Abstract
Pancreatic cancer (PCa) is one of the malignant tumors with an extremely poor prognosis. Rare biomarkers exist for predicting the outcomes of PCa patients. This study aimed to develop a nomogram model based on serum microRNA-24 (miR-24) and clinicopathological factors to predict overall survival (OS) and treatment response to conventional adjuvant chemotherapy (ACT) in patients with PCa. This retrospective study included 296 patients with PCa who underwent radical resection and were followed up every three months. The serum levels of miR-24 were analyzed with real- time polymerase chain reaction, and the clinicopathological information relevant to the patients was extracted from the medical center. By combining miR-24 with some clinicopathological factors associated with prognosis, a nomogram model was developed to predict the OS of patients with PCa. Patients with elevated miR-24 levels exhibited significantly poorer OS compared to those at low risk (P < 0.0001). miR-24 was an independent predictor of OS regardless to the patients’ age, gender, and clinical pathological characteristics. It demonstrated remarkable predictive power, with an AUC of 0.82, surpassing CA19-9 (AUC: 0.61), CA125 (AUC: 0.59), CA50 (AUC: 0.51) and CEA (0.56). When miR-24 was integrated with TNM stage, CA19-9 and CA125 in a nomogram, the prognostic accuracy was notably enhanced compared to individual factors. Furthermore, patients classified into the high-risk group who received post-operative ACT showed superior outcomes in both OS and two-year survival compared to those who did not receive ACT (P < 0.0001). A serum miR-24-based nomogram may serve as a powerful tool for predicting risk and prognosis in patients with resected pancreatic cancer, thus facilitating personalized clinical decision-making.
Keywords: Pancreatic cancer, Prognosis, MicroRNA, Serum, Overall survival
Subject terms: Prognostic markers, Cancer models, Gastrointestinal cancer
Introduction
Pancreatic cancer (PCa) is a highly malignant tumor characterized by a dismal prognosis. The pancreatic ductal adenocarcinoma (PDAC) subtype constitutes more than 90% of all pancreatic malignancies. Due to a lack of early diagnosis and effective therapeutic intervention, PCa ranks as the fourth leading cause of cancer-related mortality, accompanied by a strikingly low 5-year relative survival rate of just 12%1. While surgical resection remains the sole treatment with curative potential, approximately 80%-85% of PCa cases are deemed unresectable, as patients are often diagnosed with local infiltration or metastasis2,3. The conventional adjuvant chemotherapy (ACT), the primary treatment for PCa, faces significant challenges due to drug resistance. Even immunotherapy, which has shown promise in various cancer types, has yet to succeed in PCa, largely attributing to its immunosuppressive tumor microenvironment4. Alarmingly, individuals may obtain different benefits from a same treatment regimen, influenced by their unique genetic background and biological characteristics5. Therefore, identifying effective prognostic markers to predict patients’ response to treatment is of utmost importance.
Some molecular alterations were reported to be promising biomarkers. Traditionally, serum levels of carbohydrate antigens (CA)19–9, CA125, CA50, and carcinoembryonic antigen (CEA) are commonly used for diagnosis, metastasis assessment and prognosis in PCa with low efficacy6–8. CA19-9 is the only Food and Drug Administration- approved biomarker for diagnosis and prognosis of PCa9. However, baseline CA19-9 level was not an independent predictor of survival in a randomized phase 3 trial10. Furthermore, several studies reported the correlation between systemic inflammatory markers—specifically, neutrophil–lymphocyte ratio (NLR), platelet- lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), and neutrophil- monocyte ratio (NMR)— and the overall survival (OS) of patients with PDAC11–14. Nonetheless, a consensus regarding their prognostic value remains elusive.
Recent studies have concentrated on microRNAs (miRNAs) as predictive and prognostic biomarkers, or potential therapeutic targets, given their pivotal role in the pathogenic mechanisms underlying carcinogenesis and metastasis15,16. MiRNAs are a group of non-coding, single-stranded RNAs with around 22 nucleotides, which are considered as promising biomarkers with high stability in human fluids, ease of non-invasive detection in circulation, and a convenient screening method17. For example, miR-21, miR-155, miR-203, miR-210, miR-222, and miR-629 were identified to be potential biomarkers for prognosis in PCa18–20. Regrettably, most of these signatures were hampered by constrained performance, lacking the efficacy to predict the response to ACT.
In our preceding research, we developed a panel of four serum miRNAs as a novel, noninvasive biomarker for the early detection of pancreatic ductal adenocarcinoma (PDAC)21. As a member of this panel, miRNA-24 was speculated by a meta-analysis as a promising prognostic marker in different cancers22. However, the prognostic performance of miR-24 in PCa requires further validation. Consequently, the purpose of this study was to investigate whether serum levels of miR-24 are associated with overall survival (OS) and the response of patients with PDAC to ACT. Building upon this foundation, we endeavored to establish a nomogram model to predict the OS of patients undergoing surgical treatment.
