Abstract
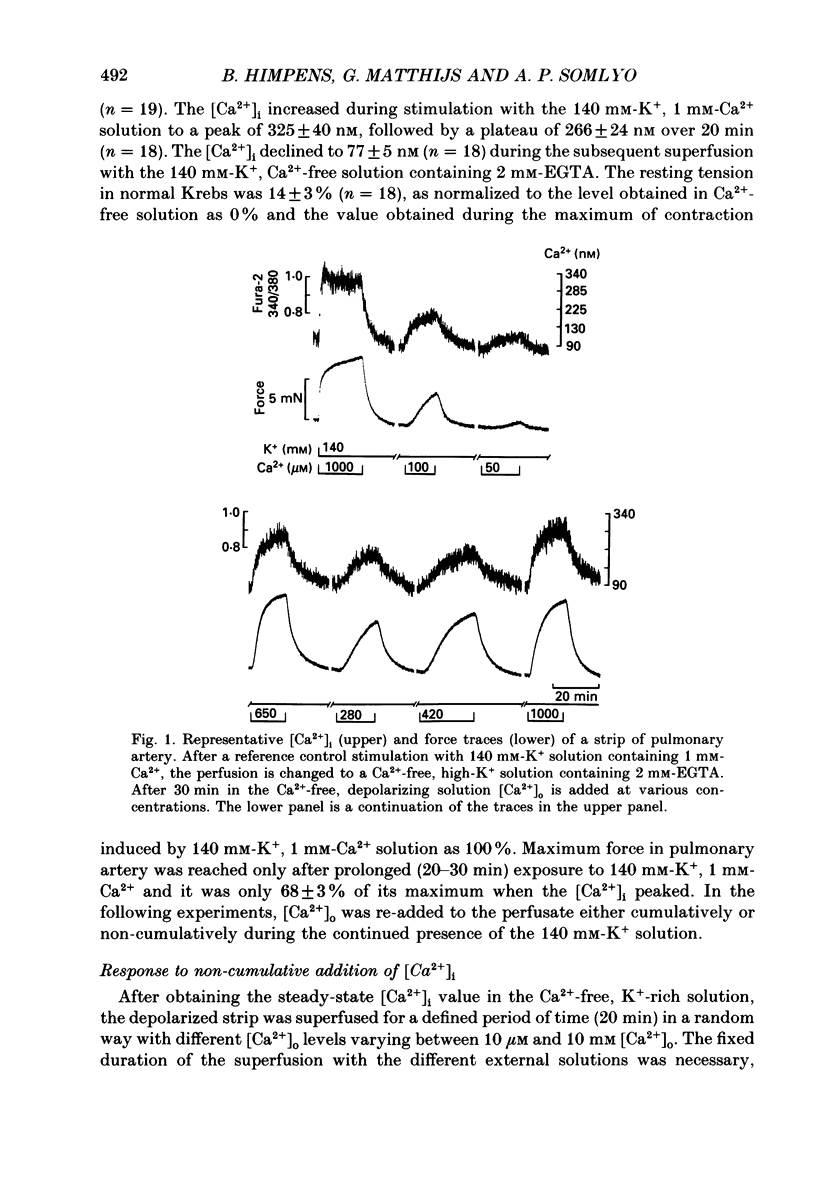
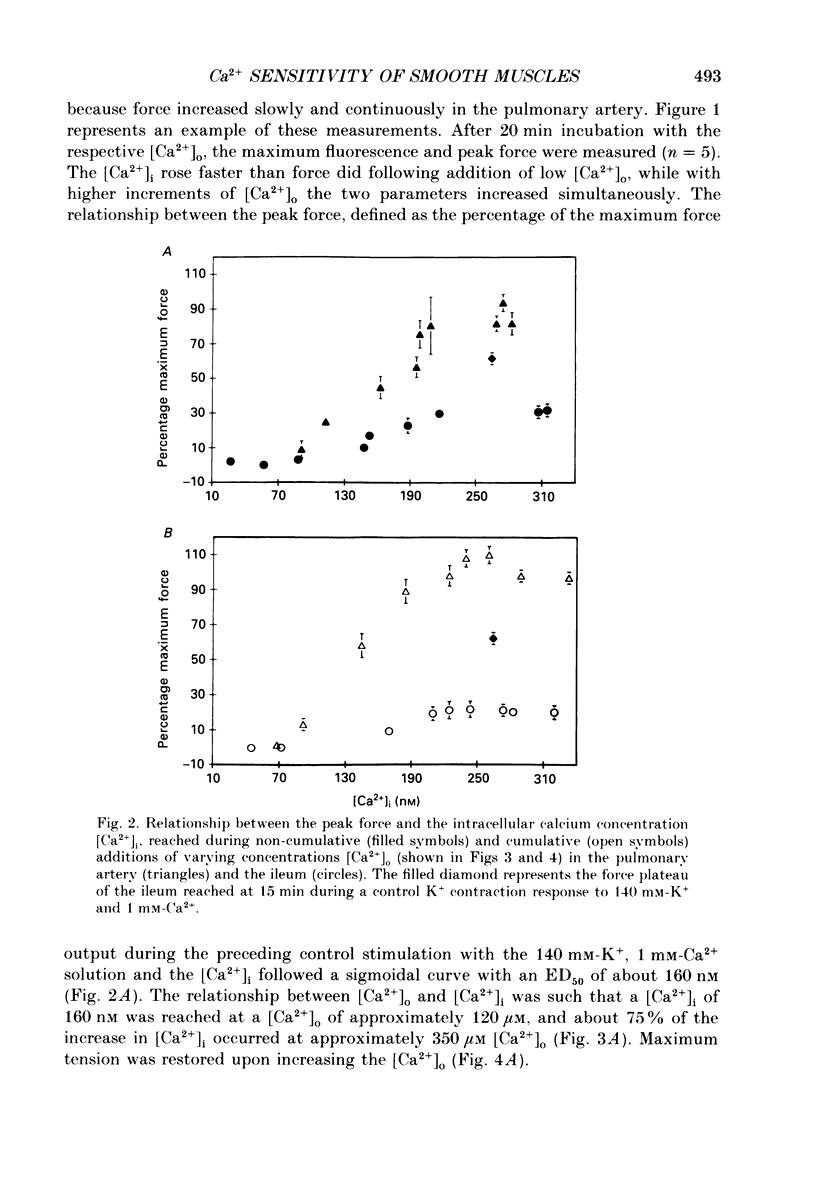
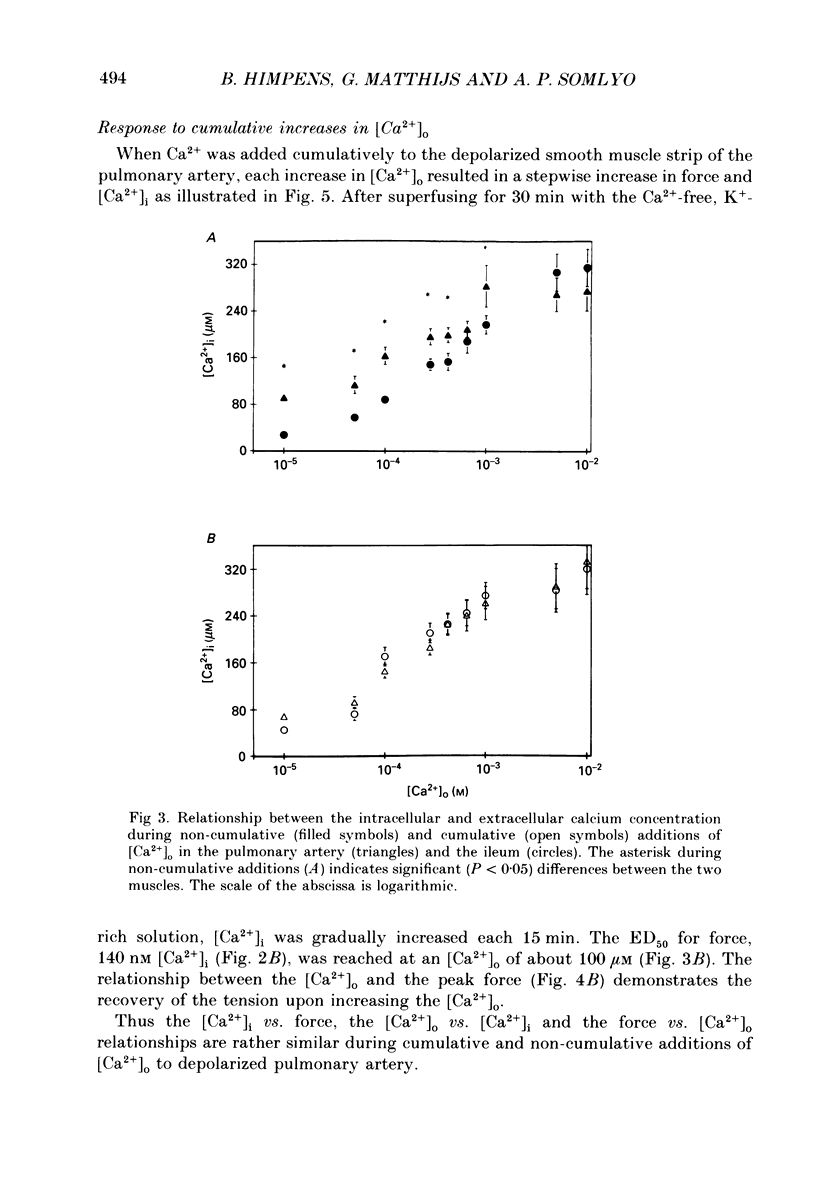
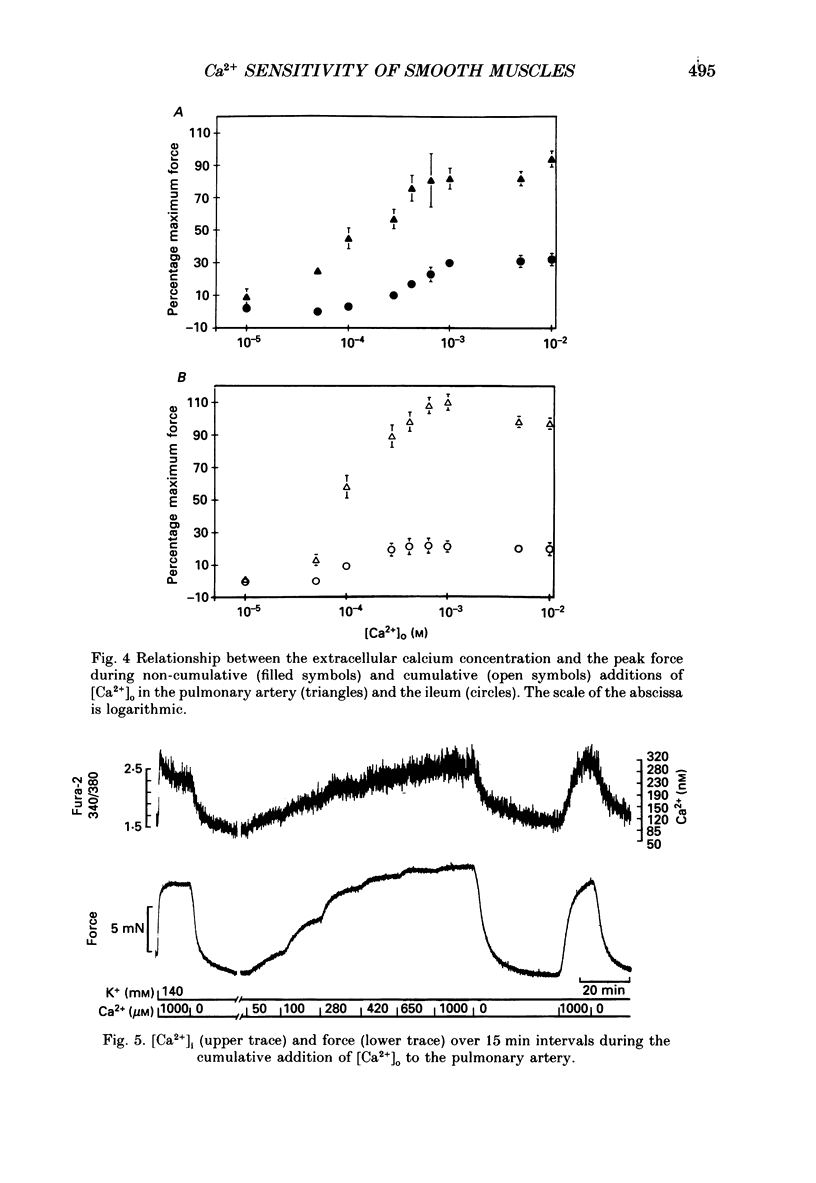
1. The free cytoplasmic Ca2+ concentration [( Ca2+]i) was measured in the tonic rabbit pulmonary artery and the phasic ileum smooth muscle. 2. Force development and [Ca2+]i were determined during either cumulative or non-cumulative additions of [Ca2+]o to smooth muscles depolarized with 140 mM-K+ solutions. 