Abstract
Calcium carbide (CaC2) is generally used as an artificial ripening agent in the agricultural and food sectors but has been prohibited due to its negative impacts on the environment and human. Therefore, in the present study, a novel and cost-effective detection technique was developed for the detection of CaC2 using L-cysteine functionalized gold nanoparticles (AuNPs). In this work, a rapid, simple, environment friendly and portable colorimetric nanosensing assay was developed using two different sizes of L-cysteine functionalized gold nanoparticles (AuNP20 and AuNP40). The sensing strategy relied on the fact that the wherein introduction of CaC2 initiates aggregation, resulting in detectable color change (red to purple) that helps in detection of CaC2. The efficiency of the developed sensor was investigated on the artificially ripened mangoes and bananas for selective detection of CaC2. The characterization of synthesized AuNPs was done using Uv–vis spectroscopy, FTIR, particle size, HR-TEM, and XRD analysis. The developed and optimized nanosensing assay was effectively utilized to detect the presences of CaC2 in carbide treated mango and banana fruits. The 20 nm and 40 nm size and stability (−32.6 mV for 20 nm and −27 mV for 40 nm) of the developed AuNPs were confirmed by TEM analysis, particle size and zeta potential analysis. The application of gold nanoparticle-based sensing assay confirms the presence of CaC2 in carbide treated mango and banana fruits by turning their color from red to purple. The developed nanosensing assay showed good sensitivity and selectivity towards CaC2 with LOD of 5 × 10−3 mL−1, reinforcing the suggestion that, this sensing assay enables rapid detection of CaC2 in fruit samples without need of a complicated laboratory setup. This simple detection method has the potential to ensure food safety, public health concerns, international standards for fruits exports, and promotes sustainable ripening methods.
Keywords: Calcium carbide detection, Gold nanoparticles, L-cysteine, Colorimetric assay, Nanosensor
Graphical abstract
Highlights
-
•
Prohibition of calcium carbide (CaC2) as an artificial ripening agent in food samples.
-
•
Functionalization of gold nanoparticles (AuNPs) with L-cysteine.
-
•
Development of a rapid, simple, environment friendly portable colorimetric nanosensing assay.
-
•
Utilization of developed sensor for the detection of CaC2 in mango and banana fruits.
-
•
An assay with good sensitivity and selectivity towards CaC2 with LOD of 5 × 10−3 mL−1.
1. Introduction
Fruits and vegetables have a vital role in providing essential nutrients for human well-being [1]. They are being contaminated with harmful compounds either during their cultivation or during post-harvest processing, all to achieve economic profit [2]. Naturally, climacteric fruits such as papaya, sapota, guava, banana, etc. undergo natural ripening but can also be artificially ripened using synthetic ripening agents [3]. Farmers harvest the climacteric fruits at the pre-mature stage of ripening and then transport them to different markets, where they undergo artificial ripening using different artificial ripening agents [4,5]. Artificial ripening is done to make the ripening process uniform and faster using artificial ripening agents such as calcium carbide, ethylene, ethephon or ethrel, ethylene glycol, ethylene, and acetylene [6]. Calcium carbide (CaC2) is also known as “masala” and classified as a carcinogen, presents in natural rocks as black to grey in its purified form and possesses a garlic-like odor [7]. It is used as an artificial ripening agent and is among the most economically feasible methods for fruit ripening in comparison to alternative approaches. However, the adverse impacts on the environment and human health, due to the presence of hazardous residues in the fruits, have led to its prohibition in numerous nations worldwide [8,9]. The interaction of CaC2 with moisture is responsible for the production of acetylene, which is an artificial analogue of ethylene (naturally occurring ripening agent). It contains trace amounts of the more lethal elements arsenic (As) and phosphorus (P), which adversely affects food [[10], [11], [12]]. These residues and acetylene gas cause multiple health issues, including headaches, dizziness, fatigue, polydipsia, vomiting, mental disorientation, memory loss, cerebral edema, seizures, and chronic hypoxia [13]. The consumption of calcium carbide (CaC2) ripened fruits is harmful to human health, particularly the nervous system, as acetylene or carbide gas reduces the amount of oxygen supplied to the brain. CaC2 can lead to acute health effects, including visual impairment, lungs swelling, and throat, mouth and skin irritation. It contains arsenic and phosphorus, which can cause emesis, diarrhea, and dysphagia. Pregnant women are more susceptible, as it may results in miscarriage. The long-term health effects of CaC2 exposure can cause cancer and reproductive hazards, as well as bronchitis [14]. As per the Food Safety and Standards Regulations (FSSR), the use of CaC2 in the artificial ripening of fruits is prohibited (FSSR, 2011) due to its negative impacts on the environment and health [15]. However, numerous unorganized merchants continue to use CaC2 as an artificial ripening agent for the ripening of fruits. The explicit prohibition of utilizing acetylene gas in the process of ripening fruits is stated in the Prevention of Food Adulteration Act (44-AA) “No person shall sell, offer, expose for sale, or have in his/her premises for the purpose of sale fruits which have been artificially ripened by use of acetylene or carbide gas.” Therefore, the detection of fruits contaminated with CaC2 is an important challenge for the Food Safety and Standards Authority of India (FSSAI). As per established protocol, fruit samples are collected and then sent to accredited laboratories for further analysis using conventional methods; this is done to detect the presence of CaC2 in the fruit samples. These conventional techniques include high-performance liquid chromatography-mass spectrometry (HPLC-MS), gas-chromatography (GC), enzyme-linked Immunosorbent assay (ELISA) [16], electrochemical methods [2], photo-acoustic spectroscopy, data processing and optical techniques [17], elemental composition analysis (EDX) (Preethi et al., 2019), spectroscopic methods such as near-infrared, mid-infrared, and laser-Raman spectroscopy [7,18], IR camera with image processing [19], inductively coupled plasma mass-spectrometry (ICP-MS) [10], headspace solid-phase micro extraction gas chromatography-mass spectrometry (HS-SPME-GC-MS) [4]. These techniques face several challenges and draw backs, including high cost, labor-intensive, complex, and inappropriate for on-site analysis [20]. Therefore, to overcome the challenges of conventional techniques, the colorimetric sensing method is one of the easiest and most affordable methods for on-site detection of calcium carbide [21,22]. Different types of nanomaterials that possess distinctive properties at the nanoscale are frequently integrated into nanosensors and can be used to fabricate the colorimetric nanosensor for detection [23]. Nanosensors utilize a variety of sensing mechanisms to detect and convert the target stimulus into observable signals. These mechanisms may involve optical, electrical, magnetic, thermal, or mechanical principles [24]. Gold nanoparticle (AuNPs) are very stable nanomaterials with a particle size ranging between 1 and 100 nm and due to their exceptional and size-dependent electronic and optical properties, colorimetric tests based on AuNPs have recently received a huge amount of research attention [25,26]. AuNPs-based sensing methods can be used for the detection of pesticides, heavy metal ions (Pb2+, Ni2+, Zn2+, Hg2+, Cd2+,Cr3+, Fe3+, and As3+), and biological molecules such as quinalphos (QP), amyloid-β oligomers, histidine, tyrosinase, insulin, spermine, and spermidine [27]. One of the most distinctive characteristics of these nanoparticles is their substantial surface plasmon resonance (SPR) absorption, which exhibits extinction coefficients ranging from 10−8-10−10 m−1 cm−1 in the visible wavelength range resulting in a reduction in detection time, a high throughput, and a high level of sensitivity in the proposed detection methods [[28], [29], [30]].
This work focuses on the ability of AuNPs to produce visually distinguishable color transition from red-wine to purple because of the "non-aggregation" and "aggregation" detection mechanism with surface plasmon resonance (SPR) band shift, which is induced by the formation of either covalent or non-covalent bonds with the target analyte. Due to regulatory measures, the use of CaC2 is prohibited in India and other countries because of its health effects, one of the major challenges that the law enforcement agencies, such as FSSAI have observed is that the conventional method for identifying artificially ripened fruits using CaC2 is to collect fruit samples and send them to the laboratories for chemical analysis. However, this approach is difficult, sophisticated, expensive, labor-intensive, and unsuitable for on-site detection. Moreover, the colorimetric nanosensing assays are used for real-time analysis of artificially ripened fruits at low-cost with improved sensitivity, mobility, and selectivity. Additionally, these assays have the potential to be commercialized and miniaturize the electrochemical biosensors. The present research focuses on the development of a portable sensor for the detection of CaC2 in artificially ripened fruits on the spot. This approach focused on the development of a colorimetric nanosensing assay using gold nanoparticles (AuNPs) of different diameters 20 nm (AuNP20) and 40 nm (AuNP40) functionalized with L-cysteine. However, the practical application of this colorimetric nanosensing assay on artificially ripened fruits (banana and mango) showed that CaC2 can be visually detected at 50 ppm. The specificity of the assay was examined using potentially interfering artificial ripening agents, such as potassium sulphate, potassium dihydrogen orthophosphate, and potassium sulphate.
2. Materials and methods
2.1. Chemical and reagents
All chemicals utilized in this research study were of analytical or high-performance liquid chromatography (HPLC) grade. Gold (III) chloride trihydrate (HAuCl4.3H2O, ≥99.9 % trace metal basis), L-cysteine (C3H7NO2S, ≥97 %), sodium citrate tribasic dehydrate (C6H5Na3O7.2H2O, ≥99 %), phosphate buffer saline (PBS, pH 7.4), and calcium carbide (CaC2, granulated, technical, ≥75 %) were procured from Sigma-Aldrich (Missouri, Germany).
2.2. Synthesis of gold nanoparticles (AuNPs)
The gold nanoparticles (AuNPs) with diameters of 20 nm (AuNP20) and 40 nm (AuNP40) were synthesized using a chemical citrate reduction method proposed by Thakkar et al. [31] with minor modifications. Gold (III) chloride trihydrate (HAuCl4.3H2O) was dissolved in ultrapure water (18.2 MΩ cm) to make a 5.0 mM HAuCl4 solution. Then, 1 mL aliquot of the prepared HAuCl4 solution was added to 18 mL of boiling ultrapure water, followed by additions of 1000 μL and 365 μL of sodium citrate tribasic dehydrate solution (0.5 % w/w) to synthesize AuNP20 and AuNP40. The gold colloidal solution was heated until turning yellow to red/wine red and allowed to cool up to 22 °C and make up a volume of up to 20 mL using ultrapure water with a final concentration of 0.25 mM. The synthesis of AuNPs is indicated by the color changing from yellow to red and the presence of CaC2 was further detected using these synthesized AuNPs. The size of the nanoparticles was verified by high resolution-transmission electron microscopy (HR-TEM).