Results
Prognostic value of miR-24 in patients with PDAC
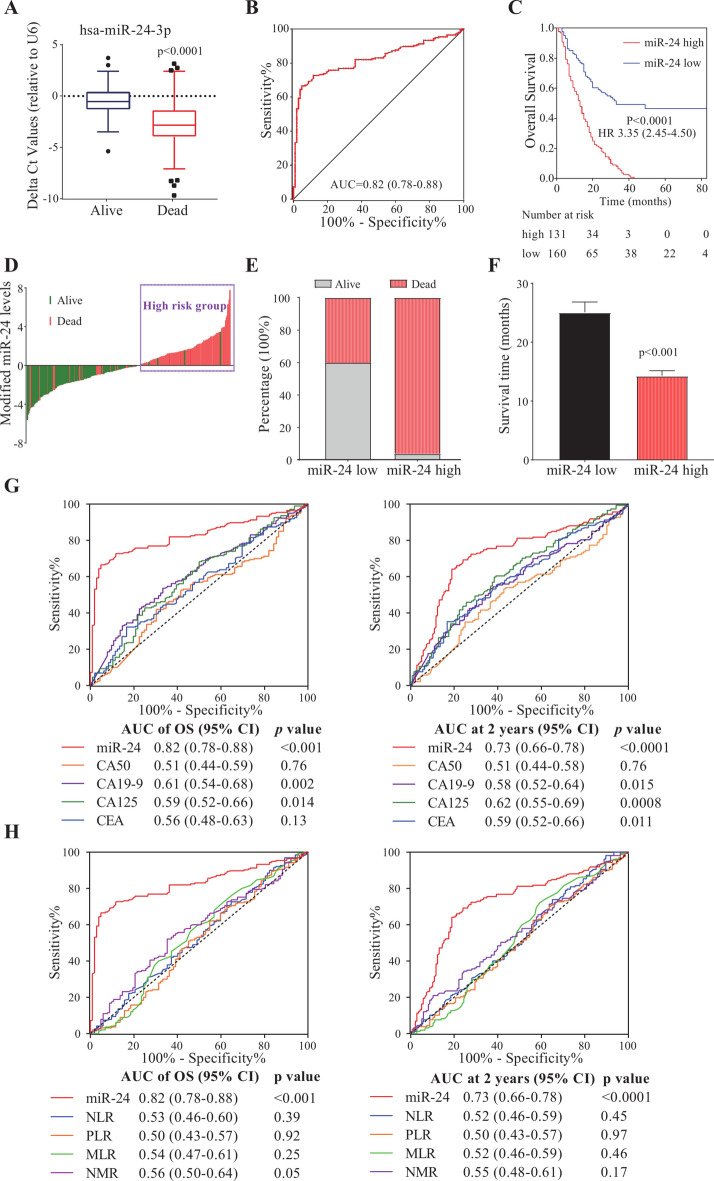
To explore the prognostic value of miR-24 in patients with PDAC, we initially compared the expression levels of miR-24 between 194 death and 102 survival patients. As illustrated in Fig. 1A, the delta Ct value of miR-24 in death patients was significantly lower than that in survivors, indicating an elevated expression of miR-24 among those who passed away (P < 0.0001). Next, ROC analysis evaluated the prognostic performance of miR-24 in predicting OS with an AUC of 0.82 (95% CI, 0.78–0.88; Fig. 1B). Based on the ROC analysis, the optimal cut-off values of miR-24 was derived as − 1.905, and patients were classified into high-risk (delta Ct < -1.905) and low-risk (delta Ct ≥ − 1.905) groups. High-risk patients had a significantly poorer OS than low-risk ones (P < 0.0001; Fig. 1C). According to the waterfall plot analysis, the proportion of dead patients with elevated miR-24 levels was significantly greater than that of those with diminished miR-24 (Fig. 1D,E). The median survival time for patients with elevated miR-24 was 14.5 months, significantly shorter than that of patients with low miR-24 levels (P < 0.001; Fig. 1F). The data presented suggest a promising prognostic value of miR-24 in patients with PDAC.
Fig. 1.
