Abstract
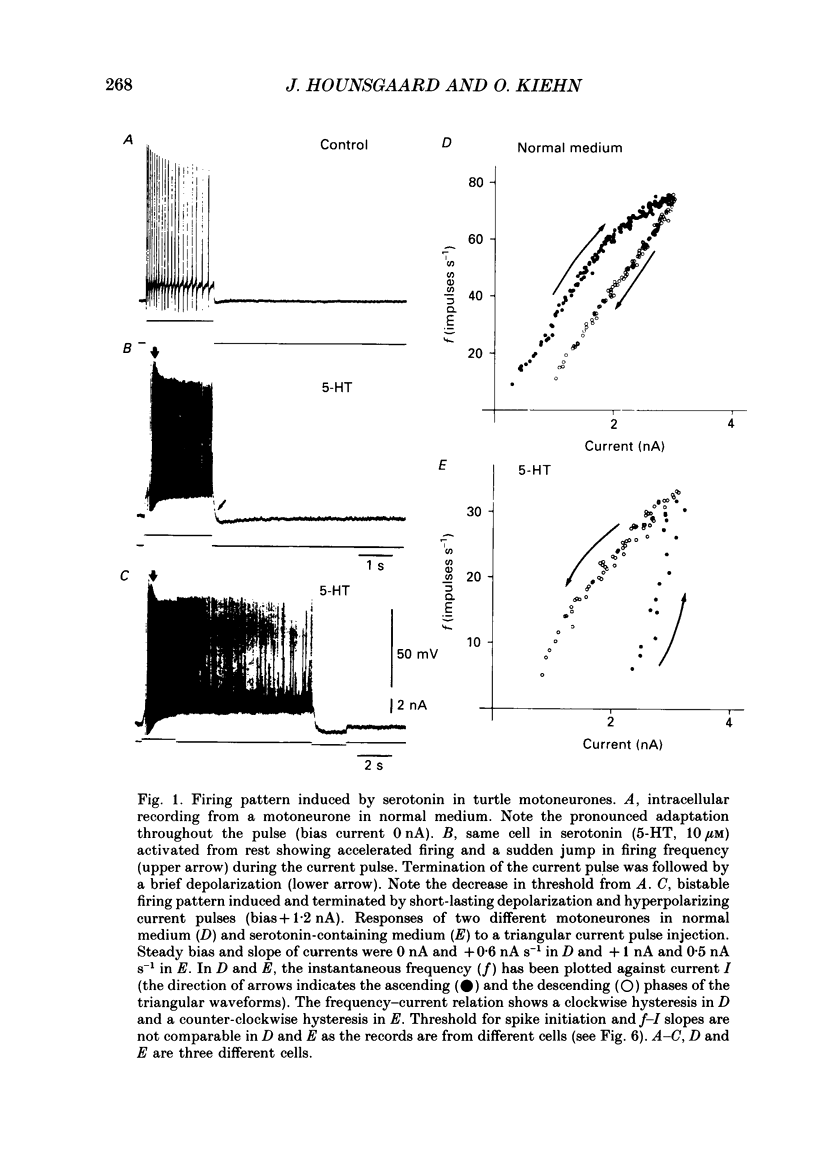
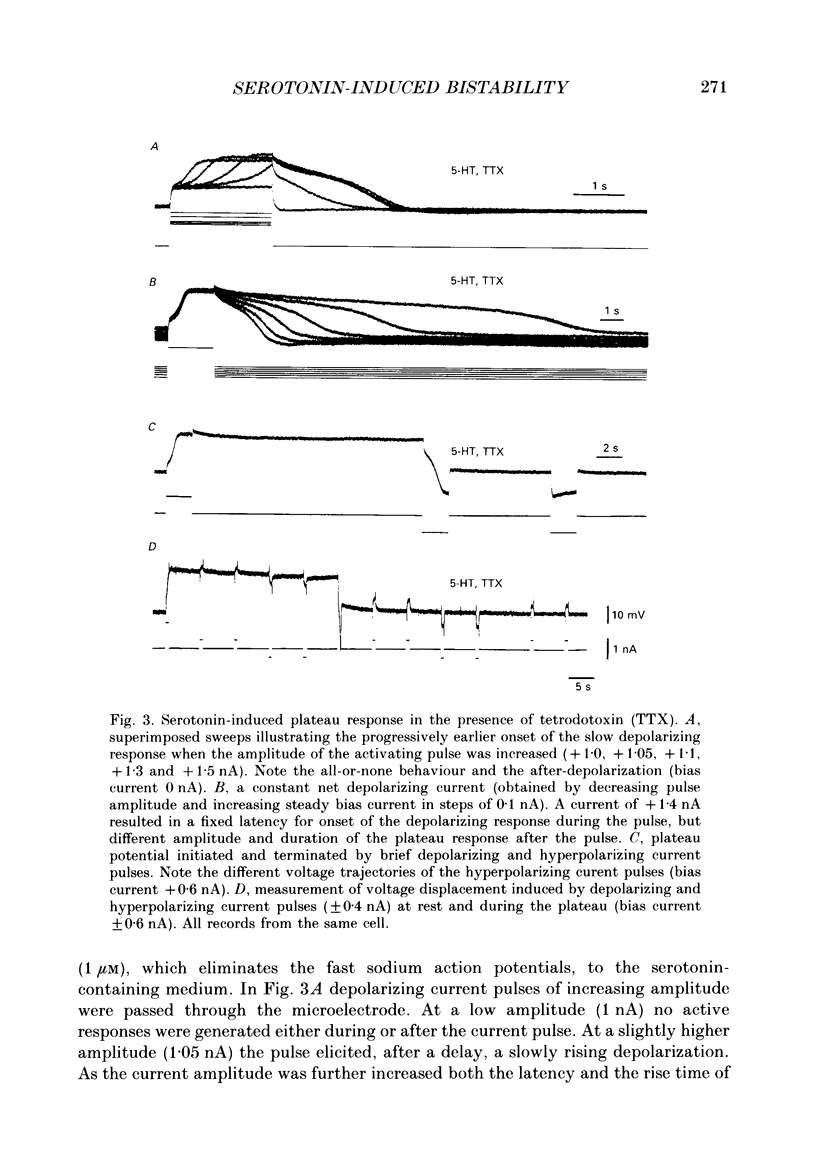
1. The effect of serotonin on the firing properties of motoneurones was studied in transverse sections of the adult turtle spinal cord in vitro with intracellular recording techniques. 2. In normal medium, turtle motoneurones adapt from an initial high frequency to a low steady firing during a depolarizing current pulse. In the presence of serotonin (4-100 microM) motoneurones responded with accelerated firing and a frequency jump during a depolarizing current pulse followed by an after-depolarization outlasting the stimulus. From a depolarized holding potential motoneuronal activity was shifted between two stable states by brief depolarizing and hyperpolarizing current pulses. As an expression of this bistable firing behaviour, the frequency-current relation in response to a triangular current injection was counter-clockwise in serotonin while clockwise in normal medium. 3. The delay to onset of the frequency jump was shortened as the amplitude of the activation pulse was increased. From a positive holding potential the after-depolarization exceeded spike threshold and its duration increased with an increase in steady bias current. The effect of serotonin on turtle motoneurones could be blocked by methysergide (10 microM). 4. When action potentials were depressed by tetrodotoxin, a voltage-dependent, non-inactivating plateau potential, intrinsic to the motoneurone, was revealed. Activation of this voltage plateau provides the motoneurones with two stable states of firing. The apparent input resistance was 2-4-fold lower during the plateau than at rest. 5. The serotonin-induced plateau potential was Ca2+-dependent and was blocked when Ca2+ was replaced by either Co2+ (3 mM) or Mn2+ (3 mM). 6. The Ca2+ plateau was blocked by nifedipine (1-15 microM). 7. Serotonin reduced the slow after-hyperpolarization following action potentials. The change in balance between inward and outward currents seems to be sufficient to reveal the plateau response. 8. Although a small plateau response was induced by Bay K 8644 (1-15 microM), this L-channel agonist could not reproduce the pronounced effect of serotonin. 9. It is concluded that serotonin induces a Ca2+-dependent and nifedipine-sensitive plateau potential in turtle motoneurones primarily by reducing a K+-current responsible for the slow after-hyperpolarization.