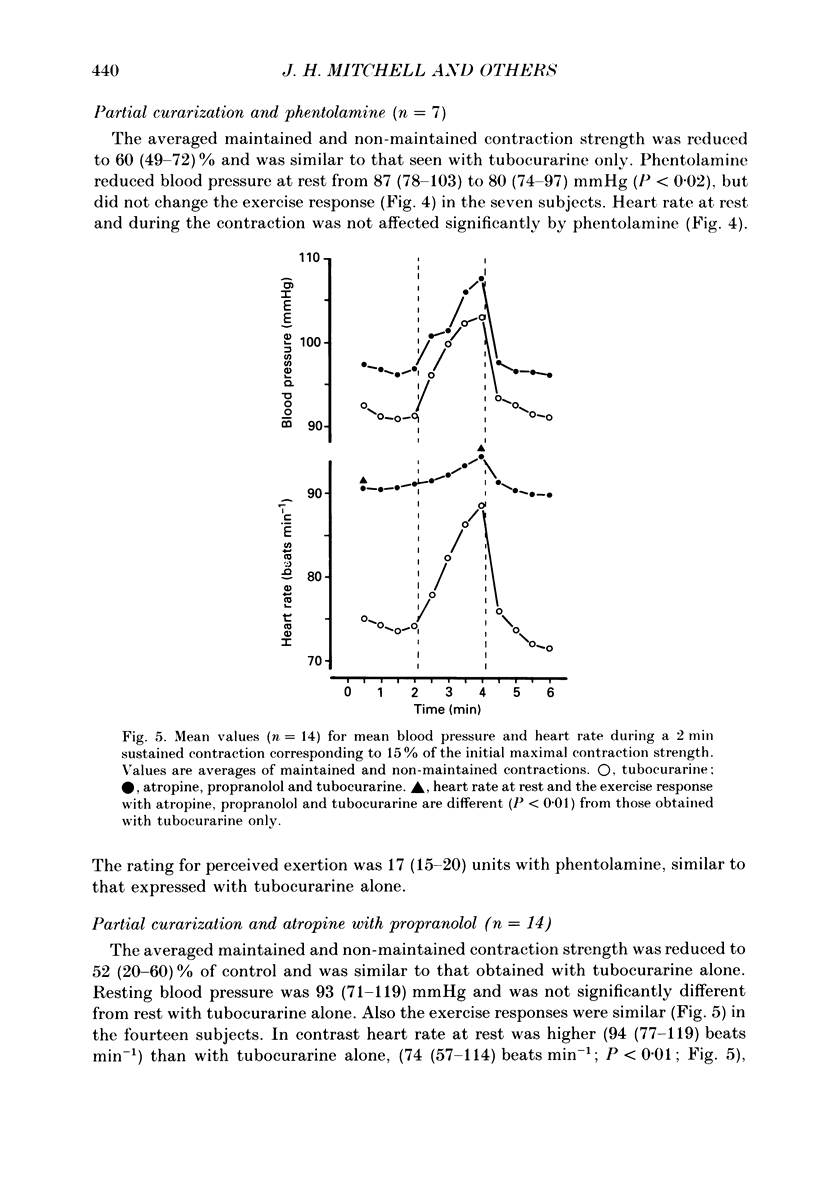
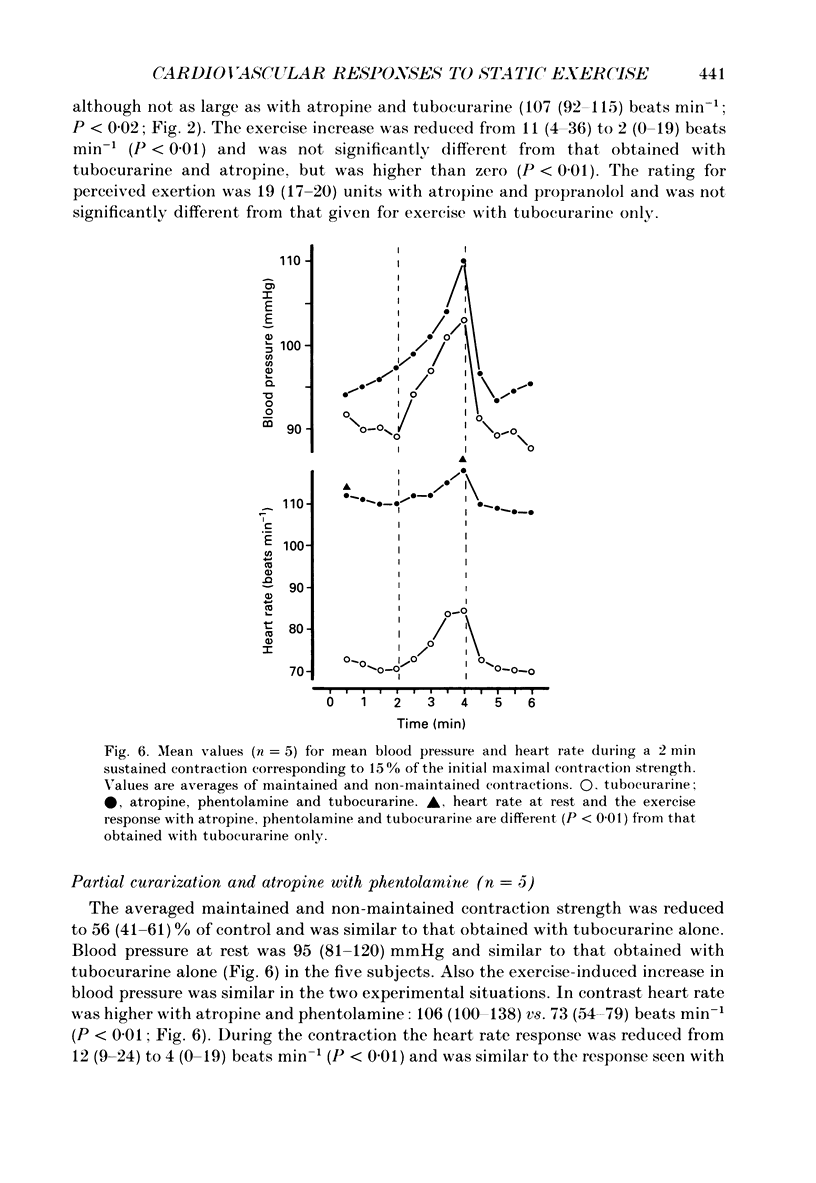
Abstract
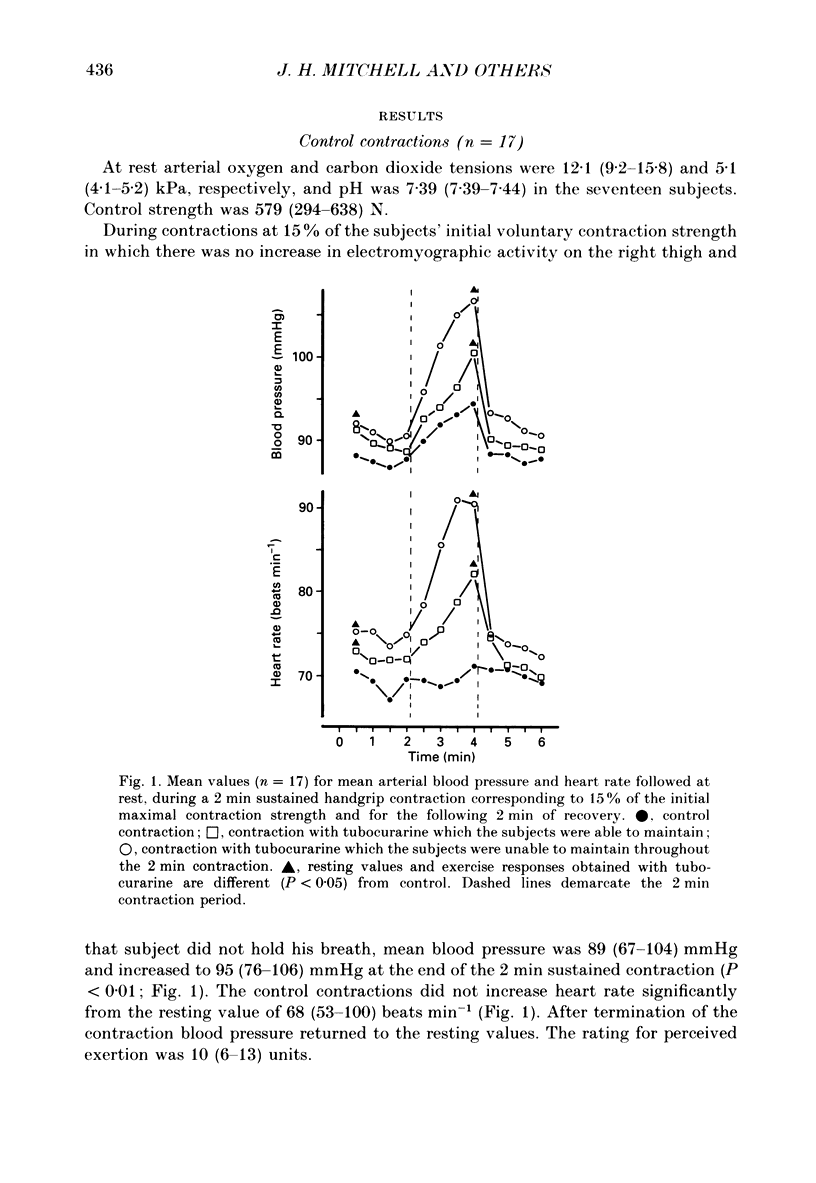
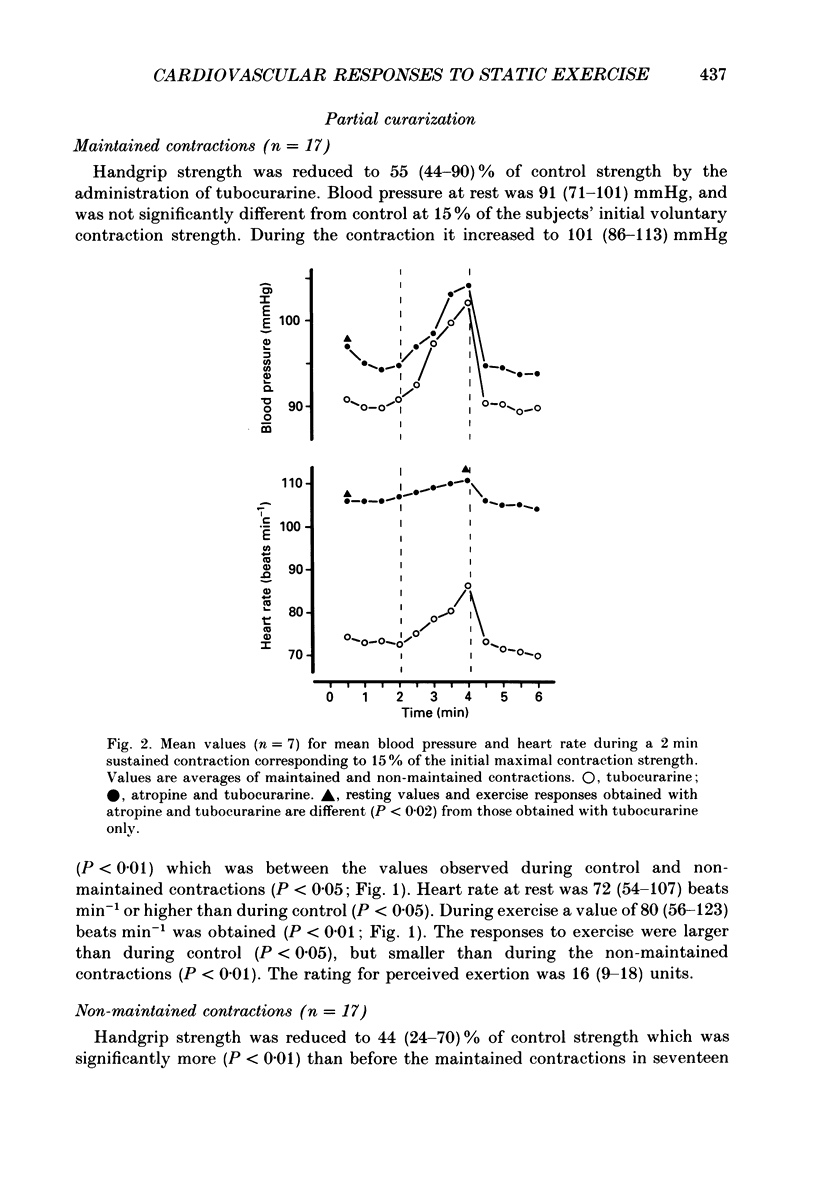
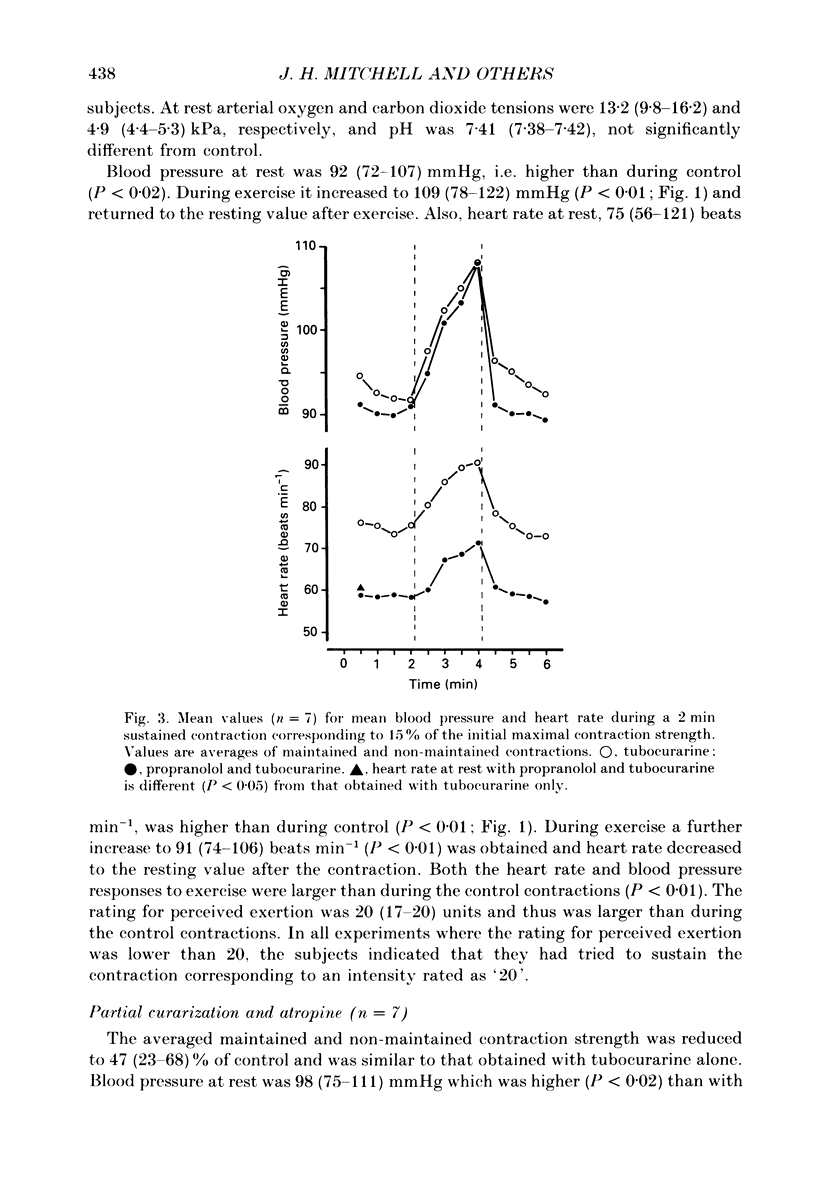
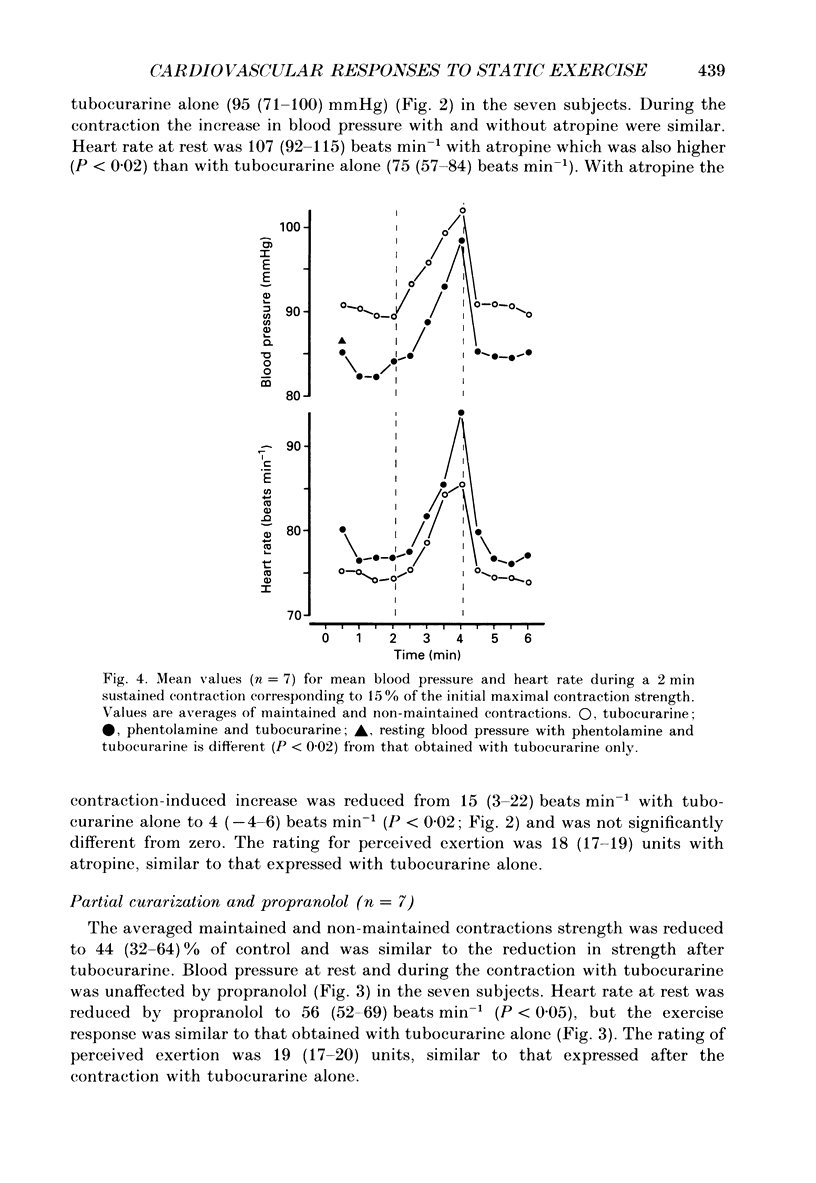
1. The cardiovascular responses, heart rate and mean arterial pressure, were followed in seventeen human subjects who performed static handgrip contractions for 2 min at the same absolute force (15% of the initial maximal voluntary contraction strength) before and during partial curarization. In control contractions the rate of perceived exertion was 10 exertion units, 16 units in contractions with tubocurarine which could be maintained and 20 units in contractions that could not be maintained. Control contractions increased mean arterial pressure by 6 mmHg from 89 mmHg while heart rate was unchanged from the resting value of 68 beats min-1. With tubocurarine, larger increases in mean arterial pressure of 11 mmHg and for heart rate of 8 beats min-1 were obtained during maintained contractions, and 15 mmHg and 16 beats min-1, respectively, during non-maintained contractions. 