Abstract
1. Retinas from channel catfish were dissociated and the cells maintained in culture. Horizontal cells that normally receive input from cone photoreceptors were identified. The conductance of the electrical junction formed between a pair of 'cone' horizontal cells was measured by controlling the membrane voltage of each cell with a voltage clamp maintained through either a micropipette or a patch pipette. The two techniques yielded similar results. 2. Transjunctional current was measured while transjunctional voltage was stepped to values between +/- 60 mV. The current (measured 5 ms after a step) was proportional to voltage over the range tested. For steps to voltages greater than +/- 45 mV, the current exhibited a slight time-dependent decline. 3. Dopamine decreased junctional conductance in a dose-dependent fashion. A 50% reduction was obtained with 10 nM-dopamine. The D1 agonist fenoldopam (100 nM) also decreased junctional conductance. The uncoupling produced by either agent was rapid and reversible. 4. The introduction of 100 microM-cyclic AMP into one cell of a pair decreased junctional conductance by, on average, 40%. Forskolin (1-10 microM), an activator of adenylate cyclase, decreased junctional conductance 50-90%. 5. The introduction of 80 microM-cyclic GMP into one cell of a pair decreased junctional conductance by, on average, 40%. Nitroprusside (1-10 microM), an activator of guanylate cyclase, reduced junctional conductance 40-65%. 6. The introduction of a peptide inhibitor specific for the cyclic AMP-dependent protein kinase reversed a decrease in junctional conductance produced by superfusion with either dopamine (1 microM), fenoldopam (100 nM) or forskolin (5-10 microM). 7. Intracellular Ca2+ concentration was measured with the fluorescent indicator Fura-2. The intracellular Ca2+ concentration was increased by activation of a Ca2+ current. Junctional conductance remained constant as the internal Ca2+ concentration changed from 100 to 700 nM. 8. Intracellular pH was measured with the fluorescent indicator bis-carboxyethylcarboxyfluorescein. The application of acetate (2.5 mM) reduced intracellular pH by 0.2-0.3 units and decreased junctional conductance by approximately 50%. A subsequent application of fenoldopam did not alter intracellular pH, but decreased junctional conductance by more than 50%. 