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974 Sep;92(1):27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Conway B. A., Hultborn H., Kiehn O., Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988 Nov;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SHORTESS G. K. QUANTITATIVE ASPECTS OF REPETITIVE FIRING OF MAMMALIAN MOTONEURONES, CAUSED BY INJECTED CURRENTS. J Physiol. 1963 Oct;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988 Nov;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57(2):422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O., Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988 Apr;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988 Apr;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura I. Determination of bacteriophage lambda tail length by a protein ruler. Nature. 1987 May 7;327(6117):73–75. doi: 10.1038/327073a0. [DOI] [PubMed] [Google Scholar]
- Kernell D., Monster A. W. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46(2):191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+-dependent K+ channels. Cell Calcium. 1983 Dec;4(5-6):421–428. doi: 10.1016/0143-4160(83)90018-0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988 Nov;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980 Jun;43(6):1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Ueda S., Takeuchi Y., Sano Y. Immunohistochemical demonstration of serotonin neurons in the central nervous system of the turtle (Clemmys japonica). Anat Embryol (Berl) 1983;168(1):1–19. doi: 10.1007/BF00305395. [DOI] [PubMed] [Google Scholar]
- Van Dongen P. A., Grillner S., Hökfelt T. 5-Hydroxytryptamine (serotonin) causes a reduction in the afterhyperpolarization following the action potential in lamprey motoneurons and premotor interneurons. Brain Res. 1986 Feb 26;366(1-2):320–325. doi: 10.1016/0006-8993(86)91310-7. [DOI] [PubMed] [Google Scholar]
- Woodward D. J., Moises H. C., Waterhouse B. D., Hoffer B. J., Freedman R. Modulatory actions of norepinephrine in the central nervous system. Fed Proc. 1979 Jun;38(7):2109–2116. [PubMed] [Google Scholar]
- Yoshimura M., Polosa C., Nishi S. Noradrenaline modifies sympathetic preganglionic neuron spike and afterpotential. Brain Res. 1986 Jan 8;362(2):370–374. doi: 10.1016/0006-8993(86)90466-x. [DOI] [PubMed] [Google Scholar]



