Abstract
1. Intracellular recordings from sympathetic neurones in the isolated coeliac ganglion of guinea-pigs have been used to define the synaptic input to three subtypes of neurone, classified on the basis of their discharge during maintained depolarizing current as phasic neurones, neurones with prolonged after-hyperpolarizations (LAH), and tonic neurones. 2. The three classes of neurone were distributed characteristically in different parts of the ganglion. 3. Passive membrane properties differed between the three neurone types. Mean input resistance was highest in phasic neurones and was inversely related to the size of the prolonged calcium-activated potassium conductance in LAH neurones. Mean input time constant was highest in tonic neurones, because of significantly higher cell capacitance. 4. Phasic and LAH neurones usually received one suprathreshold ('strong') as well as several subthreshold excitatory synaptic potentials (ESPs) from the ipsilateral splanchnic nerve. In general, the amplitude and number of splanchnic inputs were greater, and the occurrence of two strong inputs more common, in phasic than in LAH neurones. The input to tonic neurones was small and usually subthreshold, even with supramaximal splanchnic stimulation. In a few (mostly tonic) neurones lying close to the midline, small ESPs were evoked by contralateral splanchnic stimulation. 5. Antidromic action potentials were evoked in more than half of all neurones by high voltage coeliac nerve stimulation. In addition, multiple small subthreshold ESPs were recorded in virtually all tonic neurones (99%) on coeliac nerve stimulation. In contrast, coeliac stimulation rarely evoked a few very small ESPs in LAH neurones (9%), but no synaptic response in phasic neurones. 