3. The level to which [Ca2+]i declined in Ca2+-free, 140 mM-K+ solutions was significantly lower in the ileum (40 +/- 4 nM) than in pulmonary artery (77 +/- 5 nM) smooth muscle. 4. The level of [Ca2+]i reached during non-cumulative superfusion with 10 microM and 1 mM [Ca2+]o was higher in the pulmonary artery than in the ileum. 5. The force level reached for a given [Ca2+]i was also higher in the pulmonary artery than in the ileum. 6. During maintained depolarization there was a marked decrease in the sensitivity of ileum smooth muscle tension to [Ca2+]i. 7. We conclude that significant differences exist in the Ca2+ sensitivity of the regulatory/contractile apparatus among different smooth muscles; the lower sensitivity of depolarized ileum than pulmonary artery to [Ca2+]o is due to both differences in Ca2+ metabolism and in the Ca2+ sensitivity of the regulatory contractile system. We suggest that these two mechanisms also contribute to the decline in force during a phasic K+ contracture, and that desensitization to [Ca2+]i contributes to the decline of the K+ contracture in the ileum.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
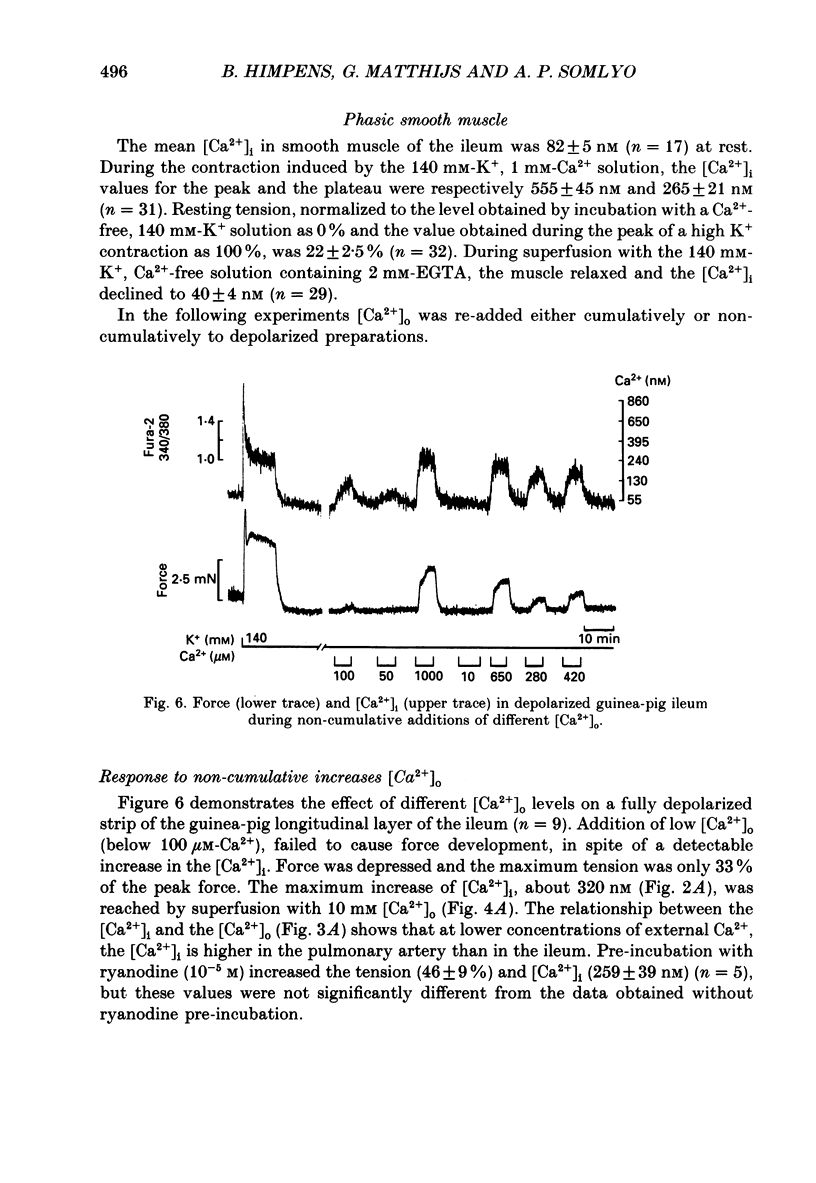
- Aickin C. C. Intracellular pH regulation by vertebrate muscle. Annu Rev Physiol. 1986;48:349–361. doi: 10.1146/annurev.ph.48.030186.002025. [DOI] [PubMed] [Google Scholar]
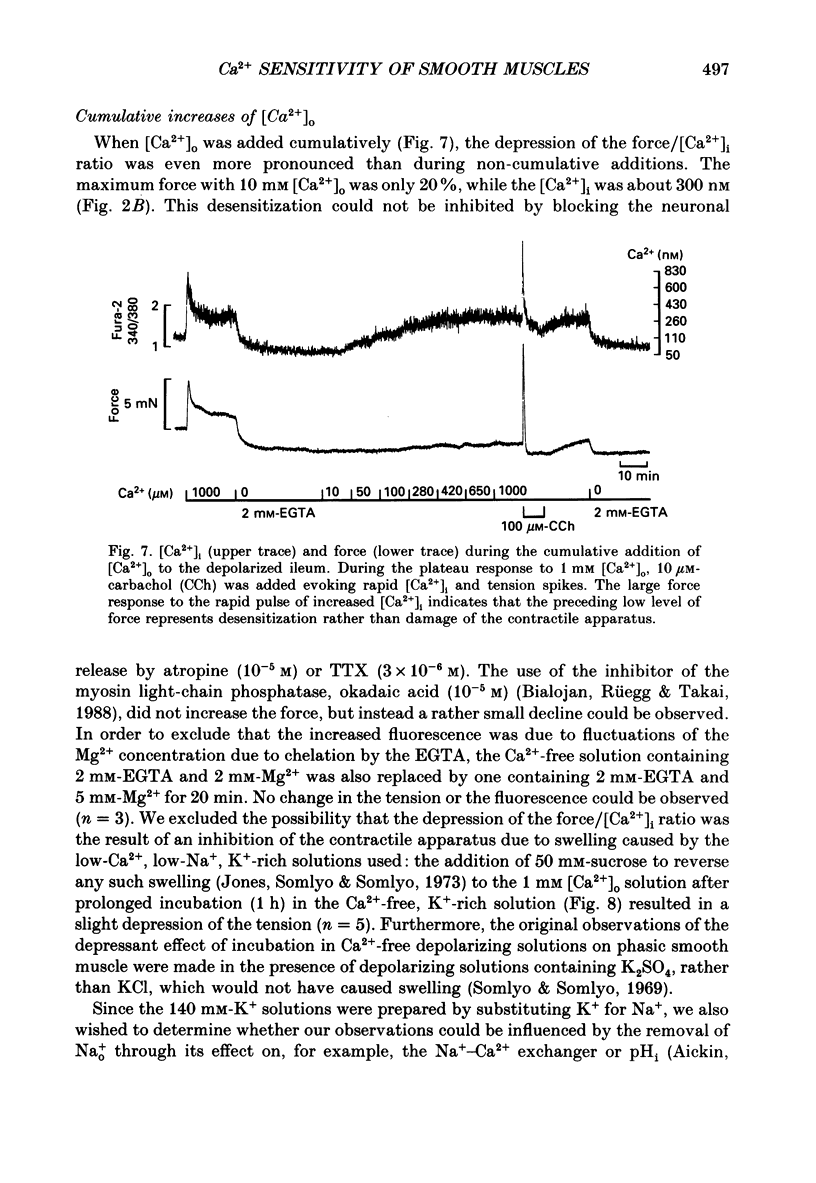
- Bialojan C., Rüegg J. C., Takai A. Effects of okadaic acid on isometric tension and myosin phosphorylation of chemically skinned guinea-pig taenia coli. J Physiol. 