Prognostic value of miR-24 in patients with PDAC. (A) Expression levels of miR-24 in the serums of survival or death patients with PDAC. (B) ROC analysis of miR-24 in survival or death patients with PDAC. (C) Kaplan–Meier survival curves of high- and low-risk groups in patients with PDAC. (D) Survival status in high- and low-risk groups of patients with PDAC. (E) The proportion of survival or death among patients exhibiting low or high levels of miR-24. (F) The mean survival time of PDAC patients with a low or high miR-24 level. Data were shown as mean ± SEM. (G) The OS and 2 year-dependent ROC curves of miR-24 and serum protein markers. (H) The OS and 2 year-dependent ROC curves of miR-24 and systemic inflammatory markers. NLR, neutrophil–lymphocyte ratio. PLR, platelet- lymphocyte ratio. MLR, monocyte-lymphocyte ratio. NMR, neutrophil-monocyte ratio.
Comparison of prognostic efficiencies between miR-24 and common biomarkers
Through time-dependent ROC analysis, miR-24 displayed a more robust predictive capability, as evidenced by significantly larger AUC compared to conventional markers. Compared to serum protein markers such as CA19-9, CA125, CA50 and CEA, miR-24 predicted OS and 2 year survival with elevated AUCs of 0.82 and 0.73, respectively (Fig. 1G). Given that some studies have reported the prognostic potential of systemic inflammatory markers determined by complete blood count, we also examined the prognostic ability of NLR, PLR, MLR and NMR. The findings revealed that these inflammatory markers failed to predicted OS or 2-year survival of patients with PDAC (Fig. 1H). These comparative results further illuminate the potential of miR-24 as a prognostic biomarker of PDAC.
The value of miR-24 in distinguishing patients who benefit from ACT in PDAC
More importantly, to develop an effective biomarker for clinical use, it is essential that we explore the response of miR-24 to ACT. In the overall patient cohort, we observed that their OS did not improve with post-operative ACT, although the 2 year survival rate did show enhancement due to ACT (Fig. 2A,B). This result may be elucidated by the notion that patients derive benefits from short-term chemotherapy while simultaneously developing long-term drug resistance. Intriguingly, the high-risk group defined by miR-24 demonstrated improved outcome for both OS and 2-year survival when treated with ACT (P < 0.0001; Fig. 2C,D). Conversely, in the low-risk group, neither OS nor 2-year survival benefitted from ACT (P > 0.05; Fig. 2E,F). These findings underscore the potential for tailoring personalized predictions of a patient’s survival cycle based on miR-24. Such an approach may effectively mitigate unnecessary medical interventions and alleviate the economic burden on patients.
Fig. 2.
The value of miR-24 in distinguishing patients who benefit from adjuvant chemotherapy (ACT) in PDAC. Kaplan–Meier curves of OS and 2-year survival in all patients (A, B), miR-24 defined high-risk group (C, D), and miR-24 defined low-risk group (E, F).
miR-24 was an independent prognostic factor of PDAC
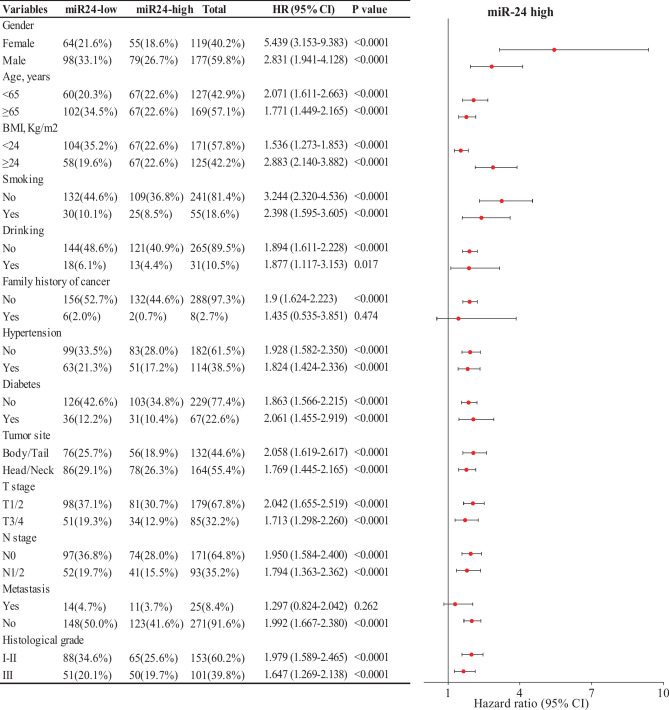
Furthermore, we identified independent prognostic factors of PDAC through univariate and multivariate Cox regression analyses on demographic and clinicopathological parameters. Results from the univariate Cox regression analysis indicated that TNM stage, CA19-9, CA125 and miR-24 were promising factors for PDAC (Table 1). However, the data from the multivariate Cox regression analysis confirmed that only miR-24 and TNM stage IV were independent prognostic factors for PDAC (P < 0.0001 and P = 0.016, respectively). As far as we know, TNM stage IV refers to cases with distant metastasis, whose survival status is notoriously grim. Therefore, it is widely acknowledged that stage IV is an independent factor influencing the prognosis of patients with PCa. Moreover, miR-24 proved effective in predicting OS across most subgroups stratified by demographic and clinicopathological parameters, including gender, age, BMI, smoking, drinking, hypertension, diabetes, tumor site, T stage, N stage and histological grade (Fig. 3). These exciting findings were further substantiated by Kaplan–Meier analysis conducted on subgroups based on T stage, N stage, histological grade and tumor site (Supplementary Fig. S1). Collectively, these results suggest that miR-24 may serve as an independent prognostic index for patients with PDAC.