6. In about half of the tonic neurones tested (but no phasic or LAH neurones), small ESPs were evoked by stimulation of the intermesenteric nerve. 7. Slow depolarization elicited by repetitive activation of splanchnic and coeliac nerve trunks, at voltages supramaximal for the fast cholinergic responses, were recorded from about half of both phasic and tonic neurones, but only one of twenty-four LAH neurones. These responses commonly faded during subsequent trials, so that it was difficult to characterize them. 8. The data indicate that the three broad groups of coeliac neurone, classified on the basis of their voltage- and calcium-dependent potassium conductances, receive different patterns of synaptic input. The differences may be related to the three major functions of vasoconstriction, motility and mucosal secretion in the small intestine.
Full text
PDF





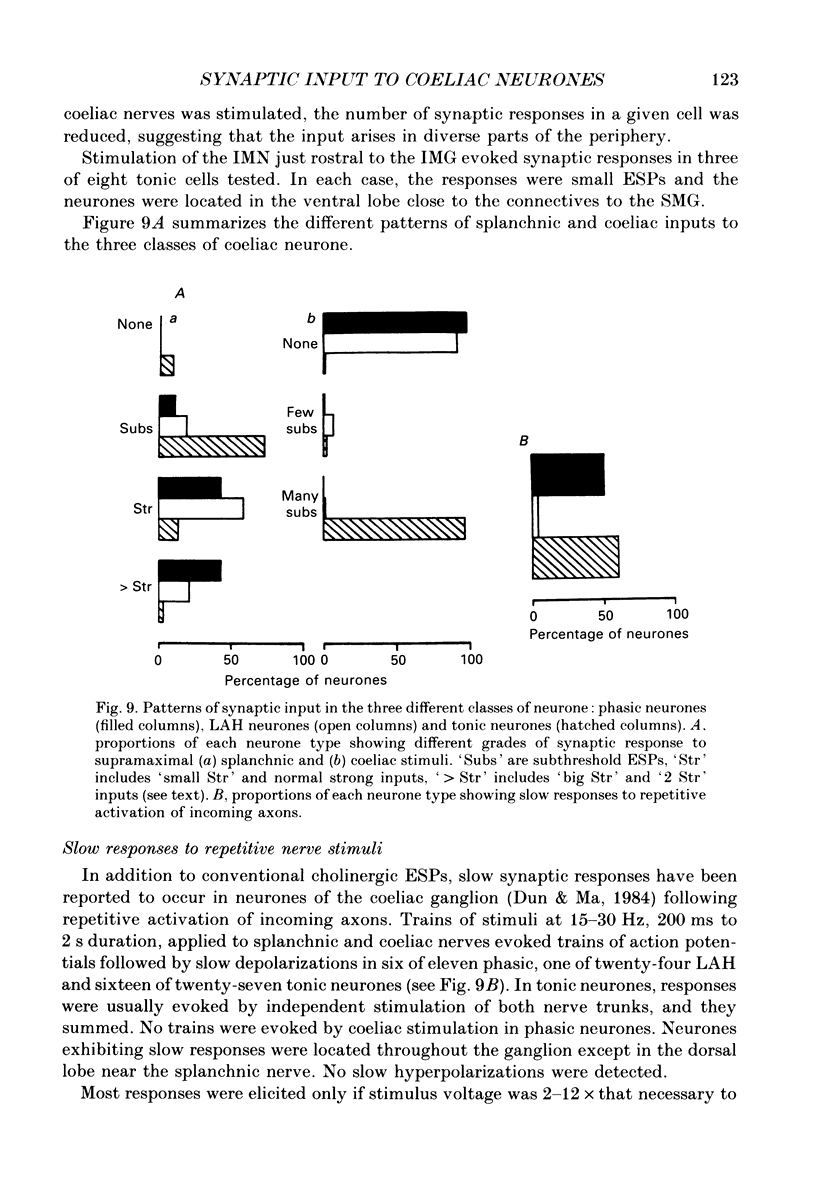
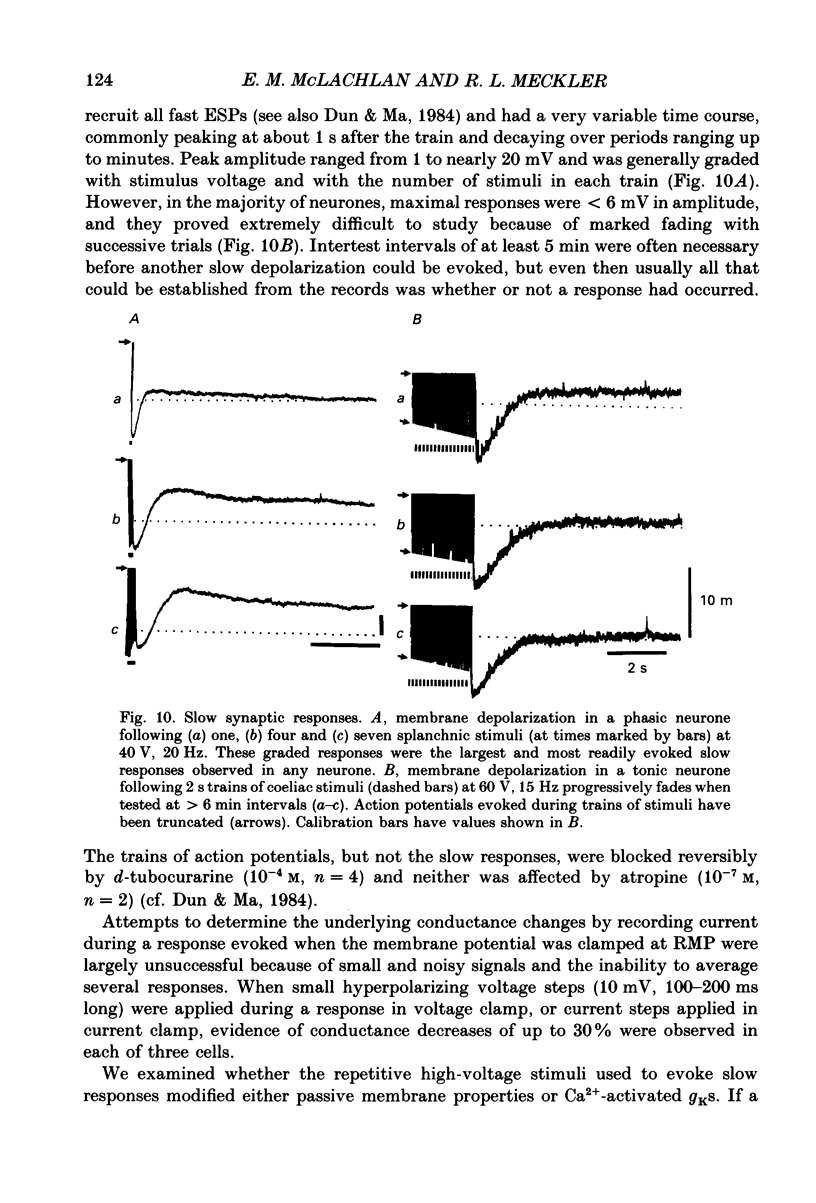
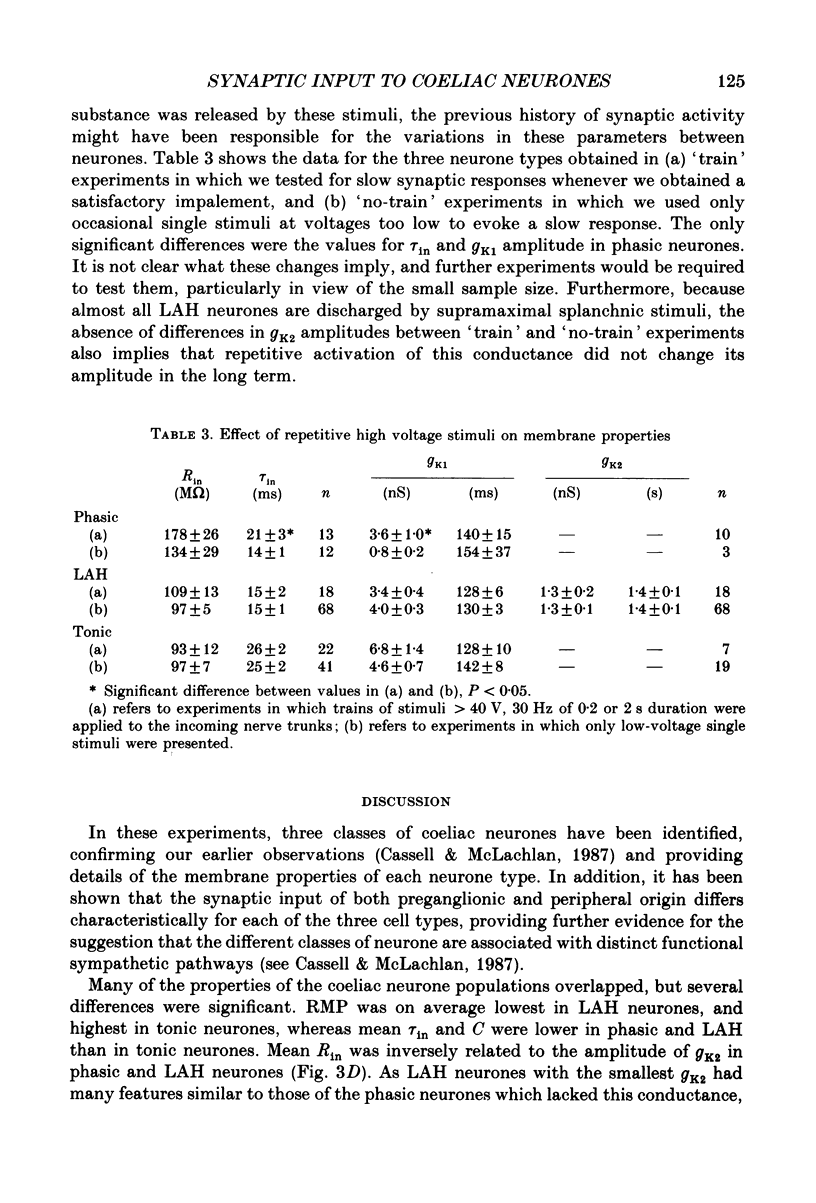




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R., Dray A., Hankins M. W. Stimulation of afferent fibres of the guinea-pig ureter evokes potentials in inferior mesenteric ganglion neurones. J Physiol. 1988 Aug;402:543–553. doi: 10.1113/jphysiol.1988.sp017220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R., Constanti A. Voltage-sensitive K-currents in sympathetic neurons and their modulation by neurotransmitters. J Auton Nerv Syst. 1982 Jul;6(1):23–35. doi: 10.1016/0165-1838(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., Clark A. L., McLachlan E. M. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 Mar;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. The effect of a transient outward current (IA) on synaptic potentials in sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 May;374:273–288. doi: 10.1113/jphysiol.1986.sp016079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. Two calcium-activated potassium conductances in a subpopulation of coeliac neurones of guinea-pig and rabbit. J Physiol. 1987 Dec;394:331–349. doi: 10.1113/jphysiol.1987.sp016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian E. P., Weinreich D. Long-duration spike afterhyperpolarizations in neurons from the guinea pig superior cervical ganglion. Neurosci Lett. 1988 Jan 22;84(2):191–196. doi: 10.1016/0304-3940(88)90406-5. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984 Nov;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Decktor D. L., Weems W. A. An intracellular characterization of neurones and neural connexions within the left coeliac ganglion of cats. J Physiol. 1983 Aug;341:197–211. doi: 10.1113/jphysiol.1983.sp014801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silinsky E. M. Interaction between inhibitory and excitatory synaptic potentials at a peripheral neurone. J Physiol. 1976 Aug;259(3):647–663. doi: 10.1113/jphysiol.1976.sp011487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McLachlan E. M. Development of dendritic calcium currents in ganglion cells of the rat lower lumbar sympathetic chain. J Physiol. 1986 Aug;377:349–368. doi: 10.1113/jphysiol.1986.sp016191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Elde R., Schultzberg M., Goldstein M., Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julé Y., Krier J., Szurszewski J. H. Patterns of innervation of neurones in the inferior mesenteric ganglion of the cat. J Physiol. 