9. The sensitivity of the junctional conductance between isolated horizontal cells to dopamine is consistent with dopamine having a direct effect on coupling in intact retina. Dopamine regulates the activity of a cyclic AMP-dependent protein kinase which in turn modulates junctional conductance. Changes in intracellular pH and Ca2+ concentration are not involved in mediating the effect of dopamine on coupling. Cyclic GMP and intracellular pH may participate in regulatory pathways independent of that used by cyclic AMP.
Full text
PDF




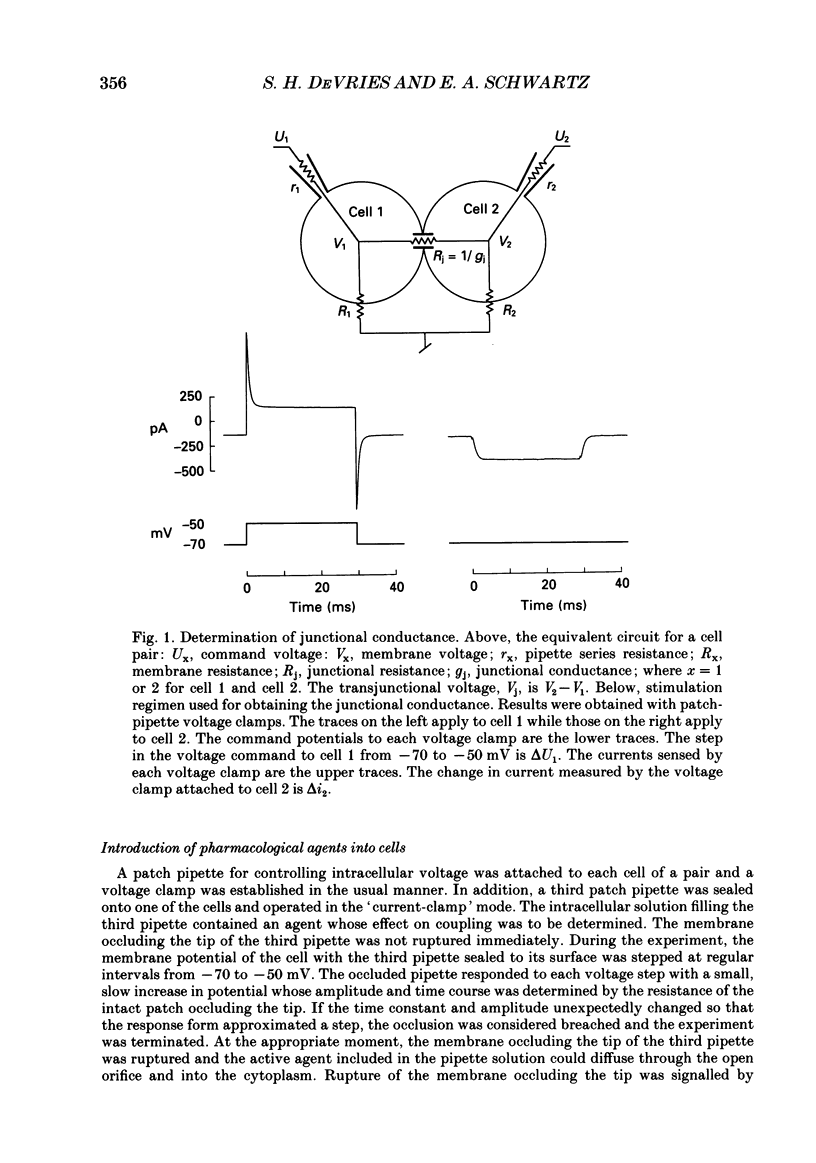



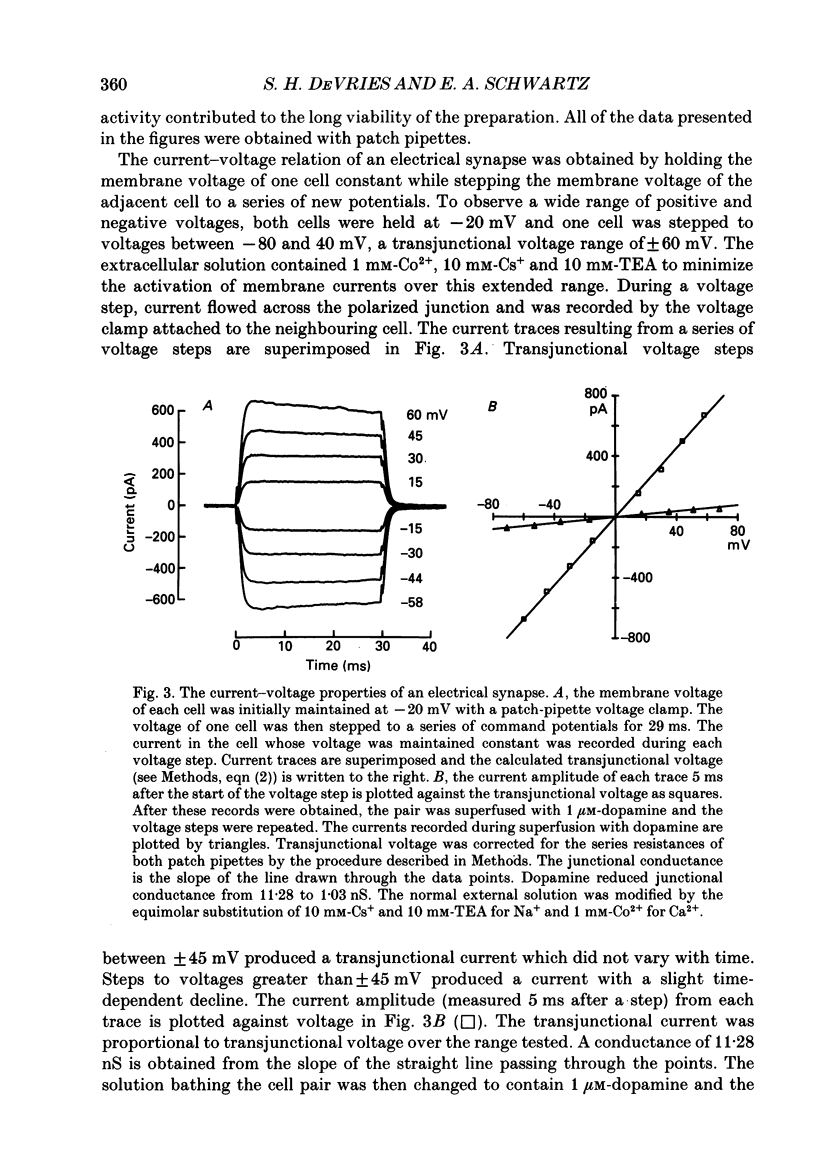






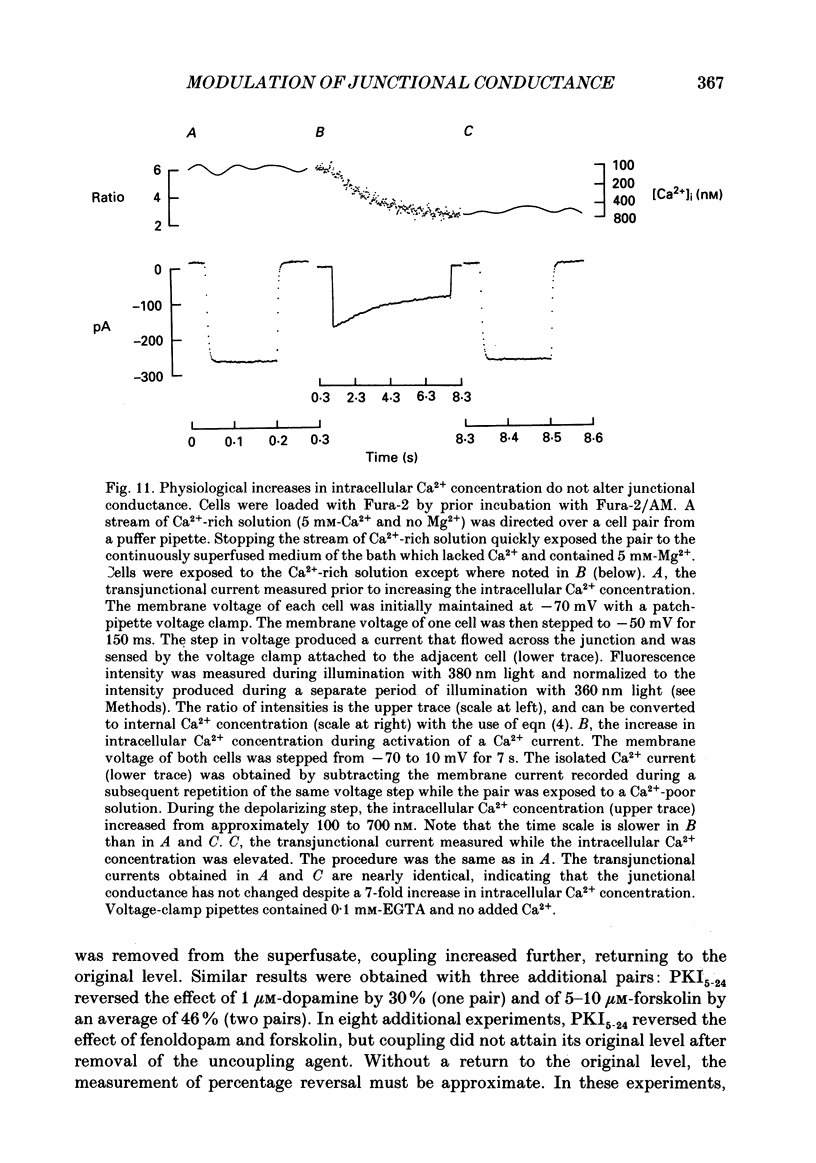








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3580–3583. doi: 10.1073/pnas.71.9.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M., Bruzzone R., Spray D. C., Regazzi R., Meda P. Cell uncoupling and protein kinase C: correlation in a cell line but not in a differentiated tissue. Am J Physiol. 1988 Nov;255(5 Pt 1):C699–C704. doi: 10.1152/ajpcell.1988.255.5.C699. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Receptors and cell activation associated with pancreatic enzyme secretion. Annu Rev Physiol. 1986;48:103–117. doi: 10.1146/annurev.ph.48.030186.000535. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Cheng H. C., Kemp B. E., Walsh D. A. Differential and common recognition of the catalytic sites of the cGMP-dependent and cAMP-dependent protein kinases by inhibitory peptides derived from the heat-stable inhibitor protein. J Biol Chem. 1986 Sep 15;261(26):12166–12171. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hahn R. A., Wardell J. R., Jr, Sarau H. M., Ridley P. T. Characterization of the peripheral and central effects of SK&F 82526, a novel dopamine receptor agonist. J Pharmacol Exp Ther. 1982 Nov;223(2):305–313. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the effect of carbon dioxide, ammonium chloride and acetylcholine on intercellular communication. J Physiol. 