Table 1.
Independent prognostic factors of PC.
| Univariate cox regression | Multivariate cox regression | |||||
|---|---|---|---|---|---|---|
| Variables | HR | (95%CI) | P value | HR | (95%CI) | P value |
| Gender | ||||||
| Female | Ref | |||||
| Male | 1.27 | 0.94–1.69 | 0.114 | |||
| Age, years | 1 | 0.99–1.01 | 0.930 | |||
| BMI, Kg/m2 | 1.01 | 0.96–1.07 | 0.682 | |||
| Smoking | 0.92 | 0.63–1.33 | 0.653 | |||
| Drinking | 0.85 | 0.52–1.38 | 0.505 | |||
| Family history of cancer | 0.57 | 0.21–1.53 | 0.264 | |||
| Hypertension | 0.99 | 0.74–1.32 | 0.950 | |||
| Diabetes | 0.83 | 0.59–1.17 | 0.282 | |||
| Tumor_site | ||||||
| Body/Tail | Ref | |||||
| Head/Neck | 1.09 | 0.82–1.45 | 0.561 | |||
| TNM stage | ||||||
| IA | Ref | |||||
| IB | 1.13 | 0.64–2.01 | 0.677 | 1.06 | 0.57–1.97 | 0.853 |
| IIA | 1.53 | 0.76–3.1 | 0.237 | 1.39 | 0.66–2.92 | 0.381 |
| IIB | 1.25 | 0.69–2.26 | 0.459 | 1.12 | 0.59–2.11 | 0.730 |
| III | 1.87 | 0.99–3.54 | 0.054 | 1.42 | 0.71–2.85 | 0.327 |
| IV | 2.47 | 1.21–5.01 | 0.013 | 2.54 | 1.19–5.42 | 0.016 |
| Chemotherapy | 1.00 | 0.75–1.34 | 0.981 | |||
| CA50 (μg/L) | 1.00 | 1.00–1.00 | 0.699 | |||
| CA19-9 (U/ml) | 1.00 | 1.00–1.00 | < 0.0001 | 1.00 | 1.00–1.00 | 0.053 |
| CA125(U/ml) | 1.00 | 1.00–1.01 | 0.009 | 1.00 | 1.00–1.01 | 0.523 |
| CEA (ng/ml) | 1.00 | 1.00–1.00 | 0.763 | |||
| miR24 | 0.81 | 0.76–0.86 | < 0.0001 | 0.80 | 0.74–0.86 | < 0.0001 |
Significance of P-values less than 0.05 are marked in bold.
Fig. 3.
Prognostic value of miR-24 in subgroups of patients with PDAC. The impact of miR-24 on OS across different subgroups of PDAC patients. Findings were examined by Cox proportional hazards regression analysis.
Nomogram based on miR-24 and clinicopathological factors
Given the significant prognostic values of miR-24 and the factors selected through univariate Cox regression analysis, we constructed a nomogram model that integrated the impact of various variables on the 1 year, 2 year and 5 year survival of patients with PDAC (Fig. 4A). Based on this model, we can make personalized predictions of patient’s survival cycle through the information regarding the patient’s TNM staging and serum levels of CA19-9, CA125 and miR-24. The concordance index and calibration curves were employed to validate the prediction power of the model. The nomogram’s concordance index was significantly higher than its constituents, including miR-24 (Fig. 4B). Observing the calibration curve at one year, we noted a substantial correlation between the actual data and the model’s predictions, though this consistency deteriorated as survival time increased (Fig. 4C–E). These findings demonstrate the promising efficacy of this model in predicting patient prognosis, although further validation of these results is warranted.
Fig. 4.
Predictive Nomogram for predicting the OS in patients with PC. (A) Predictive nomogram model for predicting the OS in patients with PC. The top scale corresponds to the variable axis. The sum of these point scores lies on the total point axis, and the overall probability of patient survival at 1, 2, and 5 years can be predicted by drawing a vertical line from total point axis downward on the survival axis. (B) Comparison of concordance index between nomogram model and its individual factors. Time-dependent calibration curves for the prognostic nomogram for predicting the 1-year (C), 2-year (D) and 5-year (E) survival, respectively.
