Abstract
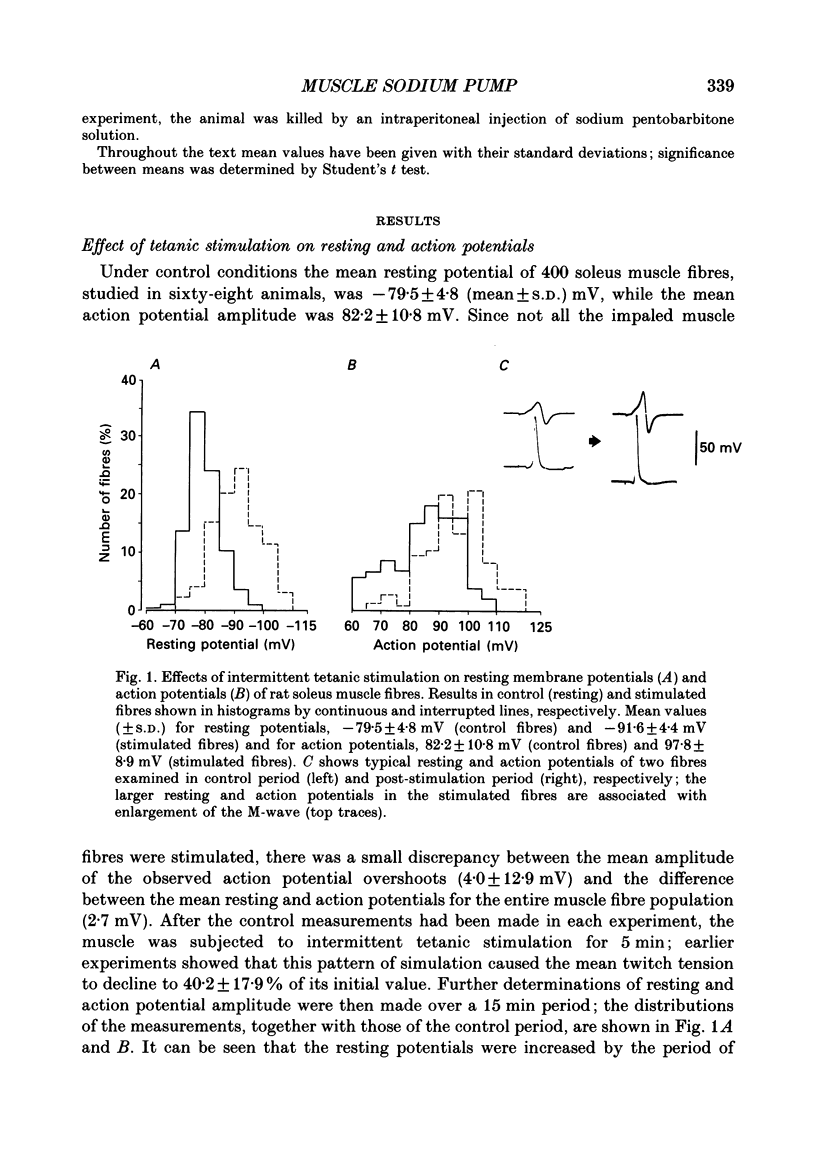
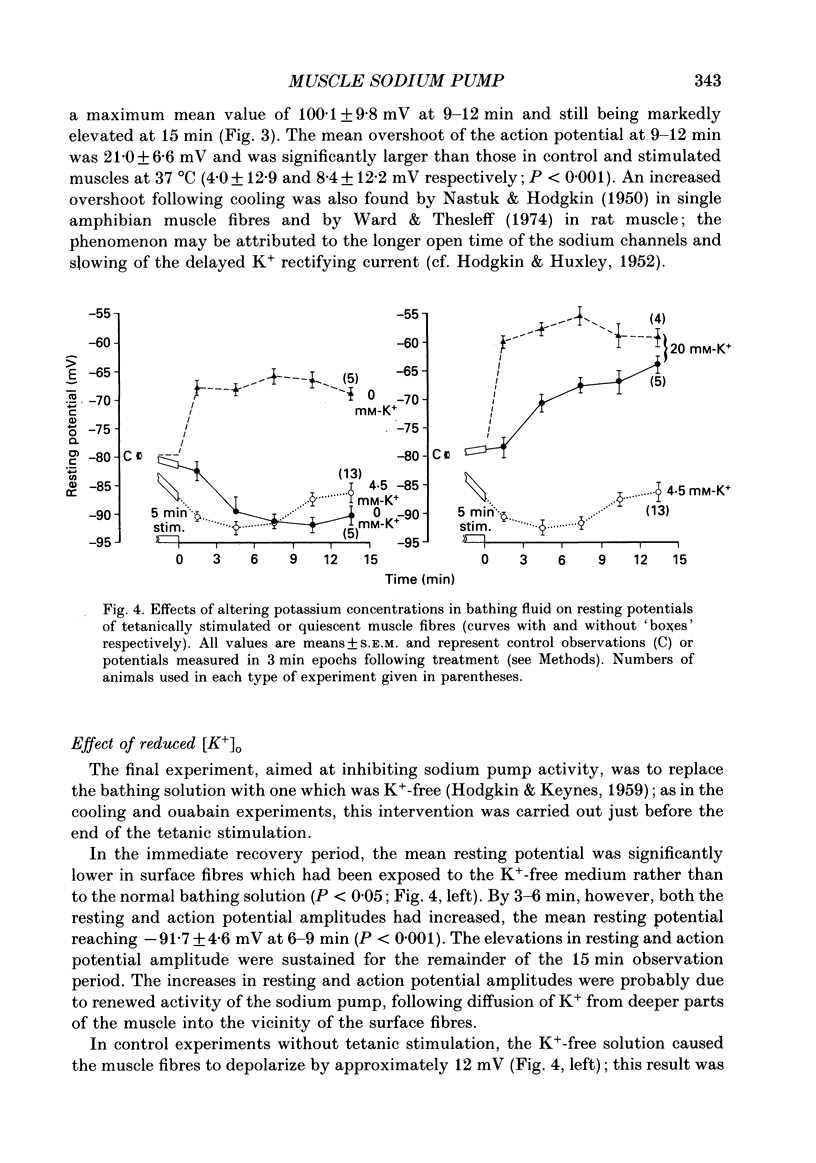
1. Soleus muscles of anaesthetized rats were stimulated tetanically (4 s at 20 Hz every 5 s for 5 min), following which the resting and action potentials were measured in surface fibres. 2. At the end of the stimulation period, the mean resting potential was found to have increased from a control value of -79.5 +/- 4.8 mV (mean +/- S.D.) to -90.5 +/- 6.3 mV. The hyperpolarization started to decline after 9 min but was still present at 15 min. 3. Associated with the membrane hyperpolarization was an increase in the mean amplitude of the muscle fibre action potential, from 82.2 +/- 10.8 to 96.8 +/- 10.0 mV. 4. Both the hyperpolarization and the enlargement of the muscle fibre action potential were abolished by 1.25 X 10(-4) M-ouabain, cooling the bathing fluid to 19 degrees C or removing K+ from the bathing fluid. 5. The results are explained in terms of an increase in electrogenic sodium pump activity resulting from tetanic stimulation. When the bathing fluid contained 20 mM-K+, the mean resting potential of stimulated fibres was approximately -30 mV greater than that calculated from the Goldman-Hodgkin-Katz equation. 6. The increase in sodium pumping not only acts to restore the concentrations of Na+ and K+ on either side of the muscle fibre membrane, but, through its electrogenic effect, enables fibres to remain excitable during continuous contractile activity.
Full text
PDF

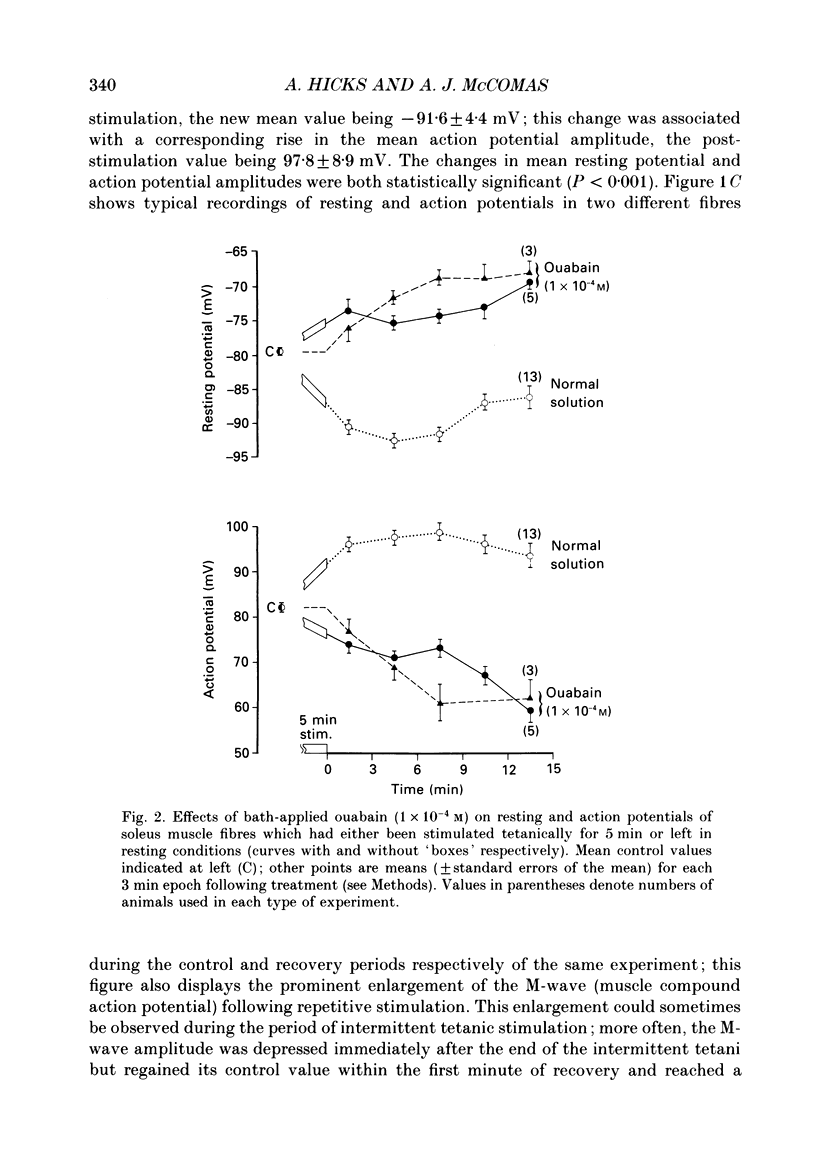