2.3. Surface functionalization of AuNPs
The AuNPs must be functionalized with recognition elements that interact specifically with the CaC2 in order to obtain excellent selectivity for CaC2 detection. The AuNPs (AuNP20 and AuNP40) were individually functionalized by continuously stirring a solution containing 10 μL of 12 μM L-cysteine per 2 mL of AuNP solution for 2 h at 25 °C. The functionalized nanoparticles (AuNP20 and AuNP40) were mixed in a 1:1 ratio and centrifuged at 4000×g for 30 min in order to remove excess L-cysteine, and the solution was then re-suspended in 1 mL ultrapure water before experimentation. The centrifugation was done to improve the quality and purity of functionalized AuNPs. The purified functionalized AuNPs exhibited prolonged stability and did not aggregate. It was done to separate the L-cysteine functionalized AuNPs from the bulk of unbounded AuNPs as the functionalized AuNPs deposited in the lower portion of the centrifuge tube. The obtained functionalized nanosensing solution was allowed to be stored in refrigerated (4 °C) condition for further analysis.
2.4. Characterization
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR) analysis
FTIR analysis was performed to examine the chemical characteristics of the interaction between gold (Au) and L-cysteine. FTIR analysis was carried out using (Alpha Bruker, Germany) equipped with an ATR device at the spectral wavelength range of 400–4000 cm−1 [32]. All the spectra were obtained by performing 24 scans per spectrum, with a resolution rate of 4 cm−1.
2.4.2. UV–visible spectrophotometer
The UV–visible spectrophotometer was used to measure the absorbance maxima of the L-cysteine functionalized AuNPs. The analysis was performed using a UV-2450, 75 Shimadzu, Japan instrument with 10 mm path length and 400–800 nm wavelength range. Quartz cuvettes were used for analysis and a sample aliquot of 1.5 mL was placed in the sample light path and distilled water was placed in the reference light path and analyzed between 400 and 800 nm wavelength range at a scan speed of 800 nm/min and a read interval of 1 nm.
2.4.3. Analysis of particle size and zeta potential distribution
Dynamic light scattering (DLS) also known as photon correlation spectroscopy or quasi-elastic light scattering and is used to determine the average size and size distribution of the AuNPs [33]. The AuNPs were also evaluated using the phase analysis electrophoretic light scattering (PALS) method for zeta potential. The particle size (Z-average), polydispersity index (PDI) and zeta potential of the L-cysteine functionalized AuNPs were determined using a Zetasizer (Nano-ZS, Malvern Instruments, UK) at room temperature with 173° and 90° scattering angles using a disposable cuvette with 1 cm of path length. The cumulant approach was used for data analysis and the mean diameter of the nanoparticles was reported as the Z-average value [34]. The Electrophoretic light scattering (ELS) method was followed to measure the zeta potential and the sample was allowed to equilibrate at room temperature for 5 min before measurement. Zeta potential capillary cuvettes (DTS1070) were used for measurement stability of L-cysteine functionalized AuNPs that contained a pair of parallel Pd electrodes responsible for an electrical trigger to charged particles. The average value of particle size (nm), polydispersity index and zeta potential (mV) of the AuNPs were reported as results.
2.4.4. Transmission electron microscopy (TEM)
Morphology, shape, size, particle distribution and selective area electron diffraction (SAED) of the synthesized AuNPs were analyzed by the high resolution-transmission electron microscopy (HR-TEM) using a JEOL HRTEM -200KeV instrument. TEM pictures of the AuNPs were captured using an ultrahigh-resolution pole piece. The AuNPs were ultrasonicated for 15–20 min and then the solution of synthesized AuNPs (5 μL) was pipetted onto a copper grid (300 mesh) and allowed to vacuum dry at room temperature. The material was vacuum dried and observed in an HR-TEM at different resolutions (10 nm, 20 nm, 100 nm, 200 nm, and 500 nm). The average diameter of AuNPs, size distribution, and standard distribution were manually computed using the average of 100 AuNPs in the TEM pictures and the HR-TEM image of each sample was captured.
2.4.5. X-ray diffraction (XRD)
The crystalline structure, phase nature, purity, lattice parameters, and crystalline grain size could be determined using XRD scans [35]. The X-ray diffraction scans were obtained from Bruker D2 phase X-ray diffractometer using CuK radiation of 1.54056A.
2.5. Colorimetric sensing assay format
About 200 μL carbide solution prepared in 0.01M PBS buffer was mixed with 200 μL L-cysteine functionalized AuNPs (pH 5) to obtain a colorimetric signal for solution-based detection of CaC2. The CaC2 and AuNPs mixture was incubated for 5 min at room temperature. The visual change in color from wine red to purple in the sensing assay in the presence of carbide solution was observed, and the UV–Vis absorption was subsequently determined.
2.6. Real-time application of colorimetric sensing assay
Unripened mangoes and bananas were used in this study as the sample model for real-time detection, and both fruits were collected from the local market of Delhi. First, these samples were washed using distilled water and then air dried to remove the impurities on the surface. Then, these samples were artificially ripened using 2 different methods, dipping in 1 % CaC2 solution and putting a sachet of 1g of CaC2 then storing them in a closed chamber in the dark at room temperature for 72 h. After 2 days, fruits were taken out to check the presence of CaC2 on the fruit surface. CaC2 was detected by the swabbing method in which a wet cotton swab was taken from the fruit surface and allowed the analyte to absorb into the cotton plug. After swabbing all the exposed areas, the cotton plug was squeezed into a vial containing the L-cysteine functionalized AuNPs solution for colorimetric detection of CaC2 in a real sample. To show the actual application of the developed sensor for CaC2 detection, CaC2 in banana fruit peels was monitored in real-time (Fig. 12).
Fig. 12.
An illustrative description of real-time detection of CaC2 using L-cysteine functionalized AuNPs colorimetric sensing assay on the carbide ripened fruit (a) banana and (b) mango.
2.7. Statistical analysis
The mean and standard deviation of three independent measurements were used to represent all the data. Microsoft Excel, ImageJ, and Origin Pro 8.5 were used to statistically analyze the data from several tests, including size assessments (DLS and TEM).
3. Results and discussions
3.1. Synthesis of size controlled AuNPs
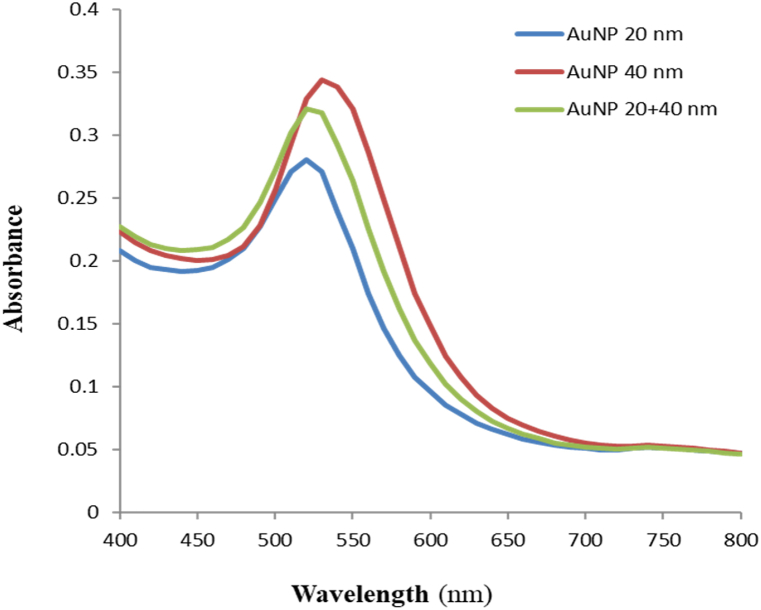
The quality and quantity of synthesized AuNPs are significantly influenced by various factors, including the synthesis method, temperature, pH, time, particle size, shape, environment, dispersity, stability, and pressure of the colloidal solution [36]. The amount of reducing agent used to reduce the gold during the synthesis determines both size and color. The reducing agent should be introduced as quickly as feasible to get monodispersity in AuNPs [37]. The reducing agent donates electrons to the oxidizing agents in order to reduce gold and silver salts to take place. Citrate concentration is inversely proportional to the size of nanoparticles, higher concentration of citrate leads to the formation of small size nanoparticles but with coalescence and fusion between the particles. A lower concentration of citrate leads to the formation of bigger sized nanoparticles due to aggregation of gold atoms [38]. The results revealed that the addition of a reducing agent (sodium citrate tribasic dehydrate) is responsible for the color change from light yellow to red to wine red in AuNP20 and AuNP40 (Fig. 1). The UV–vis spectra of synthesized AuNP20 and AuNP40 showed localized surface plasmon resonance (LSPR) peaks at 510 nm and 529 nm, respectively (Fig. 1). The synthesis of AuNPs was easily detected by a change in the color of the solution. AuNPs remained uniformly distributed in wine-red color in the absence of CaC2, but in the presence of CaC2, the AuNPs aggregated, and the colloidal solution changed into a purple colored solution that can be seen visually. This visible detection relies on the well-known metal-ligand coordination, where the ligand functions as a donor and the metal as an electronic acceptor, respectively [39]. The size, shape, and refractive index of AuNPs are among the parameters that influence their SPR absorption wavelength; hence, variations to these characteristics may result in colorimetric changes [40].
Fig. 1.
Photographic image and UV–vis spectra of synthesized AuNP20 nm and AuNP40 nm.