2. Atropine increased resting heart rate and blood pressure with tubocurarine to 107 beats min-1 and 98 mmHg, respectively, in seven subjects. The blood pressure response to exercise with tubocurarine was unaffected by atropine, but the heart rate increase was reduced from 15 to 4 beats min-1. 3. Propranolol reduced resting heart rate with tubocurarine to 56 beats min-1 with no effect on blood pressure in seven subjects. The cardiovascular responses to exercise with tubocurarine were unaffected by propranolol. In contrast, phentolamine reduced resting blood pressure with tubocurarine to 80 mmHg without affecting heart rate in seven subjects. Exercise responses with tubocurarine were unaffected by phentolamine. Combinations of atropine and propranolol in fourteen subjects or atropine and phentolamine in five subjects showed similar results during exercise with tubocurarine as with the sole use of the agents used to block autonomic receptors. 4. The results suggest that when partial curarization induces a disproportion between the signal from central command and that from exercising muscles, the larger signal arising from central command determines the magnitude of the cardiovascular responses. The centrally generated heart rate response is in part caused by vagal withdrawal. However, the blood pressure response cannot be attenuated by the sole use of alpha- or beta-receptor adrenergic blockade or combinations of these with atropine. This suggests that there may be greater redundancy in the autonomic control of blood pressure than in the vagal control of heart rate associated with central command during static exercise in man.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- Burke R. E. Motor unit properties and selective involvement in movement. Exerc Sport Sci Rev. 1975;3:31–81. [PubMed] [Google Scholar]
- Fallentin N., Sidenius B., Jørgensen K. Blood pressure, heart rate and EMG in low level static contractions. Acta Physiol Scand. 1985 Oct;125(2):265–275. doi: 10.1111/j.1748-1716.1985.tb07715.x. [DOI] [PubMed] [Google Scholar]