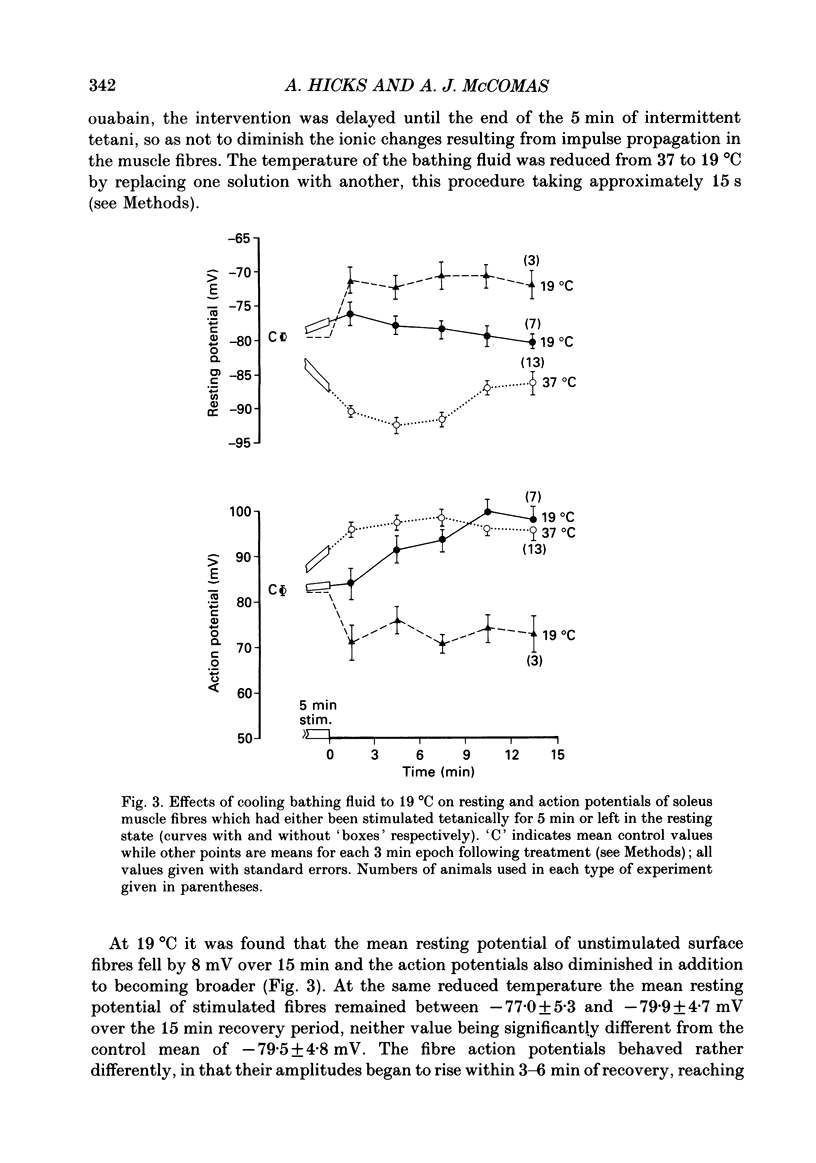





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B., Jones D. A., Woods J. J. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp Neurol. 1979 May;64(2):414–427. doi: 10.1016/0014-4886(79)90280-2. [DOI] [PubMed] [Google Scholar]
- Brodal B. P., Eeg-Larsen N. L., Iversen O. J., Jebens E., Roed A. Enhanced (Na+, K+)-activated ATPase activity after indirect electric stimulation of rat skeletal muscle in vivo. Life Sci. 1975 Aug 1;17(3):329–331. doi: 10.1016/0024-3205(75)90480-4. [DOI] [PubMed] [Google Scholar]
- CREESE R., HASHISH S. E., SCHOLES N. W. Potassium movements in contracting diaphragm muscle. J Physiol. 1958 Sep 23;143(2):307–324. doi: 10.1113/jphysiol.1958.sp006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- CREESE R., NORTHOVER J. Maintenance of isolated diaphragm with normal sodium content. J Physiol. 1961 Feb;155:343–357. doi: 10.1113/jphysiol.1961.sp006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Everts M. E., Kjeldsen K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J Physiol. 1987 Jul;388:163–181. doi: 10.1113/jphysiol.1987.sp016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Head S. D., Jenkinson D. F. The role of the sodium pump during prolonged end-plate currents in guinea-pig diaphragm. J Physiol. 1987 Mar;384:377–403. doi: 10.1113/jphysiol.1987.sp016460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch S., McComas A. Influence of human muscle length on fatigue. J Physiol. 