3.2. FTIR analysis
The FT-IR study was conducted to verify the functionalization of AuNPs with L-cysteine. A strong widening peak at spectra 3328 cm−1 appeared, which correspondences with the presence of hydroxyl group (O-H) (Fig. 2c, d, e). The FT-IR spectra of the AuNPs showed a shift and broadening of the bands from 2106 cm−1 to 2251 cm−1, which corresponds to C ≡ N stretching due to the presence of nitrile compounds (Fig. 2c, d, e). The spectra peak assigned at 1633 cm−1 showed the presence of the carbonyl group (C=O stretching) (Fig. 2c, d, e). The spectra peak at 2250 cm−1 corresponds to the S-H group and N-H group at 3194 cm−1 (Fig. 2b), indicating the presence of L-cysteine and their compatibility with gold metal (Fig. 2a). Furthermore, C=O, cysteine and spectra peaks at 1623, 2129, and 3300, respectively (Fig. 2c, d, e) were observed to be consistent with a slight shift of C ≡ N group in the FT-IR spectra of the modified gold nanoparticles (Fig. 2a). The present results are in good accordance with the previous findings [[41], [42], [43]]. AuNPs can be functionalized with functionalizing agents such as ligands, therapeutic agents, DNA, amino acids, proteins, peptides, and oligonucleotides, oligo or polyethylene glycol (PEG), antibodies, receptors, and the metal ions which are expected to bind the molecules of amino acids through cooperative metal ligand interaction [44]. It is anticipated that amino acids could interact with metal ions through cooperative metal-ligand interactions [45]. Cysteine has the mercapto group (-SH) and because of its sulfhydryl (thiol) side chain, cysteine has a very high affinity for gold which allows -SH groups to form bonds with AuNPs through Au-S bonding [46]. This property promotes the bonding of Cys-containing peptides and proteins to metal surfaces. They have been used to link proteins to gold surfaces by crosslinking them. These modified AuNPs are more stable at high ionic strength levels than bare due to stronger steric and hydration-based interparticle repulsions. These adsorbed ligands can be readily displaced by various compounds that strongly react with gold which enhances the binding of peptides and proteins to the surface of gold. The aggregation of AuNPs caused by Cys is typically attributed to the establishment of zwitterionic networks, which arise from the head-to-head interactions between the deprotonated carboxylate (COO−) and protonated amine (NH3+) groups of one L-cysteine-AuNP and the corresponding groups of AuNPs containing L-cysteine adsorbed on neighboring particles [47]. The biological significance of cysteine has led to the development of several sensors such as surface plasmon optical fiber sensor [48], electrochemical sensor [49], fiber-optic chemosensor, nanoparticles-based colorimetric sensor [[50], [51], [52]], and fluorescent sensor [53,54], many of which frequently use AuNPs for detection. L-cysteine functionalized AuNPs can be used to detect heavy metals ions, including cadmium ion [50], Hg2+ [55], and copper (II) [56], pesticide such as cypermethrin [57], amino acid like tyrosine [58], bacteria like E. coli [59], and acetone [60]. The surfaces of AuNPs underwent three rounds of washing to remove any additional thiol-rich bio-receptors and were individually functionalized to prevent undesirable interactions.
Fig. 2.
FT-IR spectra of surface functionalized gold nanoparticles. (a) L-cysteine of AuNP20 nm + AuNP40 nm, (b) L-cysteine, (c) AuNP20 nm + AuNP40 nm, (d) AuNP40 nm, and (e) AuNP20 nm.
3.3. Characterization of gold nanoparticles
3.3.1. Uv–vis spectroscopy
Uv–vis spectroscopy is the most important technique to examine the optical properties of synthesized nanoparticles. The Uv–vis spectra of L-cysteine functionalized AuNPs are shown in Fig. 3. The Uv–vis spectra of AuNP20, AuNP40 and AuNP20 +AuNP40 (mixed in 1:1 ratio) were taken after 15 min of synthesis. The localized surface plasmon resonance (LSPR) peaks for AuNP20, AuNP40 and AuNP20 +AuNP40 were observed at 510 nm, 529 nm, and 520 nm with absorbance of 0.28, 0.34, and 0.32, respectively. The greatest absorption peak of the synthesized AuNPs is seen around 510–530 nm, which is in agreement with previous findings for AuNPs that indicated absorption maxima between 510 and 550 nm [61]. The size distribution range is correlated with the absorption peak and the breadth of the absorption spectra as particle size increases. Gold nano colloidal typically exhibits a single absorption peak in the visible region between 510 and 550 nm due to the SPR. When the ligand functionalized AuNPs connect to their target, the SPR peak broadens and shifts, resulting in a colorimetric response [20]. According to Jeon et al. [62], the size, three-dimensional (3D) structure, and refractive index (RI) of the surrounding media are all important factors that affect the LSPR of AuNPs. The plasmonic responses of nanoparticles are affected by surrounding refractive index, size, and shape, resulting in AuNP aggregation [63]. Thus, pH changes can influence AuNPs' chemical and physical structure (size and shape), affecting their dielectric environment and surface charge [64]. Increased reaction time decreases solution pH, hence increasing AuNP size [65]. Increasing the reaction temperature speeds up synthesis but produces larger AuNPs [66]. The LSPR peak shifted toward the shorter wavelength region as pressure increased, AuNPs reduced in size and density, and they remained isolated [67]. Localized surface plasmon resonance (LSPR), the collective effect of free electron oscillations near the metal surface, gives AuNPs their electrical, magnetic, and optical properties. The SPR peak depends on AuNP form, size, dispersity, and environment. Changes in the SPR band can reveal the functionalization of AuNPs and their particle interactions. Functionalization of nanoparticles has changed their surface dielectric constant, shifting the band maxima [68].
Fig. 3.
UV–Vis spectra of the synthesized AuNP20 nm, AuNP40 nm and AuNP20 nm + AuNP40 nm.
3.3.2. Particle size and zeta potential
The synthesized AuNPs are in a moderate polydispersity state, as the PDI values for AuNP20 nm and AuNP40 nm were 0.359 and 0.392, respectively (Fig. 4a and b). PDI describes the degree of non-uniformity of a size distribution of synthesized AuNPs, therefore, a wide variety of particle sizes possess higher PDI values, whereas samples with uniform particle sizes have lower PDI values [69]. The PDI values range from 0.0 (monodispersed nanoparticles) to 1.0 (highly polydispersed) and PDI values for aggregated nanoparticles with broad particle size distribution were generally greater than 0.5 [70]. The measured PDI values of AuNP20 and AuNP40 were 0.359 and 0.392, respectively, which indicate a broad particle size distribution and classified as medium polydispersity, reflecting a satisfactory level of moderate homogeneity. Despite the presence of a tiny secondary peak associated with agglomeration, the primary focus is on the first peak to assess particle size; hence, the agglomeration peak will be neglected [71]. A sample is classified as polydisperse if it is larger than 0.4, moderately polydisperse if it is between 0.1 and 0.4, and monodisperse if it is less than 0.1 [72]. As reported earlier, the PDI increases as the particle size increases and the results are in accordance with the previous findings [73] (Fig. 5a and b), and indicate zeta potential for AuNP20 nm and AuNP40 nm as −32.6 ± 7.92 mV and −27.6 ± 6.81 mV, respectively, which shows that the AuNPs are moderately stable. The large negative charge on the surface of both AuNPs indicates the presence of a citrate molecule that provides electrostatic stability to AuNPs [74]. Therefore, anions are adsorbed onto the surface of nanoparticles, forming a negative protective layer that provides stability and prevents the AuNPs from aggregation [75]. Zeta potential values ranging from 0 to ±5 mV indicate rapid agglomeration and precipitation in a suspension, values between ±10 and ± 30 mV indicate the onset of instability, while values between ±30 and ± 40 mV indicate considerable stability and ±40 to ±60 mV indicate a high level of suspension stability. In the formulation and suspension stability study, the surface charge is measured using the zeta potential [71]. The nanoparticles possess zeta potential greater than +30 mV or less than −30mV and are considered a stable system that prevents aggregation [76]. The dispersion stability of AuNPs is affected by several factors, including solution temperature, pH, salt concentration, ionic strength, electrolyte type, and protein adsorption [77]. Therefore, AuNPs are frequently maintained in a citrate solution or functionalized with thiolated compounds to achieve dispersion stability [78]. The hydrodynamic sizes (Z-average ± S.D.) of AuNP20 nm and AuNP40 nm were 23.87 ± 10.16 nm and 28.88 ± 13.42 nm, respectively. The Stokes-Einstein equation is used to calculate the hydrodynamic size of AuNPs by considering the diffusivity of the particles in suspension. In DLS, the hydrodynamic radius (RH) for the spherical nanoparticles can be determined from the diffusion coefficients using the Stokes-Einstein equation: Df = kBT/(6πηRH), where kB represents the Boltzmann constant, T denotes the temperature of the suspension, and η signifies the viscosity of the surrounding medium [79]. This equation is used to calculate the hydrodynamic size of AuNPs by considering the diffusivity of the particles in suspension. However, this equation takes into account both the gold core and the surface charge that exist in the stern layer of the particles [80].
Fig. 4.
Particle size analysis of (a) AuNP20 nm and (b) AuNP40 nm.
Fig. 5.
Zeta potential results of (a) AuNP20 nm and (b) AuNP40 nm.