1979 Jun;291:317–326. doi: 10.1113/jphysiol.1979.sp012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Spray D. C., Saez J. C., Bennett M. V. Determination of synaptic phenotype: insulin and cAMP independently initiate development of electrotonic coupling between cultured sympathetic neurons. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6235–6239. doi: 10.1073/pnas.81.19.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P. J., Smith K., Angelides K. A comparative analysis of glial and neuronal markers in the retina of fish: variable character of horizontal cells. J Comp Neurol. 1985 Jul 8;237(2):264–272. doi: 10.1002/cne.902370210. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Meda P., Bruzzone R., Chanson M., Bosco D., Orci L. Gap junctional coupling modulates secretion of exocrine pancreas. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4901–4904. doi: 10.1073/pnas.84.14.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K., Garraway N. R. Morphological and functional identifications of catfish retinal neurons. I. Classical morphology. J Neurophysiol. 1975 Jan;38(1):53–71. doi: 10.1152/jn.1975.38.1.53. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J Physiol. 1986 Aug;377:283–295. doi: 10.1113/jphysiol.1986.sp016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987 Jan;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Witkovsky P., Trimarchi C. Dopaminergic mechanisms underlying the reduction of electrical coupling between horizontal cells of the turtle retina induced by d-amphetamine, bicuculline, and veratridine. J Neurosci. 1987 Aug;7(8):2273–2284. [PMC free article] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Fischer E. H., Demaille J. G., Krebs E. G. Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4379–4383. doi: 10.1073/pnas.82.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Fujita M., Saez J. C., Choi H., Watanabe T., Hertzberg E., Rosenberg L. C., Reid L. M. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987 Jul;105(1):541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol. 1983 Dec;345:329–351. doi: 10.1113/jphysiol.1983.sp014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci. 1984 May;4(5):1271–1280. doi: 10.1523/JNEUROSCI.04-05-01271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]