Discussion
At present, surgery remains the first-line treatment for patients with PCa. Consequently, there is no doubt concerning the significance of evaluating the postoperative prognosis of these patients. In this study, we found that serum levels of miR-24 may serve as an independent prognostic factor for OS in patients with resected PDAC. To the best of our knowledge, this is the first study to elucidate the relationship between serum levels of miR-24 and survival outcomes in patients with PCa.
We chose to focus on this molecule because the dysregulation of miR-24 has been implicated in various human cancers, where it can function either as a tumor suppressor or an oncogene. For instance, circulating miR-24 has been shown to be upregulated in patients with advanced non-small cell lung cancer, serving as one of the novel predictive biomarkers for pemetrexed-based chemotherapy23. Serum miR-24 levels were investigated in hepatitis B viral-related hepatocellular carcinoma (HCC), revealing significantly elevated expressions in HCC patients compared to healthy controls24. Fang et al. discovered that plasma levels of miR-24, miR-320a, and miR-423-5p were all decreased in patients with colorectal cancer (CRC) and benign lesions relative to healthy controls, thus establishing them as novel biomarkers for CRC detection25. Furthermore, miR-24 has been reported to regulate cell growth and chemosensitivity in CRC by targeting the RNA-binding protein DND126. In the context of PCa, miR-24-3p may influence epithelial-mesenchymal transition and negatively regulate ASF1B expression, thereby modulating the malignant phenotype of PDAC cells27. Additionally, a study indicated that miR-24 promotes tumor growth and angiogenesis by suppressing Bim expression in vivo, a factor involved in numerous crucial biological behaviors associated with cancer28. Thus, the prognostic significance of this pathway-representative molecule in PDAC may be readily comprehended.
Nevertheless, the prognostic role of miR-24 in PCa has rarely been studied. Our previous work identified a panel of serum miRNAs including miR-24 whose diagnostic efficacy was fully assessed with the AUCs of 0.971 and 0.924, respectively, in discriminating patients with early-stage PCa from healthy controls or chronic pancreatitis as well as pancreatic cystic neoplasms21. Subsequently, the present study broadens the clinical application of miR-24 for patients with PDAC in predicting survival regardless of their chemotherapy regimens. It is indubitable that ACT can significantly enhance the survival of PDAC patients following radical resection, as evidenced by a well-designed randomized controlled trial29. However, the implications may differ, given that ACT has not demonstrated a survival benefit in certain retrospective studies30,31. In this study, although ACT did not improve OS in the entire cohort, it significantly enhanced survival among high-risk patients, suggesting that these individuals are more responsive to ACT. The high-risk patients exhibited exceedingly poor survival rates, thereby likely necessitating a more urgent intensification of ACT.
In addition, this marker showed superiority over conventional protein markers (CA19-9, CA125, CA50 and CEA), as well as systemic inflammatory markers (NLR, PLR, MLR and NMR). Although numerous studies have indicated their prognostic value, debate persists regarding their efficacy as prognostic markers. The synthesis of CA19-9 is influenced by common variants in the fucosyltransferase (FUT) enzymes32. A recent study found that higher preoperative CA19-9 levels were associated with recurrence and mortality for patients with PCa within the higher-FUT groups, yet not in their lower-FUT counterparts33. Elevations in CA125 levels beyond three months postoperatively, rather than early postoperative elevations, emerged as predictors of poor prognosis. Our findings in this study illuminated the prognostic significance of CA19-9 and CA125, while CA50 and CEA revealed no prognostic significance for OS in patients with PDAC. Nonetheless, CEA demonstrated prognostic significance for 2-year survival (P = 0.011). In addition, systemic inflammatory markers such as NLR, PLR, MLR and NMR exhibited no significant differences between the dead and survival patients, as per our findings. This result outcome stands in contrast to several studies, although validation through the expansion of cohorts remains imperative11,34–36. Given that some literature also proposed the contradictory results, this finding should not be deemed disappointing37.
More importantly, our study effectively created nomogram model by the combination of miR-24 and individual clinicopathological factors by nomogram model could markedly enhance their predictive powers. Studies demonstrating constructed nomograms, created from baseline clinical factors readily available before chemotherapy, have exhibited commendable performance and provide convenience in aiding clinicians with individualized patient management38,39. Therefore, the miRNA, especially the combination, provided a potential as the supplemental prognostic tool for precision medicine of PDAC in the clinic.