3.3.3. TEM analysis
Transmission electron micrographs of the gold nanoparticles (AuNPs 20 nm, AuNPs 40 nm and AuNPs 20 + 40 nm) are shown in Fig. 6. The results provide comprehensive details on the size, morphology and crystallinity of the synthesized gold nanoparticles. TEM images of the AuNPs synthesized using two distinct concentrations of sodium citrate tribasic dehydrate were responsible for the synthesis of two different sizes of AuNPs. ImageJ software was used to calculate the size of synthesized AuNP20 and AuNP40 and the calculated mean sizes were 24.58 nm and 44.30 nm, respectively. When the concentration of C6H5Na3O7.2H2O was high (1000 μL) monodispersed, isotropic and spherical nanoparticles in the size range of 20–25 nm AuNPs were produced. The clear separation and luminous hue present on the surface of AuNPs indicate the presence of L-cysteine which might act as a capping substance to stop the agglomeration. Similarly, AuNPs synthesized with low concentration (365 μL) of C6H5Na3O7.2H2O produced moderately polydispersed and anisotropic AuNPs with a size range of 40–45 nm in rod and spherical shape. The electron micrograph of mixed AuNPs (1:1 ratio) showed heterogeneous distribution and small-sized AuNPs were arranged around the large-sized AuNPs (Fig. 6c). The spectroscopic measurements of the AuNPs (increase in SPR peak from 510 to 530) and the TEM images are corroborated with DLS findings. The concentration of C6H5Na3O7.2H2O used in the synthesis of AuNPs is inversely proportional to the size of AuNPs. A single gold nanoparticle was investigated using the selected area electron diffraction (SAED) analysis to determine the single crystalline nature of AuNPs in the face-centered cubic (FCC) phase [81]. The Scherer ring pattern, which consists of sparkling spots, represents the FCC gold crystalline structure of AuNPs [82]. The bright circular ring in the SAED pattern of AuNP20 correspondences to (111), (200), (220), (311) and (222) planes which represents the monocrystalline nature and the partial concentric rings in the SAED pattern of AuNP40 and AuNP20+AuNP40 strongly indicate the polycrystalline structure of the particles (Fig. 6b and c).
Fig. 6.
High resolution-transmission electron microscopy (HR-TEM) images and selected area diffraction (SAED) pattern of (a) AuNP20 nm (b) AuNP40 nm and (c) AuNP20 + AuNP40.
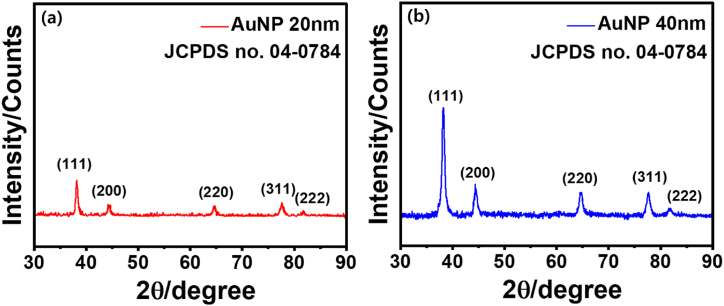
3.3.4. XRD (X-ray diffraction) analysis
The AuNPs identified by the XRD pattern displayed Bragg reflections, which are well exhibited by the face-centered cubic (FCC) gold nanostructures [83]. The properties of synthesized AuNPs were determined by comparing the obtained diffractogram with Joint Committee on Powder Diffraction Standards (JCPDS) file (JCPDS no. 04–0784) [84]. Results revealed that the 111 facet of the FCC structure is thought to make up a very high diffraction peak at 38° compared to typical AuNPs [85]. XRD patterns for both AuNPs are similar because the structural properties are same except their size. As the size of the nanoparticle increases the intensity of peaks also increases. The XRD spectra of AuNPs 40 nm showed similar pattern of AuNPs 20 nm with a (111) main diffraction peak. As expected, the peak intensity increased as the layer thickness increased (Fig. 7a and b). These peaks were identical to the typical Bragg reflections (111), (200), (220), (311), and (222) of FCC lattice. The strong diffraction at peak 38.1° demonstrates zero-valent gold's preferred growth orientation which was fixed in the (111) direction. This represents molecular-sized solids of equivalent spacing between each atom and molecule in a repeating 3D pattern. Pure gold nanocrystals typically have XRD patterns similar to those of AuNP20 and AuNP40 and the results correspond to SAED analysis [102].
Fig. 7.
XRD analysis of AuNPs. (a) 20 nm and (b) 40 nm.
3.4. Sensing assay for CaC2 detection
The presented formulation of an AuNP-based chemical sensor was used to detect the CaC2. Upon adding CaC2 to the sensing solution, the color of the solution changed from red wine to purple which is a colorimetric signal for real time application. The nanosensing assay was developed by optimizing the impact of different combinations of AuNP20 and AuNP40 nm, the overall concentration of L-cysteine, and the pH in presence of CaC2 using Uv–vis spectroscopy. Single-sized AuNPs solution (AuNP20 nm or AuNP40 nm) and a mixed solution (1:1) of AuNP 20 + AuNP40 were used in sensing of CaC2 in a concentration range of 0–750 ppm and were recorded in Uv–vis spectroscopy. Based on this study, the optimal results were achieved by combining AuNP20 and AuNP40 nm in 1:1 (v/v) ratio solution, as the second peak at 640 nm was observed in the minimum amount of time. The 520 nm peak reduced but the 640 nm peak developed gradually, representing the aggregation of nanoparticles from their dispersed state. However, in case of single-sized AuNPs (AuNP 20 nm and AuNP40 nm) minimal variations were observed, suggesting that AuNPs were randomly aggregating [86]. The ideal ratio of AuNP20 nm was projected to match the quantity required for entirely covering the surface of AuNP40 nm. A particle number ratio of approximately 40:1 for both the sizes of AuNPs can be inferred from the diameters of the AuNPs in a 1:1 (v/v) solution of AuNP20 and AuNP40 nm. Therefore, the nanosensing assay comprises a mixture of AuNP20 and AuNP40, exhibiting a red-wine color, which indicates that the nanoparticles are in a dispersed form. The LSPR peak of developed nanosensing assay solution occurs between the peaks of AuNP20 nm and AuNP40 nm (Fig. 3). The nanosensing assay comes into contact with CaC2, which contains arsenic (As) as a residue. This leads to formation of nano-assemblies of nanoparticles at the molecular level. These assemblies pre-concentrate more As ions than single-sized AuNPs, forming a network, and change the color of the solution from red to purple which reflects aggregation. Therefore, this method produces a greater number of active sites for As ion binding and significant signal production, compared to conventional single-sized AuNP-mediated biosensing [31]. Carboxylic and hydroxyl groups present in sodium citrate assembled around the AuNPs responsible for negatively charged citrate layers. The AuNPs remain dispersed in the colloidal solution due to electrostatic force which causes repulsion and prevents aggregation of AuNPs. Upon functionalization of AuNPs with L-Cysteine, the -SH group on the side chain of L-cysteine forms covalent bond (Au-S) and binds with AuNPs [87]. The carboxyl (-COO-) and amino (-NH2) groups were exposed and Au-S bond formed by ligand exchange reaction between AuNPs and L-cysteine. The L-cysteine functionalized AuNPs were used to detect CaC2 which contain arsenic ions (AS) as residue and this L-cysteine has two functions: at one end, Au-S bond is formed and at the other end, it binds with As ions (As-S or As-O linkage) [88,89]. Further, UV–vis spectroscopy was performed to detect the presence of CaC2. Fig. 8 indicates the UV–visible spectra of synthesized L-cystine functionalized AuNPs (L-cys-(AuNP20 +AuNP40)) in the absence of CaC2 and L-cys-(AuNP20 +AuNP40) and in the presence of CaC2 (L-cys-(AuNP20 +AuNP40) + CaC2). A very well-defined absorption band was seen in the UV visible absorption spectra of AuNPs solution at 520 nm. Additionally, the binding of CaC2 and AuNPs responsible for aggregation of AuNPs resulted in a red shift in the plasmon band energy and broadening of the SPR peak [57]. Due to reduced intensity, a new wider absorption peak appears in the UV–vis spectrum at longer wavelength in the 650 nm region due to the aggregation of AuNPs in the presence of CaC2, the red color peak indicated the aggregation of nanoparticles as shown in Fig. 8.
Fig. 8.
UV–Visible spectra of synthesized L-cysteine functionalized AuNPs (L-cys-(AuNP20 +AuNP40)) and L-cysteine functionalized AuNPs with calcium carbide (L-cys-(AuNP20 +AuNP40) + CaC2).
3.5. Limit of detection (LOD)
The limit of detection (LOD) is the lowest concentration of an analyte that can be reliably detected in a sample with a given probability (usually at 95 % confidence). The limit of detection (LOD) for CaC2 for this sensor was established by exposing them to a range of CaC2 concentrations, from 0 to 750 ppm. Fig. 9a shows the UV–Vis spectra of L-cysteine functionalized AuNP solution containing different concentrations of CaC2 after a mixed time interval of 5 min. After the addition of CaC2, the intensity of the SPR band of the AuNPs at 520 nm decreased, and a new absorption band appeared around 720 nm, indicating the successful conjugation of CaC2with the AuNPs. As shown in Fig. 9b, the color of the solution changed shifting from wine-red to purple at the same time. Therefore, the visual detection limit was estimated to be 50 ppm. To calculate the limit of detection (LOD) and limit of quantification (LOQ), the following formulas were used; LOD = 3.3 x S.D./m and LOQ = 10 x S.D./m, respectively, where S.D. is the value obtained from the absorption maxima of different reading of synthesized nanoparticles and m is the slope of the graph. The calculated value for concentration-dependent LOD and LOQ are 37.744 and 114.37, respectively (Fig. 9c). As the concentration of CaC2 increases from 250 to 750 ppm color changes from purple to red due to anti-aggregation. The aggregation of AuNPs was effectively suppressed in the presence of a mixture of ions and aggregation reagents, leading to significant spectrum and color changes [90,91]. The comparison between the pre-existing sensing method and the developed gold nanoparticle-based sensing assay in the present research for on-site detection of ripening agents in fruits and vegetables is depicted in Table 1.
Fig. 9.
(a and b) UV–Vis spectra and image of nanosensing assay based colorimetric detection of CaC2 at different concentrations at pH 5. (c) Linear fitting for concentration-dependent sensing and LOD and LOQ values.
Table 1.