1988 Apr;398:81–95. doi: 10.1113/jphysiol.1988.sp017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
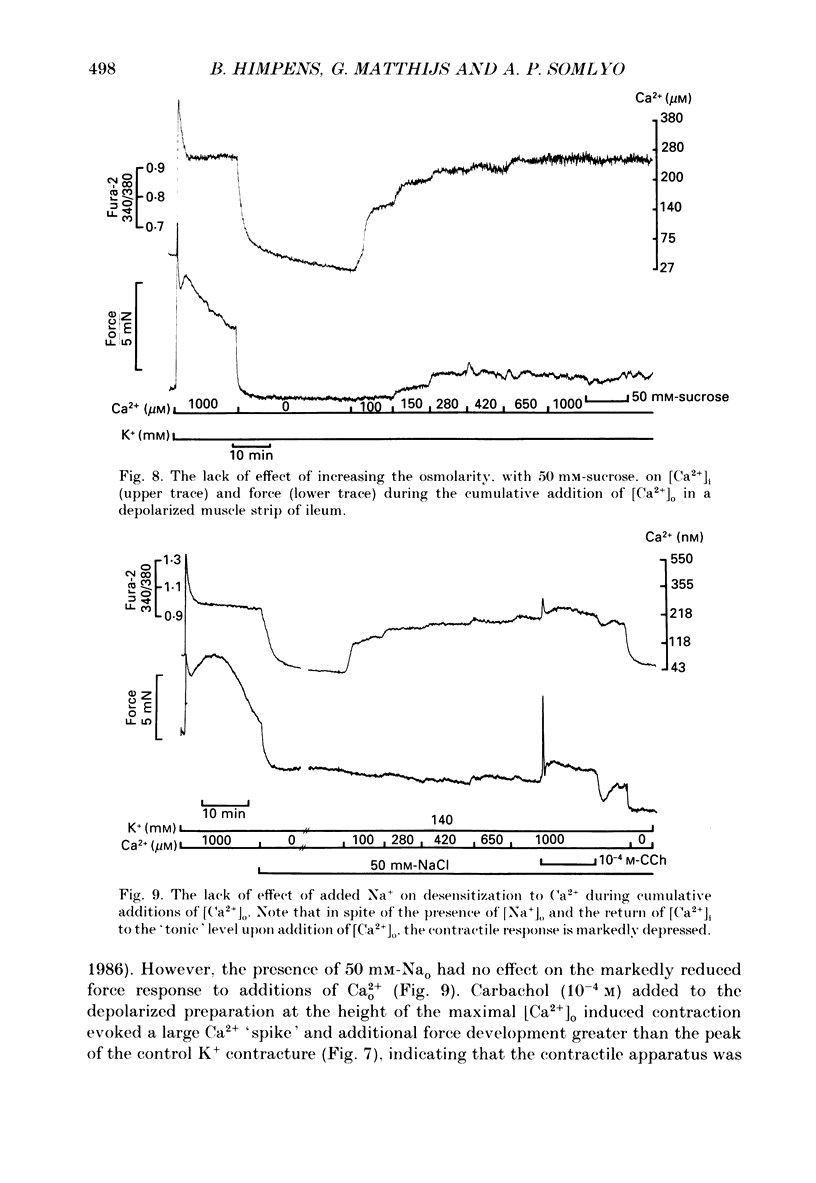
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
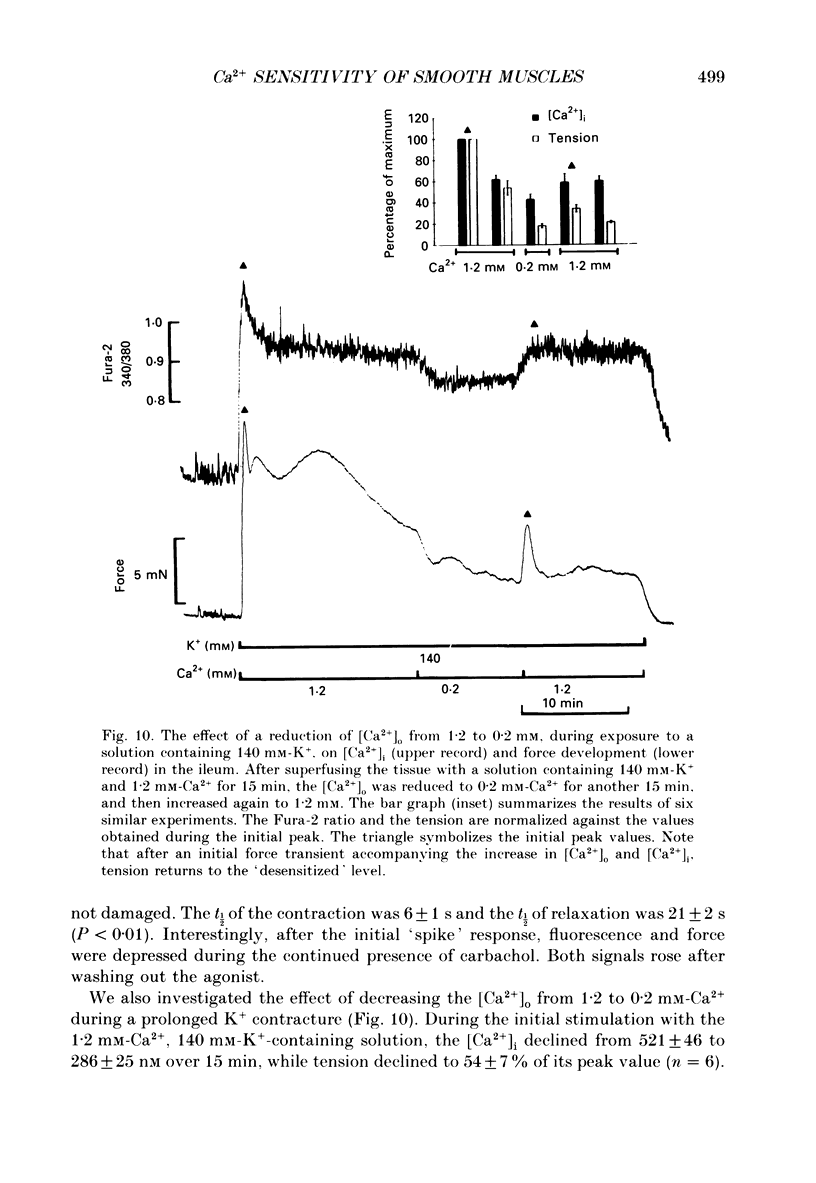
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. V., Butler T. M., Somlyo A. P. Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. J Gen Physiol. 1988 Dec;92(6):713–729. doi: 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W., Somlyo A. P., Somlyo A. V. Potassium accumulation in smooth muscle and associated ultrastructural changes. J Physiol. 1973 Jul;232(2):247–273. doi: 10.1113/jphysiol.1973.sp010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzer G., Rüegg J. C., Zimmer M., Hofmann F. Relaxation of skinned coronary arteries depends on the relative concentrations of Ca2+, calmodulin and active cAMP-dependent protein kinase. Pflugers Arch. 1985 Sep;405(1):70–76. doi: 10.1007/BF00591100. [DOI] [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Kuriyama H. Dependence on Ca2+ of the activities of phosphatidylinositol 4,5-bisphosphate phosphodiesterase and inositol 1,4,5-trisphosphate phosphatase in smooth muscles of the porcine coronary artery. Biochem J. 1985 Nov 1;231(3):497–503. doi: 10.1042/bj2310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Pharmacology of excitation-contraction coupling in vascular smooth muscle and in avian slow muscle. Fed Proc. 1969 Sep-Oct;28(5):1634–1642. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]