1985 May;362:205–213. doi: 10.1113/jphysiol.1985.sp015671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. N., Atwood H. L., Charlton M. P. Intracellular sodium-activity at rest and after tetanic stimulation in muscles of normal and dystrophic (dy2J/dy2J) C57BL/6J mice. Exp Neurol. 1986 Aug;93(2):359–368. doi: 10.1016/0014-4886(86)90196-2. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Hyperpolarization following long-lasting tetanic activation of hippocampal pyramidal cells. Brain Res. 1983 Sep 19;275(1):159–163. doi: 10.1016/0006-8993(83)90429-8. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. The effects of repetitive stimulation on the action potential and the twitch of rat muscle. Acta Physiol Scand. 1974 Feb;90(2):387–400. doi: 10.1111/j.1748-1716.1974.tb05600.x. [DOI] [PubMed] [Google Scholar]
- Hirche H., Schumacher E., Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflugers Arch. 1980 Sep;387(3):231–237. doi: 10.1007/BF00580975. [DOI] [PubMed] [Google Scholar]
- Hník P., Holas M., Krekule I., Kŭriz N., Mejsnar J., Smiesko V., Ujec E., Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976 Mar 11;362(1):85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflugers Arch. 1986 May;406(5):458–463. doi: 10.1007/BF00583367. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. RESTING POTENTIAL OF ISOLATED RAT MUSCLES MEASURED IN PLASMA. Nature. 1963 Nov 2;200:474–475. doi: 10.1038/200474a0. [DOI] [PubMed] [Google Scholar]