Comparison of performance characteristics of fabricated nanosensor with the pre-existing sensing methods for the detection of artificially ripened fruits.
| S. No. | Sensing Methods | Agro products | Target analyte | LOD | Limitations | References |
|---|---|---|---|---|---|---|
| 1 | aDTNB based colorimetric sensing assay | Banana | CaC2 | 50 ppm | Highly pH sensitive | [17] |
| 2 | bLS-capped AUNPs based colorimetric assay | Mango | CaC2 | 50 ppm | Complicated handling | [92] |
| 3 | cPt/CeO2/AChE electrode based electrochemical sensor | Mango | CaC2 | 0.6 nM | Require on-site power source, pH and temperature sensitive, invasive and destructive |
[2] |
| 4 | eDHFIP-QCM based sensor (Aroma e-sense) | Mango | dDHF (artificial ripening biomarker) | 12 ppm | Indirect sensing | [93] |
| 5 | fNIR spectroscopy method | Mango | Carbide ripened fruit | – | Surface-sensitive and non-portable | [7] |
| 6 | Image processing based android application | Banana | Carbide ripened fruit | – | Low accuracy | [94] |
| 7 | gDy2O3 @AMB-IP based sensor | Mango | Ambroxol (artificial ripening marker) | 6.48 μM/L | Indirect sensing | [95] |
| 8 | Thiol functionalized polydiacetylene film based colorimetric sensor | Kiwi | Ethylene | 600 ppm | Low sensitivity | [96] |
| 9 | hAgBF4/PVP coated QCM composite based piezoelectric sensor | Pear, orange and banana | Ethylene | 420 ppb | Expensive, Labor intensive for fabrication |
[97] |
| 10 | iIR thermal emission based sensor | Mango and banana | Ethylene | 5 ppm | Expensive | [98] |
| 11 | Multispectral imaging | Banana | Differentiate natural and carbide ripened fruits | – | Low accuracy | [99] |
| 12 | jMATLAB based Image processing technology | Mango and Banana | Carbide ripened mango | – | Low accuracy | [100,101] |
| 13 | L-Cysteine Functionalized AuNPs based colorimetric nanosensing assay | Mango and Banana | CaC2 | 50 ppm | Cost effective, highly stable, rapid, high selectivity, high accuracy at low concentrations of ripening agents, portable and user friendly. | Present work |
DTNB = 5,5’ -dithiobis-(2- nitrobenzoic acid).
LS = Lauryl Sulphate.
Pt/CeO2/AChE = (Platinum/Ceria/acetylcholinesterase).
DHF = 2,5-Dimethyl-4-hydroxy-3(2H)- Furanone.
DHFIP-QCM = DHF Imprinted Polymer-Quartz Crystal Microbalance.
NIR = Near Infrared Spectroscopy.
Dy2O3 @AMB-IP = Dysprosium oxide @Polyacrylamide cross-linked Ambroxol imprinted.
AgBF4/PVP-QCM = Silver Tetrafluoroborate/Polyvinylpyrrolidone-Quartz Crystal Microbalance.
IR = Infrared.
MATLAB = Matrix Laboratory.
3.6. Interference tests
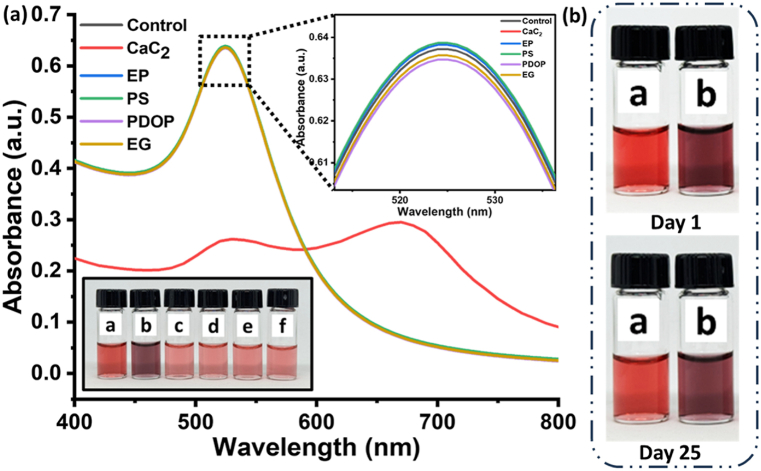
The selectivity of the sensor may be assessed using an interference test to evaluate the activity of molecular recognition. The specificity test of the colorimetric kit was done against some common artificial ripening agents (ARAs) such as ethylene glycol (EG), potassium dihydrogen orthophosphate (PDOP), ethephon (EP), and potassium sulphate [17] (Sonwal et al., 2023). These ARAs (100 ppm) were added to different vials containing nanosensing assay in a 1:1 ratio. A vial containing CaC2 changed to grey, and the color of other ARAs such as PDOP and ethephon changed into blue color and EG and PS remained as such (Fig. 10). Additionally, spectral analysis was also carried out for all interfering compounds (Fig. 10).
Fig. 10.
Interference test of nano sensing assay and Uv–vis spectra analysis with different ARAs. (a) Control (b) calcium carbide (c) ethephon, (d) ethylene Glycol, (e) potassium dihydrogen orthophosphate, and (f) Potassium sulphate.
In aspect to interference, ethephon and PDOP are not used at mass level to ripe the fruits. PDOP is not a fruit ripening agent, it is a flower blooming agent or fruit setting agent and was only used to check the interference in the present study. As it is not a fruit ripening agent, the interference of PDOP is not considerable in point of AFRA sensing. Furthermore, the interference is observed at a high concentration of all AFRAs (100 ppm) which is again a point of no consideration in aspect of sensing at low levels of analytes. Thus, to prove the developed sensor as a promising sensor for detection of AFRA, we performed the interference studies at lower concentrations which do not interfere at the LOD (50 ppm) (Fig. 11). Moreover, spectroscopic data depict the quantitative analysis of AFRAs and prove that there is no interference effect of ethephon and PDOP as there is no peak appeared for the interfering compounds ethephon and PDOP.
Fig. 11.
(a) Interference test of nanosensing assay at lower limit of detection concentration (50 ppm) (a) Control (b) Calcium carbide (c) Ethylene Glycol (d) Potassium dihydrogen orthophosphate (e) Potassium sulphate. (b) Time dependent degradation of color till 25 days.
Another set of experiments was performed to check the selectivity at lowest limit of detection concentration (50 ppm) and time-dependent degradation of color till 25 days. It was observed that none of the other ARAs showed any sensing signal apart from CaC2. Hence, the presented detection formulation is highly specific at a lower limit of detection concentration (50 ppm) (Fig. 11a) and time-dependent degradation of color showed good stability even after 25 days (Fig. 11b).
3.7. Application of colorimetric sensing assay and its validation
The result revealed that no color change was observed in controlled fruit wash (no treatment) and the wine-red colloidal solution of AuNPs changed into purple color upon adding real-time sample collected from artificially ripened fruits, which indicates the presence of CaC2 in both the carbide treated fruit samples (dipping and sachet). Fig. 12 (a, b) illustrates the complete real time detection of CaC2 from the surface of artificially ripened fruit peels (banana and mango).
4. Conclusion
Nano-biosensors are very rapid and sensitive tools for detecting harmful food contaminants in food and the environment. Colorimetric biosensors have caught the interest of several researchers in this field due to their sensitivity, applicability, handiness, affordability, and user-friendliness in comparison to traditional analytical methods. The future commercialization of colorimetric sensing techniques may fulfill the requirement for detecting food contaminants essential for food safety. In conclusion, an easy, sensitive and on-spot detection colorimetric kit was developed utilizing L-cysteine functionalized gold nanoparticles for the detection of calcium carbide in carbide-treated fruits commodities. In this study a rapid, simple, environment friendly and portable colorimetric nanosensing assay was developed using a mixture of different sizes of gold nanoparticle (AuNP 20 nm + AuNP 40 nm) and functionalized with L-cysteine. The sensing strategy relied on the fact that the addition of CaC2 will induce the aggregation of functionalized AuNPs, resulting in color change from red to purple. The characterization of synthesized AuNPs was conducted using UV–Vis spectroscopy, which exhibited a peak at 520 nm; Fourier Transform Infrared Spectroscopy (FTIR) confirmed the presence of L-cysteine on the AuNPs' surface; particle size analysis, High-Resolution Transmission Electron Microscopy (HR-TEM), and X-ray Diffraction (XRD) were used as well. The sizes and stability of the synthesized AuNPs were validated using TEM, particle size analysis, and zeta potential test. This method exhibits exceptional sensitivity and robust selectivity for CaC2, with a detection limit of 5 × 10−3 mL L−1. The developed and optimized nanosensing assay was effectively utilized to detect the presences of CaC2 in carbide treated mango and banana fruits, confirming that the developed colorimetric nanosensing assay changes color from red to purple in the presence of CaC2 (carbide-treated fruits), but no color change occurs in naturally ripened fruits. Our approach could offer fresh perspectives for developing highly sensitive and precise assays for the selective detection of target analyte and this is tested by its selectivity test.
CRediT authorship contribution statement
Aishwarya Dixit: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Sonam Sonwal: Writing – review & editing, Methodology. Ashutosh Upadhyay: Writing – review & editing, Validation, Supervision, Investigation, Funding acquisition. Vivek K. Bajpai: Writing – review & editing, Validation, Supervision, Methodology, Investigation. Yun Suk Huh: Writing – review & editing, Methodology. Shruti Shukla: Writing – review & editing, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the National Institute of Food Technology Entrepreneurship and Management, Kundli, Sonipat, Haryana-131028 for providing the research facility and infrastructure (NIFTEM-K communication number: NIFTEM-P-2024-67). First authors (Aishwarya Dixit) wish to express gratitude to the University Grant Commission (UGC-SRF) for providing funding for research work and fellowship.
Contributor Information
Ashutosh Upadhyay, Email: ashutosh@niftem.ac.in.
Vivek K. Bajpai, Email: vbiotech04@gmail.com.
Yun Suk Huh, Email: yunsuk.huh@inha.ac.kr.
Shruti Shukla, Email: shrutishukla@nehu.ac.in.