1983 Nov;344:293–304. doi: 10.1113/jphysiol.1983.sp014940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef K. D., Kreulen D. L. Venous mechanoreceptor input to neurones in the inferior mesenteric ganglion of the guinea-pig. J Physiol. 1986 Aug;377:49–59. doi: 10.1113/jphysiol.1986.sp016176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen D. L., Peters S. Non-cholinergic transmission in a sympathetic ganglion of the guinea-pig elicited by colon distension. J Physiol. 1986 May;374:315–334. doi: 10.1113/jphysiol.1986.sp016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae I. M., Furness J. B., Costa M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 1986;244(1):173–180. doi: 10.1007/BF00218395. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Llewellyn-Smith I. J. The immunohistochemical distribution of neuropeptide Y in lumbar pre- and paravertebral sympathetic ganglia of the guinea pig. J Auton Nerv Syst. 1986 Dec;17(4):313–324. doi: 10.1016/0165-1838(86)90097-4. [DOI] [PubMed] [Google Scholar]
- Meckler R. L., McLachlan E. M. Axons of peripheral origin preferentially synapse with tonic neurones in the guinea pig coeliac ganglion. Neurosci Lett. 1988 Mar 31;86(2):189–194. doi: 10.1016/0304-3940(88)90569-1. [DOI] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978 Mar;41(2):305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Yarom Y., Sugimori M., Llinás R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985 Dec;16(4):719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Polosa C., Nishi S. Electrophysiological properties of sympathetic preganglionic neurons in the cat spinal cord in vitro. Pflugers Arch. 1986 Feb;406(2):91–98. doi: 10.1007/BF00586668. [DOI] [PubMed] [Google Scholar]


