticomotoneuronal cells on post-spike facilitation of muscles and motor units. Neurosci Lett. 1989 Jan 2;96(1):76–81. doi: 10.1016/0304-3940(89)90246-2. [DOI] [PubMed] [Google Scholar]