References
- 1.Patrick O.I., Rosemary A.I.O. Calcium carbide-induced alterations of some haematological and serum biochemical parameters of wistar rats. Asian J. Pharmaceut. Health Sci. 2016;6(1) https://ajphs.com/article/2016/6/1/1396-1400 [Google Scholar]
- 2.Ramachandra B.L., Gumpu M.B., Nesakumar N., Krishnan U.M., Rayappan J.B.B. Calcium carbide in mangoes: an electrochemical way for detection. Anal. Methods. 2016;8(23):4590–4599. doi: 10.1039/c6ay01314g. [DOI] [Google Scholar]
- 3.Umesh A.R., Venkatesh H.N., Manjunath K., Mohana D.C. Artificial ripening of fruits-misleading ripe and health risk. Everyman's Science. 2016;6:364–369. https://www.researchgate.net/publication/326207587_Artificial_ripening_of_fruits-misleading_ripe_and_health_risk [Google Scholar]
- 4.Vemula M., Shaikh A.S., Chilakala S., Tallapally M., Upadhyayula V. Identification of calcium carbide-ripened sapota (Achras sapota) fruit by headspace SPME-GC-MS. Food Addit. Contam. 2020;37(10):1601–1609. doi: 10.1080/19440049.2020.1794055. [DOI] [PubMed] [Google Scholar]
- 5.Kereth G.A., Lyimo M., Mbwana H.A., Mongi R.J., Ruhembe C.C. Assessment of post-harvest handling practices: knowledge and losses of fruits in Bagamoyo district of Tanzania. Food Sci. Qual. Manag. 2013;11:8–15. https://www.researchgate.net/publication/281297046_Assessment_of_postharvest_handling_practices_knowledge_and_losses_of_fruits_in_Bagamoyo_district_of_Tanzania [Google Scholar]
- 6.Lee Y., Yu M., Yen C., Tsay J., Hou C., Li P., Huang P., Liang Y. Exploitation of Post-Ripening treatment for improving cold tolerance and storage period of Jin Huang Mango. Horticulturae. 2024;10(1):103. doi: 10.3390/horticulturae10010103. [DOI] [Google Scholar]
- 7.Lakade A.J.V.V., Ramasamy R., Shetty P.H. NIR spectroscopic method for the detection of calcium carbide in artificial ripening of mangoes (Magniferaindica) Food Addit. Contam. 2019;36(7):989–995. doi: 10.1080/19440049.2019.1605206. [DOI] [PubMed] [Google Scholar]
- 8.Okeke E.S., Okagu I.U., Okoye C.O., Ezeorba T.P.C. The use of calcium carbide in food and fruit ripening: potential mechanisms of toxicity to humans and future prospects. Toxicology. 2022;468 doi: 10.1016/j.tox.2022.153112. [DOI] [PubMed] [Google Scholar]
- 9.Oladipupo A.R. The use of calcium carbide in fruit ripening: health risks and arsenic index as a quantitative marker for calcium carbide residue. 2022. www.pcbiochemres.com [DOI]
- 10.Hassan S., Mazhar W., Farooq S., Ali A., Musharraf S.G. Assessment of heavy metals in calcium carbide treated mangoes by inductively coupled plasma-mass spectrometry (ICP-MS) Food Addit. Contam. 2019;36(12):1769–1776. doi: 10.1080/19440049.2019.1671990. [DOI] [PubMed] [Google Scholar]
- 11.Islam M.N., Imtiaz M.Y., Alam S.S., Nowshad F., Shadman S.A., Khan M.S. Artificial ripening on banana (Musa Spp.) samples: analyzing ripening agents and change in nutritional parameters. Cogent Food Agric. 2018;4(1) doi: 10.1080/23311932.2018.1477232. [DOI] [Google Scholar]
- 12.Chandel R., Sharma P.C., Gupta A. Method for detection and removal of arsenic residues in calcium carbide ripened mangoes. J. Food Process. Preserv. 2017;42(2) doi: 10.1111/jfpp.13420. [DOI] [Google Scholar]
- 13.Asif M. Physico-chemical properties and toxic effect of fruit-ripening agent calcium carbide. Ann. Trop. Med. Publ. Health. 2012;5(3):150. doi: 10.4103/1755-6783.98602. [DOI] [Google Scholar]
- 14.Bhattarai U.K., Shrestha K. Use of calcium carbide for artificial ripening of fruits : its application and hazards. J. Food Sci. Technol. Nepal. 2005;1 https://biblio.ugent.be/publication/914672/file/930976.pdf [Google Scholar]
- 15.Food Safety and Standards (Prohibition and Restriction of Sales) Regulation, 2011 section 2.3.5. https://fssai.gov.in/cms/food-safety-and-standards-regulations.php.
- 16.Liu Y., Wan C., Ye B., Hao Y., Lan Y., Ouyang A. Rapid quantitative analysis of dimethoate pesticide using surface-enhanced Raman spectroscopy. Transactions of the ASABE. 2013;56(3):1043–1049. doi: 10.13031/trans.56.10009. [DOI] [Google Scholar]
- 17.Sonwal S., Shukla S., Alhammadi M., Umapathi R., Sudhani H.P.K., Cho Y., Huh Y.S. Colorimetric sensing of calcium carbide over banana peels using 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) as a rapid chemoreceptor: a point of care tool for food fraud analysis. Materials Advances. 2023;4(19):4390–4399. doi: 10.1039/d3ma00212h. [DOI] [Google Scholar]
- 18.Huang Y., Kangas L.J., Rasco B. Applications of artificial neural networks (ANNs) in food science. Crit. Rev. Food Sci. Nutr. 2007;47(2):113–126. doi: 10.1080/10408390600626453. [DOI] [PubMed] [Google Scholar]
- 19.Hallur V., Atharga B., Hosur A., Binjawadagi B., Bhat K. International Conference on Circuits, Communication, Control and Computing. 2014. Design and development of a portable instrument for the detection of artificial ripening of banana fruit. [DOI] [Google Scholar]
- 20.Chai F., Wang C., Wang T., Ma Z., Su Z. L-cysteine functionalized gold nanoparticles for the colorimetric detection of Hg2+induced by ultraviolet light. Nanotechnology. 2009;21(2) doi: 10.1088/0957-4484/21/2/025501. [DOI] [PubMed] [Google Scholar]
- 21.Vafabakhsh M., Dadmehr M., Noureini S.K., Es’haghi Z., Malekkiani M., Hosseini M. Paper-based colorimetric detection of COVID-19 using aptasenor based on biomimetic peroxidase like activity of ChF/ZnO/CNT nano-hybrid. Spectrochim. Acta Part a Molecular and Biomolecular Spectroscopy. 2023;301 doi: 10.1016/j.saa.2023.122980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullah N., Mansha M., Khan I., Qurashi A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. TrAC, Trends Anal. Chem. 2018;100:155–166. doi: 10.1016/j.trac.2018.01.002. [DOI] [Google Scholar]
- 23.Kolya H., Hashitsume K., Kang C.W. Recent advances in colorimetric detection of arsenic using metal-based nanoparticles. Toxics. 2021;9(6):143. doi: 10.3390/toxics9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khazaei M., Hosseini M.S., Haghighi A.M., Misaghi M. Nanosensors and their applications in early diagnosis of cancer. Sensing and Bio-sensing Research. 2023;41 doi: 10.1016/j.sbsr.2023.100569. [DOI] [Google Scholar]
- 25.Hammami I., Alabdallah N.M., Jomaa A.A., Kamoun M. Gold nanoparticles: synthesis properties and applications. J. King Saud Univ. Sci. 2021;33(7) doi: 10.1016/j.jksus.2021.101560. [DOI] [Google Scholar]
- 26.Wu S., Li D., Wang J., Zhao Y., Dong S., Wang X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sensor. Actuator. B Chem. 2017;238:427–433. doi: 10.1016/j.snb.2016.07.067. [DOI] [Google Scholar]
- 27.Kusuma S.a.F., Harmonis J.A., Pratiwi R., Hasanah A.N. Gold Nanoparticle-Based Colorimetric Sensors: properties and application in detection of heavy metals and biological molecules. Sensors. 2023;23(19):8172. doi: 10.3390/s23198172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dadmehr M., Korouzhdehi B. Nanobiosensors for aflatoxin B1 detection, current research trends and future outlooks. Microchem. J. 2023;194 doi: 10.1016/j.microc.2023.109344. [DOI] [Google Scholar]
- 29.Farahani S.M., Dadmehr M., Karimi M.A., Korouzhdehi B., Karimi M.A., Rajabian M. Fluorometric detection of phytase enzyme activity and phosphate ion based on gelatin supported silver nanoclusters. Food Chem. 2022;396 doi: 10.1016/j.foodchem.2022.133711. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y., Zhao H., Wu Z., Li X., He Y., Yuan Z. Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and l-Cysteine. Analyst. 2011;136(18):3725. doi: 10.1039/c1an15238f. [DOI] [PubMed] [Google Scholar]
- 31.Thakkar S., Liu J., Dumée L.F., Singh B.R., Shukla S., Yang W. Arsenic ion assisted core–satellites nano-assembly of gold nanoparticles for its colorimetric determination in water. J. Water Proc. Eng. 2022;48 doi: 10.1016/j.jwpe.2022.102833. [DOI] [Google Scholar]
- 32.Rana R., Langenfeld-Heyser R., Finkeldey R., Polle A. FTIR spectroscopy, chemical and histochemical characterisation of wood and lignin of five tropical timber wood species of the family of Dipterocarpaceae. Wood Sci. Technol. 2009;44(2):225–242. doi: 10.1007/s00226-009-0281-2. [DOI] [Google Scholar]
- 33.Zheng T., Bott S., Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl. Mater. Interfaces. 2016;8(33):21585–21594. doi: 10.1021/acsami.6b06903. [DOI] [PubMed] [Google Scholar]
- 34.Stawny M., Gostyńska A., Dettlaff K., Jelińska A., Główka E., Ogrodowczyk M. Effect of lipid emulsion on stability of ampicillin in total parenteral nutrition. Nutrients. 2019;11(3):559. doi: 10.3390/nu11030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mourdikoudis S., Pallares R.M., Thanh N.T.K. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10(27):12871–12934. doi: 10.1039/c8nr02278j. [DOI] [PubMed] [Google Scholar]
- 36.Patra J.K., Baek K. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J. Nanomater. 2014;2014:1–12. doi: 10.1155/2014/417305. [DOI] [Google Scholar]