- Knochel J. P., Blachley J. D., Johnson J. H., Carter N. W. Muscle cell electrical hyperpolarization and reduced exercise hyperkalemia in physically conditioned dogs. J Clin Invest. 1985 Feb;75(2):740–745. doi: 10.1172/JCI111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke S., Solomon H. C. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J Exp Zool. 1967 Dec;166(3):377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Meadows J. C., Merton P. A. Isolated single motor units in human muscle and their rate of discharge during maximal voluntary effort. J Physiol. 1971;217 (Suppl):12P–13P. [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRETER F. A. Distribution of water, sodium, and potassium in resting and stimulated mammalian muscle. Can J Biochem Physiol. 1963 Apr;41:1035–1045. [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Hník P., Rehfeldt H., Vejsada R., Ujec E. The measurement of K+e concentration changes in human muscles during volitional contractions. Pflugers Arch. 1983 Nov;399(3):235–237. doi: 10.1007/BF00656721. [DOI] [PubMed] [Google Scholar]
- Ward M. R., Thesleff S. The temperature dependence of action potentials in rat skeletal muscle fibres. Acta Physiol Scand. 1974 Aug;91(4):574–576. doi: 10.1111/j.1748-1716.1974.tb05714.x. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Withrow C. D., Woodbury D. M. Effects of ouabain and diphenylhydantoin on transmembrane potentials, intracellular electrolytes, and cell pH of rat muscle and liver in vivo. J Physiol. 1971 Jan;212(1):101–115. doi: 10.1113/jphysiol.1971.sp009312. [DOI] [PMC free article] [PubMed] [Google Scholar]