In conclusion, we elucidated the prognostic significance of miR-24, and based on this marker, we developed a nomogram model to predict the OS of patients with resected PCa. Our findings suggest that serum miR-24 is associated with OS, and may serve as an independent prognostic factor for patients with resected PDAC. Moreover, those patients classified as high-risk by miR-24 may derive greater benefit from ACT. This marker warrants further validation in larger cohorts and prospective studies.
Methods
Patient selection
We conducted a retrospective analysis of 296 patients with stage I-IV PDAC who underwent radical resection at the Renji Hospital affiliated with Shanghai Jiaotong University, between October, 2012 and July, 2019. The patient characteristics are detailed in Table 2. This study comprised 177 men (59.8%) and 119 women (40.2%). The median age of the patients was 66 years (range, 24–89 years). Patients were selected according to the following exclusion criteria: 1) patients with concurrent primary tumors; 2) those who received pre-surgical therapies; 3) those lacking baseline or follow-up data; 4) patients who died from causes unrelated to PCa, such as complications, other malignant diseases or accidents. The patients were followed up every three months until death or the end of follow-up. OS (in months) was defined as the duration from the date of surgical operation to the date of death or the last follow-up.
Table 2.
Baseline characteristics of patients.
| Variables | Number (%) | Variables | Number (%) |
|---|---|---|---|
| Age, years | TNM stage | ||
| < 65 | 127 (42.9) | IA | 25 (8.4) |
| ≥ 65 | 169 (57.1) | IB | 87 (29.4) |
| Gender | IIA | 26 (8.8) | |
| Male | 177 (59.8) | IIB | 67 (22.6) |
| Female | 119 (40.2) | III | 37 (12.5) |
| BMI, Kg/m2 | IV | 22 (7.4) | |
| < 24 | 214 (72.3) | Unknown | 32 (10.8) |
| ≥ 24 | 82 (27.7) | CA50, ug/L | |
| Smoking | > 20 | 192 (64.9) | |
| Yes | 55 (18.6) | ≤ 20 | 57 (19.3) |
| No | 241 (81.4) | Unknown | 47 (15.9) |
| Drinking | CA19-9, U/ml | ||
| Yes | 31 (10.5) | > 37 | 240 (81.1) |
| No | 265 (89.5) | ≤ 37 | 56 (18.9) |
| Family history of cancer | CA125, U/ml | ||
| Yes | 8 (2.7) | > 35 | 54 (18.2) |
| No | 288 (97.3) | ≤ 35 | 215 (72.6) |
| Hypertension | Unknown | 27 (9.1) | |
| Yes | 114 (38.5) | CEA, ng/ml | |
| No | 182 (61.5) | > 5 | 86 (29.1) |
| Diabetes | ≤ 5 | 184 (62.2) | |
| Yes | 67 (22.6) | Unknown | 26 (8.8) |
| No | 229 (77.4) | Chemotherapy | |
| Tumor site | Yes | 149 (50.3) | |
| Head/Neck | 147 (49.7) | No | 147 (49.7) |
| Body/Tail | 118 (39.9) | ||
| Unknown | 31 (10.5) |
This work was approved by the local ethics committees of Dalian University of Technology (reference number: 2020-075), and informed consent was acquired from all the patients. PDAC staging was carried out according to the TNM classification (AJCC eighth edition). The study was conducted according to the principles of the Declaration of Helsinki regarding studies involving human participants. All experiments were performed in accordance with Reporting Recommendations for Tumor Marker Prognostic Studies.
Serum levels of miR-24 detected by real time polymerase chain reaction (RT-PCR)
The serum levels of miR-24 were measured by RT-PCR with TaqMan probes, conducted as previously detailed21. Briefly, total RNA was extracted from 0.25 mL serum samples using RNAiso Blood (Takara, Japan) according to the manufacturer’s instruction. Then RNA was transcribed into cDNA in a scaled-down (10 μL) reverse transcription reaction. Next, RT-PCR was performed on the LightCycler 480 II System (Roche, Switzerland) through 45-cycle amplification. As an internal control, serum levels of small nuclear RNA U6 were measured simultaneously. Each sample was tested in triplicate, and the Ct value of miRNA or U6 was calculated as the mean of the three repeated values. Endogenous level of miRNA in serum was calculated using delta Ct value, representing the relative Ct value of miR-24 normalized to that of U6.
Statistical analysis
Continuous variables were compared with the student’s t-test or one-way ANOVA, while categorical variables were tested by the Chi-square test. The prognostic utility of variables was assessed using receiver operating characteristic (ROC) curve analysis. Endogenous level of miRNA expression was calculated as delta Ct method (ΔCtsample = CtmiRNA–Ct U6). Patients were split into high- and low-risk groups due to miR-24 levels, and the potential differences in OS were analyzed using Kaplan–Meier curves and compared using the Log rank test. We conducted a univariate Cox proportional hazards regression analysis on all variables and incorporated those with a P < 0.05 into a multivariate Cox proportional hazards regression analysis to ascertain independent prognostic factors. In addition, we established a nomogram based on risk factors to predict the risk and OS of PDAC patients at 1, 2, and 5 years. The accuracy of nomogram was evaluated using C-index, and the discrimination of nomogram was verified using calibration plots. Statistical analyses were conducted using R Studio (version 4.3.1) or GraphPad Prism 9 software. P < 0.05 was considered statistically significant.