- 37.Lata K., Jaiswal A.K., Naik L., Sharma R. Gold nanoparticles: preparation, characterization and its stability in buffer. A Journal of Nanotechnology and Its Applications. 2014;17(1):1–10. https://www.researchgate.net/publication/274195747_Gold_Nanoparticles_Preparation_Characterization_and_Its_Stability_in_Buffer [Google Scholar]
- 38.Tran M., DePenning R., Turner M., Padalkar S. Effect of citrate ratio and temperature on gold nanoparticle size and morphology. Mater. Res. Express. 2016;3(10) doi: 10.1088/2053-1591/3/10/105027. [DOI] [Google Scholar]
- 39.Jeong U., Kim Y. Colorimetric detection of heavy metal ions using aminosilane. J. Ind. Eng. Chem. 2015;31:393–396. doi: 10.1016/j.jiec.2015.07.014. [DOI] [Google Scholar]
- 40.Domínguez-González R., Varela L.G., Bermejo-Barrera P. Functionalized gold nanoparticles for the detection of arsenic in water. Talanta. 2014;118:262–269. doi: 10.1016/j.talanta.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Devi S., Singh B., Paul A., Tyagi S. Highly sensitive and selective detection of trinitrotoluene using cysteine-capped gold nanoparticles. Anal. Methods. 2016;8(22):4398–4405. doi: 10.1039/c6ay01036a. [DOI] [Google Scholar]
- 42.Aswathy J.M., Jahnavi S., Krishna R., Manzoor K., Nair S., Menon D. Targeted labeling of cancer cells using biotin tagged Avidin Functionalized biocompatible fluorescent nanocrystals. J. Nanosci. Nanotechnol. 2011;11(9):7611–7620. doi: 10.1166/jnn.2011.4726. [DOI] [PubMed] [Google Scholar]
- 43.Hemmalakshmi S., Priyanga S., Devaki K. Fourier transform infra-red spectroscopy analysis of Erythrina variegata L. J. Pharmaceut. Sci. Res. 2017;9(11):2062–2067. https://www.researchgate.net/publication/321889637_Fourier_transform_infra-red_spectroscopy_analysis_of_Erythrina_variegata_L [Google Scholar]
- 44.Amina S.J., Guo B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle</p> international. Journal of Nanomedicine. 2020;15:9823–9857. doi: 10.2147/ijn.s279094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng Z., Wang H., Vongsvivut J., Li R., Glushenkov A.M., He J., Chen Y., Barrow C.J., Yang W. Self-assembly of core-satellite gold nanoparticles for colorimetric detection of copper ions. Anal. Chim. Acta. 2013;803:128–134. doi: 10.1016/j.aca.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S., Zhou H., Kong N., Wang Z., Fu H., Zhang Y., Xiao Y., Yang W., Yan F. L-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact. Mater. 2021;6(10):3288–3299. doi: 10.1016/j.bioactmat.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acres R.G., Feyer V., Tsud N., Carlino E., Prince K.C. Mechanisms of aggregation of cysteine functionalized gold nanoparticles. J. Phys. Chem. C. 2014;118(19):10481–10487. doi: 10.1021/jp502401w. [DOI] [Google Scholar]
- 48.Thawany P., Khanna A., Tiwari U.K., Deep A. L-cysteine/MoS2 modified robust surface plasmon resonance optical fiber sensor for sensing of Ferritin and IgG. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-31152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Gahouari T., Bodkhe G., Sayyad P., Ingle N., Mahadik M., Shirsat S.M., Deshmukh M., Musahwar N., Shirsat M. Electrochemical sensor: L-Cysteine induced selectivity enhancement of electrochemically reduced graphene Oxide–Multiwalled Carbon nanotubes hybrid for detection of lead (PB2+) ions. Frontiers in Materials. 2020;7 doi: 10.3389/fmats.2020.00068. [DOI] [Google Scholar]
- 50.Sonia N., Seth R. L-Cysteine functionalized gold nanoparticles as a colorimetric sensor for ultrasensitive detection of toxic metal ion cadmium. Mater. Today Proc. 2020;24:2375–2382. doi: 10.1016/j.matpr.2020.03.767. [DOI] [Google Scholar]
- 51.Poosinuntakul N., Parnklang T., Sitiwed T., Chaiyo S., Kladsomboon S., Chailapakul O., Apilux A. Colorimetric assay for determination of Cu (II) ions using l-cysteine functionalized silver nanoplates. Microchem. J. 2020;158 doi: 10.1016/j.microc.2020.105101. [DOI] [Google Scholar]
- 52.Dizman H.M., Kazancioglu E.O., Shigemune T., Takahara S., Arsu N. High sensitivity colorimetric determination of L-cysteine using gold nanoparticles functionalized graphene oxide prepared by photochemical reduction method. Spectrochim. Acta Part a Molecular and Biomolecular Spectroscopy. 2021;264 doi: 10.1016/j.saa.2021.120294. [DOI] [PubMed] [Google Scholar]
- 53.Irshad H., Assiri M.A., Khadija N., Rafique S., Khan A.M., Imran M., Shahzad S.A. Triazine based fluorescent sensor for sequential detection of Hg2+ and L-Cysteine in real samples and application in logic Gate: a combination of Extensive experimental and theoretical analysis. Spectrochim. Acta Part a Molecular and Biomolecular Spectroscopy. 2023;300 doi: 10.1016/j.saa.2023.122934. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H., Huang Y., Hu S., Huang Q., Wei C., Zhang W., Kang L., Huang Z., Hao A. Fluorescent probes for “off–on” sensitive and selective detection of mercury ions and l-cysteine based on graphitic carbon nitride nanosheets. J. Mater. Chem. C. 2014;3(9):2093–2100. doi: 10.1039/c4tc02394c. [DOI] [Google Scholar]
- 55.Tang J., Wu P., Hou X., Xu K. Modification-free and N-acetyl-L-cysteine-induced colorimetric response of AuNPs: a mechanistic study and sensitive Hg2+ detection. Talanta. 2016;159:87–92. doi: 10.1016/j.talanta.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 56.Srivatzen S., Kavitha B.S., Prasad A., Asokan S. L-Cysteine Modified Gold nanoparticles for Copper (II) ion detection using etched fiber Bragg grating Sensor. IEEE Sens. J. 2024;24(9):14253–14260. doi: 10.1109/jsen.2024.3374514. [DOI] [Google Scholar]
- 57.Rujiralai T., Leelaharat N., Cheewasedtham W. Highly specific colorimetric detection based on aggregation of l-cysteine functionalized gold nanoparticles for cypermethrin in water samples. RSC Adv. 2024;14(13):9175–9183. doi: 10.1039/d3ra07626a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H., Luo Y., Cai W., Xu L., Li J., Kong Y. Colorimetric discrimination and spectroscopic detection of tyrosine enantiomers based on melamine induced aggregation of l-cysteine/Au nanoparticles. Talanta. 2024 doi: 10.1016/j.talanta.2024.125758. [DOI] [PubMed] [Google Scholar]
- 59.Muguteeswaran R., Evarshini A., Samuel V.R., Pondurai S. A label free nanobiosensor for the detection of E.coli using cysteine capped gold nanoparticle. Phys. Scri. 2024;99(5) doi: 10.1088/1402-4896/ad38e0. [DOI] [Google Scholar]
- 60.Keshvari F., Bahram M., Farhadi K. Sensitive and selective colorimetric sensing of acetone based on gold nanoparticles capped with l-cysteine. J. Iran. Chem. Soc. 2016;13(8):1411–1416. doi: 10.1007/s13738-016-0856-4. [DOI] [Google Scholar]
- 61.Jameel Z.N. Synthesis of the gold nanoparticles with novel shape via chemical process and evaluating the structural, morphological and optical properties. Energy Proc. 2017;119:236–241. doi: 10.1016/j.egypro.2017.07.075. [DOI] [Google Scholar]
- 62.Jeon H.B., Tsalu P.V., Ha J.W. Shape effect on the refractive index sensitivity at localized surface plasmon resonance inflection points of single gold nanocubes with vertices. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-50032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martín-Sánchez C., Sánchez-Iglesias A., Barreda-Argüeso J.A., Polian A., Liz-Marzán L.M., Rodríguez F. Behavior of Au nanoparticles under pressure observed by in situ small-angle X-ray scattering. ACS Nano. 2022;17(1):743–751. doi: 10.1021/acsnano.2c10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Thai P., Abe S., Kosugi K., Saito N., Takahashi K., Sasaki T., Kikuchi T. Size/shape control of gold nanoparticles synthesized by alternating current glow discharge over liquid: the role of pH. Mater. Res. Express. 2019;6(9) doi: 10.1088/2053-1591/ab3038. [DOI] [Google Scholar]
- 65.Yazdani S., Daneshkhah A., Diwate A., Patel H., Smith J., Reul O., Cheng R., Izadian A., Hajrasouliha A.R. Model for Gold Nanoparticle synthesis: effect of pH and reaction time. ACS Omega. 2021;6(26):16847–16853. doi: 10.1021/acsomega.1c01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ariski R.T., Lee K.K., Kim Y., Lee C. The impact of pH and temperature on the green gold nanoparticles preparation using Jeju Hallabong peel extract for biomedical applications. RSC Adv. 2024;14(21):14582–14592. doi: 10.1039/d4ra00614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gezgin S.Y., Kepceoğlu A., Gündoğdu Y., Zongo S., Zawadzka A., Kiliç H.Ş., Sahraoui B. Effect of AR gas pressure on LSPR property of AU nanoparticles: comparison of experimental and theoretical studies. Nanomaterials. 2020;10(6):1071. doi: 10.3390/nano10061071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakellari G.I., Hondow N., Gardiner P.H. Factors influencing the surface functionalization of citrate stabilized gold nanoparticles with cysteamine, 3-mercaptopropionic acid or l-selenocystine for sensor applications. Chemosensors. 2020;8(3):80. doi: 10.3390/chemosensors8030080. [DOI] [Google Scholar]
- 69.Oliveira A.E.F., Pereira A.C., Resende M.a.C., Ferreira L.F. Gold nanoparticles: a didactic step-by-step of the synthesis using the turkevich method, mechanisms, and characterizations. Analytica—A Journal of Analytical Chemistry and Chemical Analysis. 2023;4(2):250–263. doi: 10.3390/analytica4020020. [DOI] [Google Scholar]