- Galbo H., Kjaer M., Secher N. H. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987 Aug;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander A. P., Bouman L. N. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975 Feb;38(2):272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Iwamoto G. A., Botterman B. R. Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol (1985) 1985 May;58(5):1676–1682. doi: 10.1152/jappl.1985.58.5.1676. [DOI] [PubMed] [Google Scholar]
- Juhlin-Dannfelt A., Frisk-Holmberg M., Karlsson J., Tesch P. Central and peripheral circulation in relation to muscle-fibre composition in normo- and hyper-tensive man. Clin Sci (Lond) 1979 Apr;56(4):335–340. doi: 10.1042/cs0560335. [DOI] [PubMed] [Google Scholar]
- Leonard B., Mitchell J. H., Mizuno M., Rube N., Saltin B., Secher N. H. Partial neuromuscular blockade and cardiovascular responses to static exercise in man. J Physiol. 1985 Feb;359:365–379. doi: 10.1113/jphysiol.1985.sp015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. E., Shaver J. A., Leon D. F., Thompson M. E., Reddy P. S., Leonard J. J. Autonomic mechanisms in hemodynamic responses to isometric exercise. J Clin Invest. 1974 Jul;54(1):104–115. doi: 10.1172/JCI107731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. G., Jr Effect of adrenergic receptor blockade on the responses to isometric handgrip: studies in normal and hypertensive subjects. J Cardiovasc Pharmacol. 1979 Mar-Apr;1(2):253–263. doi: 10.1097/00005344-197903000-00008. [DOI] [PubMed] [Google Scholar]
- McCloskey D. I. Centrally-generated commands and cardiovascular control in man. Clin Exp Hypertens. 1981;3(3):369–378. doi: 10.3109/10641968109033671. [DOI] [PubMed] [Google Scholar]
- Mitchell J. H., Kaufman M. P., Iwamoto G. A. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell J. H., Payne F. C., Saltin B., Schibye B. The role of muscle mass in the cardiovascular response to static contractions. J Physiol. 1980 Dec;309:45–54. doi: 10.1113/jphysiol.1980.sp013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. J. The action of d-tubocurarine and of decamethonium on respiratory and other muscles in the cat. J Physiol. 1951 Feb;112(3-4):311–331. doi: 10.1113/jphysiol.1951.sp004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. R., Washburn R. A., Hanson P. G., Painter P. L., Nagle F. J. Increased cardiovascular response to static contraction of larger muscle groups. J Appl Physiol Respir Environ Exerc Physiol. 1983 Feb;54(2):434–437. doi: 10.1152/jappl.1983.54.2.434. [DOI] [PubMed] [Google Scholar]
- Secher N. H. Heart rate at the onset of static exercise in man with partial neuromuscular blockade. J Physiol. 1985 Nov;368:481–490. doi: 10.1113/jphysiol.1985.sp015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher N. H., Rube N., Secher O. Effect of tubocurarine on human soleus and gastrocnemius muscles. Acta Anaesthesiol Scand. 1982 Jun;26(3):231–234. doi: 10.1111/j.1399-6576.1982.tb01760.x. [DOI] [PubMed] [Google Scholar]
- de Troyer A., Bastenier J., Delhez L. Function of respiratory muscles during partial curarization in humans. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1049–1056. doi: 10.1152/jappl.1980.49.6.1049. [DOI] [PubMed] [Google Scholar]