Supplementary Information
Author contributions
Jing Huang: Writing—original draft, Investigation, Formal analysis, Software, Visualization. Qian Zhang: Data curation, Investigation, Methodology. Yang Ge: Data curation, Methodology. Ren Zheng: Investigation. Minwei Yan: Resources. Yongwei Sun: Resources. Vay Liang W. Go: Conceptualization. Zhigang Zhang: Supervision. Huilong Fang: Project administration. Jianzhou Liu: Validation. Junchao Guo: Project administration. Gary Guishan Xiao: Writing—review & editing, Funding acquisition.
Funding
This work was founded by Natural National Science Foundation of China [grant numbers 81770846 and 81642006], US Hirshberg Foundation for Pancreatic Cancer Research [grant number AH201901083], and Shenzhen Weilan Foundation for Cancer Research [grant number KD2021030001]. The funders had no role in relation to the study design, collection, analysis and interpretation of data, writing of the report and decision to submit the article for publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Huang and Qian Zhang contributed equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82369-9.
References
- 1.Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin.73, 17–48. 10.3322/caac.21763 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Kolbeinsson, H. M., Chandana, S., Wright, G. P. & Chung, M. Pancreatic cancer: A review of current treatment and novel therapies. J. Investig. Surg.36, 2129884. 10.1080/08941939.2022.2129884 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Lan, Y., Jia, Q., Feng, M., Zhao, P. & Zhu, M. A novel natural killer cell-related signatures to predict prognosis and chemotherapy response of pancreatic cancer patients. Front. Genet.14, 1100020. 10.3389/fgene.2023.1100020 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar, S. et al. Interplay between MAP kinases and tumor microenvironment: Opportunity for immunotherapy in pancreatic cancer. Adv. Cancer Res.159, 113–143. 10.1016/bs.acr.2023.02.003 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Ricci, V. et al. Pancreatic cancer: Beyond Brca mutations. J. Pers. Med.10.3390/jpm12122076 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu, H. X. et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery161, 373–384. 10.1016/j.surg.2016.08.005 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Lucarotti, M. E. et al. Clinical evaluation of combined use of CEA, CA19-9 and CA50 in the serum of patients with pancreatic carcinoma. Eur. J. Surg. Oncol.17, 51–53 (1991). [PubMed] [Google Scholar]
- 8.Gu, Y. L. et al. Applicative value of serum CA19-9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac. J. Cancer Prev.16, 6569–6573. 10.7314/apjcp.2015.16.15.6569 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ramalhete, L., Vigia, E., Araújo, R. & Marques, H. P. Proteomics-Driven Biomarkers in Pancreatic Cancer. Proteomes10.3390/proteomes11030024 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabernero, J. et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist20, 143–150. 10.1634/theoncologist.2014-0394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang, Z. J. et al. Neutrophil-lymphocyte ratio (NLR) was associated with prognosis and immunomodulatory in patients with pancreatic ductal adenocarcinoma (PDAC). Biosci. Rep. 10.1042/BSR20201190 (2020). [DOI] [PMC free article] [PubMed]
- 12.Toledano-Fonseca, M. et al. The combination of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with liquid biopsy biomarkers improves prognosis prediction in metastatic pancreatic cancer. Cancers (Basel)10.3390/cancers13061210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer122, 2158–2167. 10.1002/cncr.30057 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Panni, R. Z. et al. Association of preoperative monocyte-to-lymphocyte and neutrophil-to-lymphocyte ratio with recurrence-free and overall survival after resection of pancreatic neuroendocrine tumors (US-NETSG). J. Surg. Oncol.120, 632–638. 10.1002/jso.25629 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Daoud, A. Z., Mulholland, E. J., Cole, G. & McCarthy, H. O. MicroRNAs in Pancreatic Cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer19, 1130. 10.1186/s12885-019-6284-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, S., Fesler, A., Wang, H. & Ju, J. microRNA based prognostic biomarkers in pancreatic cancer. Biomark. Res.6, 18. 10.1186/s40364-018-0131-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, P. T. B. et al. MicroRNA-based diagnosis and therapy. Int. J. Mol. Sci.10.3390/ijms23137167 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannetti, E. et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res.70, 4528–4538. 10.1158/0008-5472.Can-09-4467 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Greither, T. et al. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int. J. Cancer126, 73–80. 10.1002/ijc.24687 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Shi, W., Lu, Y., Gong, R., Sun, J. J. & Liu, G. Serum miR-629 is a novel molecular marker for diagnosis and the prognosis of pancreatic cancer. Eur. Rev. Med. Pharmacol. Sci.22, 5187–5193. 10.26355/eurrev_201808_15715 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Huang, J. et al. Development of a serum-based MicroRNA signature for early detection of pancreatic cancer: A multicenter cohort study. Dig. Dis. Sci.69, 1263–1273. 10.1007/s10620-024-08338-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, R. et al. Prognostic significance of microRNA miR-24 in cancers: a meta-analysis. Bioengineered12, 450–460. 10.1080/21655979.2021.1875662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchina, T. et al. Circulating miR-22, miR-24 and miR-34a as novel predictive biomarkers to pemetrexed-based chemotherapy in advanced non-small cell lung cancer. J. Cell Physiol.229, 97–99. 10.1002/jcp.24422 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Meng, F. L., Wang, W. & Jia, W. D. Diagnostic and prognostic significance of serum miR-24-3p in HBV-related hepatocellular carcinoma. Med. Oncol.31, 177. 10.1007/s12032-014-0177-3 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Fang, Z. et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J. Exp. Clin. Cancer Res.34, 86. 10.1186/s13046-015-0198-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Q., Li, W., Liu, G. & Tang, W. MicroRNA-24 regulates the growth and chemosensitivity of the human colorectal cancer cells by targeting RNA-binding protein DND1. J. Buon24, 1476–1481 (2019). [PubMed] [Google Scholar]
- 27.Huang, W. et al. miR-24-3p regulates epithelial-mesenchymal transition and the malignant phenotype of pancreatic adenocarcinoma by regulating ASF1B expression. Biochem. Genet.61, 742–761. 10.1007/s10528-022-10278-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, R. et al. The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget6, 43831–43842. 10.18632/oncotarget.6257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oettle, H. et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curativeintent resection of pancreatic cancer: a randomized controlled trial. JAMA297, 267–277. 10.1001/jama.297.3.267 (2007). [DOI] [PubMed] [Google Scholar]
- 30.You, D. D. et al. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J. Gastrointest. Surg.13, 1699–1706. 10.1007/s11605-009-0969-5 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Kim, C., Owen, D. & Gill, S. Real-world impact of availability of adjuvant therapy on outcomes in patients with resected pancreatic adenocarcinoma: a Canadian cancer agency experience. Am. J. Clin. Oncol.35, 212–215. 10.1097/COC.0b013e318209d36c (2012). [DOI] [PubMed] [Google Scholar]
- 32.Dbouk, M. et al. Diagnostic performance of a tumor marker gene test to personalize serum CA19-9 reference ranges. Clin. Cancer Res.29, 4178–4185. 10.1158/1078-0432.Ccr-23-0655 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando, Y. et al. Using a CA19-9 tumor marker gene test to assess outcome after pancreatic cancer surgery. Ann. Surg. Oncol.31, 2902–2912. 10.1245/s10434-024-14942-5 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo, G. et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann. Surg. Oncol.22, 670–676. 10.1245/s10434-014-4021-y (2015). [DOI] [PubMed] [Google Scholar]
- 35.Abu-Shawer, O. et al. The clinical value of peripheral immune cell counts in pancreatic cancer. PLoS ONE15, e0232043. 10.1371/journal.pone.0232043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, Y. et al. Meaningful nomograms based on systemic immune inflammation index predicted survival in metastatic pancreatic cancer patients receiving chemotherapy. Cancer Med.13, e7453. 10.1002/cam4.7453 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merlo, I. et al. Prognostic factors in resected pancreatic ductal adenocarcinoma: Is neutrophil-lymphocyte ratio a useful marker?. J. Gastrointest. Cancer54, 580–588. 10.1007/s12029-022-00839-7 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Deng, G. C. et al. Nomogram to predict survival of patients with advanced and metastatic pancreatic cancer. BMC Cancer21, 1227. 10.1186/s12885-021-08943-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, W. et al. Nomogram predicts risk and prognostic factors for bone metastasis of pancreatic cancer: A population-based analysis. Front. Endocrinol. (Lausanne)9, 752176. 10.3389/fendo.2021.752176 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Xiang, Z. J. et al. Neutrophil-lymphocyte ratio (NLR) was associated with prognosis and immunomodulatory in patients with pancreatic ductal adenocarcinoma (PDAC). Biosci. Rep. 10.1042/BSR20201190 (2020). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.