- 70.Lanza G., Betancourth D., Avila A., Riascos H., Perez-Taborda J. Control of the size distribution of AuNPs for colorimetric sensing by pulsed laser ablation in liquids. Kuwait Journal of Science. 2024;52(1) doi: 10.1016/j.kjs.2024.100294. [DOI] [Google Scholar]
- 71.Dheyab M.A., Aziz A.A., Jameel M.S., Khaniabadi P.M., Oglat A.A. Rapid Sonochemically-Assisted synthesis of highly stable gold nanoparticles as computed tomography contrast agents. Appl. Sci. 2020;10(20):7020. doi: 10.3390/app10207020. [DOI] [Google Scholar]
- 72.Takechi-Haraya Y., Ohgita T., Demizu Y., Saito H., Izutsu K., Sakai‐Kato K. Current status and challenges of analytical methods for evaluation of size and surface modification of Nanoparticle-Based drug formulations. AAPS PharmSciTech. 2022;23(5) doi: 10.1208/s12249-022-02303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong J., Carpinone P.L., Pyrgiotakis G., Demokritou P., Moudgil B.M. Synthesis of precision gold nanoparticles using Turkevich method. Kona Powder and Particle Journal. 2020;37(0):224–232. doi: 10.14356/kona.2020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chotithammakul S., Cortie M.B., Pissuwan D. Comparison of Single- and Mixed-Sized gold nanoparticles on lateral flow assay for albumin detection. Biosensors. 2021;11(7):209. doi: 10.3390/bios11070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irfan M., Ahmad T., Moniruzzaman M., Bhattacharjee S., Abdullah B. Size and stability modulation of ionic liquid functionalized gold nanoparticles synthesized using Elaeis guineensis (oil palm) kernel extract. Arab. J. Chem. 2017;13(1):75–85. doi: 10.1016/j.arabjc.2017.02.001. [DOI] [Google Scholar]
- 76.Ramakrishna M., Dandamudi R.B., Gengan R.M., Chandra S., Rao G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. Journal of Nanostructure in Chemistry. 2015;6(1):1–13. doi: 10.1007/s40097-015-0173-y. [DOI] [Google Scholar]
- 77.Ngernpimai S., Puangmali T., Kopwitthaya A., Tippayawat P., Chompoosor A., Teerasong S. Enhanced stability of gold nanoparticles with thioalkylated carboxyl-terminated ligands for applications in biosensing. ACS Appl. Nano Mater. 2024;7(11):13124–13133. doi: 10.1021/acsanm.4c01631. [DOI] [Google Scholar]
- 78.Wang Y., Quinsaat J.E.Q., Ono T., Maeki M., Tokeshi M., Isono T., Tajima K., Satoh T., Sato S., Miura Y., Yamamoto T. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol) Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim J., Yeap S.P., Che H.X., Low S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013;8(1) doi: 10.1186/1556-276x-8-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma A., Shankhwar S., Gaur M.S. PEI-conjugated AuNPs as a sensing platform for arsenic (AS-III) J. Exp. Nanosci. 2013;9(9):892–905. doi: 10.1080/17458080.2012.736641. [DOI] [Google Scholar]
- 81.Punuri J.B., Sharma P., Saranya S., Tamuli R., Bora U. Piper betle-mediated green synthesis of biocompatible gold nanoparticles. Int. Nano Lett. 2012;2(1) doi: 10.1186/2228-5326-2-18. [DOI] [Google Scholar]
- 82.Borse V., Konwar A.N. Synthesis and characterization of gold nanoparticles as a sensing tool for the lateral flow immunoassay development. Sensors International. 2020;1 doi: 10.1016/j.sintl.2020.100051. [DOI] [Google Scholar]
- 83.Song Y., Zhu A., Song Y., Cheng Z., Xu J., Zhou J. Experimental and theoretical study on the synthesis of gold nanoparticles using ceftriaxone as a stabilizing reagent for and its catalysis for dopamine. Gold Bull. 2012;45(3):153–160. doi: 10.1007/s13404-012-0059-4. [DOI] [Google Scholar]
- 84.Oza G., Pandey S., Mewada A., Sharon M. ResearchGate; 2012. Facile Biosynthesis of Gold Nanoparticles Exploiting Optimum pH and Temperature of Fresh Water Algae.https://www.researchgate.net/publication/230707309_Facile_biosynthesis_of_gold_nanoparticles_exploiting_optimum_pH_and_temperature_of_fresh_water_algae_Chlorella_pyrenoidusa [Google Scholar]
- 85.Krishnamurthy S., Esterle A., Sharma N., Sahi S.V. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res. Lett. 2014;9(1) doi: 10.1186/1556-276x-9-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu W., Zhang Y., Li D., Barrow C.J., Wang H., Yang W. A biomimetic sensor for the detection of lead in water. Biosens. Bioelectron. 2014;67:621–624. doi: 10.1016/j.bios.2014.09.077. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Yi Z., Liang Q., Zhen J., Wang R., Li M., Zeng L., Li Y. In situ deposition of gold nanoparticles and L-Cysteine on Screen-Printed carbon electrode for rapid electrochemical determination of AS(III) in water and tea. Biosensors. 2023;13(1):130. doi: 10.3390/bios13010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ditta S.A., Yaqub A., Tanvir F., Rashid M., Ullah R., Zubair M., Ali S., Anjum K.M. Gold nanoparticles capped with L-glycine, L-cystine, and L-tyrosine: toxicity profiling and antioxidant potential. J. Mater. Sci. 2023;58(6):2814–2837. doi: 10.1007/s10853-023-08209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan J., Liu B., Liu J. Interactions between gold, thiol and As(iii) for colorimetric sensing. Analyst (London. 1877. Online)/Analyst. 2020;145(15):5166–5173. doi: 10.1039/d0an00946f. [DOI] [PubMed] [Google Scholar]
- 90.Shellaiah M., Sun K. Review on anti-aggregation-enabled colorimetric sensing applications of gold and silver nanoparticles. Chemosensors. 2022;10(12):536. doi: 10.3390/chemosensors10120536. [DOI] [Google Scholar]
- 91.Najafzadeh F., Ghasemi F., Hormozi-Nezhad M.R. Anti-aggregation of gold nanoparticles for metal ion discrimination: a promising strategy to design colorimetric sensor arrays. Sensor. Actuator. B Chem. 2018;270:545–551. doi: 10.1016/j.snb.2018.05.065. [DOI] [Google Scholar]
- 92.Lakade A.J., Sundar K., Shetty P.H. Gold nanoparticle-based method for detection of calcium carbide in artificially ripened mangoes (Magniferaindica) Food Addit. Contam. 2018;35(6):1078–1084. doi: 10.1080/19440049.2018.1449969. [DOI] [PubMed] [Google Scholar]
- 93.Ghatak B., Banerjee S., Ali S.B., Das N., Tudu B., Pramanik P., Mukherji S., Bandyopadhyay R. Development of a low-cost portable aroma sensing system for identifying artificially ripened mango. Sensor Actuator Phys. 2021;331 doi: 10.1016/j.sna.2021.112964. [DOI] [Google Scholar]
- 94.Subramanya G.C., Misba A.K.N., Sumedha S., Niharika U.H. Android application for detection of artificially ripened bananas. International Journal of Advance Research, Ideas and Innovations in Technology. 2019;5(4):542–550. https://www.ijariit.com/manuscripts/v5i4/V5I4-1353.pdf [Google Scholar]
- 95.Ghatak B., Naskar H., Ali S.B., Banerjee S., Chakraborty A.K., Das N., Tudu B., Mukherji S., Bandyopadhyay R. Dysprosium particles decorated Ambroxol imprinted polymer sensor to detect carbide-treated mango. Sensor Actuator Phys. 2023;358 doi: 10.1016/j.sna.2023.114420. [DOI] [Google Scholar]
- 96.Nguyen L.H., Oveissi F., Chandrawati R., Dehghani F., Naficy S. Naked-Eye detection of ethylene using Thiol-Functionalized Polydiacetylene-Based flexible sensors. ACS Sens. 2020;5(7):1921–1928. doi: 10.1021/acssensors.0c00117. [DOI] [PubMed] [Google Scholar]
- 97.Tolentino M.a.K.P., Albano D.R.B., Sevilla F. Piezoelectric sensor for ethylene based on silver(I)/polymer composite. Sensor. Actuator. B Chem. 2018;254:299–306. doi: 10.1016/j.snb.2017.07.015. [DOI] [Google Scholar]
- 98.Kathirvelan J., Vijayaraghavan R. An infrared based sensor system for the detection of ethylene for the discrimination of fruit ripening. Infrared Phys. Technol. 2017;85:403–409. doi: 10.1016/j.infrared.2017.07.022. [DOI] [Google Scholar]
- 99.Vetrekar N., Prabhu A., Naik A., Ramachandra R., Raja K., Desai A., Gad R.S. Collaborative representation of convolutional neural network features to detect artificial ripening of banana using multispectral imaging. J. Food Process. Preserv. 2022;46(10) doi: 10.1111/jfpp.16882. [DOI] [Google Scholar]
- 100.Thakare M.N., Hatmode N.S. Identification of artificially ripened fruits using MATLAB. International Journal of Trend in Scientific Research and Development. 2020 https://www.ijtsrd.com/papers/ijtsrd33428.pdf [Google Scholar]
- 101.Khandarkar S.S., Wadhankar V.R., Dabhade D.S. Detection and identification of artificially ripened fruits using MATLAB. International Journal of Innovative Research in Computer and Communication Engineering. 2018;6(12):9136–9140. https://www.semanticscholar.org/paper/Detection-and-Identification-of-Artificially-Fruits-Khandarkar-Wadhankar/f5611a3890dd39573fb64b76a692b6e4e253625d [Google Scholar]
- 102.Gaspar D., Pimentel A.C., Mateus T., Leitão J.P., Soares J., Falcão B.P., Araújo A., Vicente A., Filonovich S.A., Águas H., Martins R., Ferreira I. Influence of the layer thickness in plasmonic gold nanoparticles produced by thermal evaporation. Scientific report. 2013;3:1469. doi: 10.1038/srep01469. [DOI] [PMC free article] [PubMed] [Google Scholar]