Abstract
1. The electrophysiological properties of three subtypes of granulocytes obtained from the circulating blood of the newt, Triturus pyrrhogaster, were studied using the whole-cell variation of the patch-electrode voltage-clamp technique. 2. Neutrophils were identified by their multilobulated nucleus in the cytoplasm. Basophils and eosinophils, having characteristic granular structures in their cells, could definitely be distinguished from other leucocytes. The reliability of cellular identification using phase-contrast microscopy was confirmed by fixing and staining the granulocytes with Wright's solution. 3. In neutrophils under current-clamp conditions, a hyperpolarization-induced conductance increase was observed. With depolarization, however, no changes in regenerative potential were detected. When voltage clamped in standard saline (containing 96 mM-NaCl), hyperpolarizing voltage pulses to a potential more negative than -90 mV evoked slowly decaying inward currents. 4. The hyperpolarization-evoked membrane currents in neutrophils were identified as anomalous rectifying K+ currents, since (1) externally applied Cs+ (0.1 or 1 mM) or Ba2+ (1 mM) produced suppressive effects on the currents, (2) replacement of external Na+ with choline ions eliminated the decay of macroscopically observed currents, and (3) both the amplitude and kinetic properties of the currents were strongly dependent on membrane potential as well as on external K+ concentration; the activation of the conductance depended on the electrochemical force for K+ rather than on membrane potential alone. The magnitude of steady-state conductance was roughly proportional to the square root of the external K+ concentration. 5. In basophils and eosinophils, no major time- or voltage-dependent increase in conductance was detected at voltages between +20 and -130 mV. However, under current-clamp conditions, spontaneous fluctuation of zero-current potentials was clearly apparent, presumably due to the activities of some ion channels generating a small amount of current flux through the membranes of these cells. 6. It was concluded that the three subtypes of granulocytes in the newt differ considerably not only in appearance and structure but also in the electrical properties of their membranes. The anomalous rectifying K+ channels in neutrophils may serve to determine the resting potential of the cell at K+ equilibrium potential. The closure of the channels at depolarization might facilitate the maintenance of depolarization triggered by stimuli accompanying current influx.
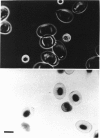
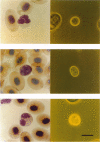
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G., DeCoursey T. E., Cahalan M. D., Gupta S. Electroimmunology: the physiologic role of ion channels in the immune system. J Immunol. 1985 Aug;135(2 Suppl):787s–791s. [PubMed] [Google Scholar]
- Choquet D., Sarthou P., Primi D., Cazenave P. A., Korn H. Cyclic AMP-modulated potassium channels in murine B cells and their precursors. Science. 1987 Mar 6;235(4793):1211–1214. doi: 10.1126/science.2434998. [DOI] [PubMed] [Google Scholar]
- Decoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 1987 Mar;89(3):405–420. doi: 10.1085/jgp.89.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Krause D., Lee S. C. Voltage-gated potassium conductance in human T lymphocytes stimulated with phorbol ester. J Physiol. 1986 Mar;372:405–423. doi: 10.1113/jphysiol.1986.sp016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO K., KAMEZAWA S., KATO K., MITSUI T. A contribution to the study of the comparative hematology with reference chiefly to the cytochemical reactions. Okajimas Folia Anat Jpn. 1956 Sep;28(1-6):45–58. doi: 10.2535/ofaj1936.28.1-6_45. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Saxton R. E. Variation of calcium current during the cell growth cycle in mouse hybridoma lines secreting immunoglobulins. J Physiol. 1984 Oct;355:313–321. doi: 10.1113/jphysiol.1984.sp015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K. Ionic channels in leukocytes. J Leukoc Biol. 1986 Mar;39(3):241–254. doi: 10.1002/jlb.39.3.241. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Sheehy P. A. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. J Physiol. 1985 Dec;369:475–499. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Hiraoka M. Barium-induced automatic activity in isolated ventricular myocytes from guinea-pig hearts. J Physiol. 1988 Jan;395:455–472. doi: 10.1113/jphysiol.1988.sp016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizeki K. [Differentiation of granulocytes in the newt (Triturus pyrrhogaster), with special reference to the granule formation (author's transl)]. Kaibogaku Zasshi. 1980 Aug;55(4):305–319. [PubMed] [Google Scholar]
- Kawa K. Existence of calcium channels and intercellular couplings in the testosterone-secreting cells of the mouse. J Physiol. 1987 Dec;393:647–666. doi: 10.1113/jphysiol.1987.sp016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Transient outward currents and changes of their gating properties after cell activation in thrombocytes of the newt. J Physiol. 1987 Apr;385:189–205. doi: 10.1113/jphysiol.1987.sp016491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Voltage-gated sodium and potassium currents and their variation in calcitonin-secreting cells of the chick. J Physiol. 1988 May;399:93–113. doi: 10.1113/jphysiol.1988.sp017070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Weissmann G. Neutrophil stimulation: receptor, membrane, and metabolic events. Fed Proc. 1984 Sep;43(12):2749–2754. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. The plasticity of ion channels: parallels between the nervous and immune systems. Trends Neurosci. 1988 May;11(5):214–218. doi: 10.1016/0166-2236(88)90129-4. [DOI] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. A patch-clamp study of histamine-secreting cells. J Gen Physiol. 1986 Sep;88(3):349–368. doi: 10.1085/jgp.88.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUI T. Application of the electron microscope to the cytochemical peroxidase reaction in salamander leukocytes. J Biophys Biochem Cytol. 1960 Apr;7:251–260. doi: 10.1083/jcb.7.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Lichtenstein L. M. The purification of human basophils. J Immunol. 1980 May;124(5):2519–2521. [PubMed] [Google Scholar]
- Malech H. L., Root R. K., Gallin J. I. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977 Dec;75(3):666–693. doi: 10.1083/jcb.75.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Kanoh T., Uchino H. Membrane potential change of human polymorphonuclear leukocytes stimulated with formyl-methionyl-leucyl-phenylalanine. Nihon Ketsueki Gakkai Zasshi. 1984 Feb;47(1):48–58. [PubMed] [Google Scholar]
- Okada Y., Doida Y., Roy G., Tsuchiya W., Inouye K., Inouye A. Oscillations of membrane potential in L cells. I. Basic characteristics. J Membr Biol. 1977 Aug 4;35(4):319–335. doi: 10.1007/BF01869957. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Pecht I. Membrane potential changes during IgE-mediated histamine release from rat basophilic leukemia cells. J Membr Biol. 1983;75(2):97–104. doi: 10.1007/BF01995629. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. J Physiol. 1979 Sep;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Melnick D. A., Malech H. L., Meshulam T., Simons E. R., Lazzari K. G., Proto P. J., Gadenne A. S., Leavitt J. L., Griffin J. D. The effects of phorbol myristate acetate and chemotactic peptide on transmembrane potentials and cytosolic free calcium in mature granulocytes evolve sequentially as the cells differentiate. J Biol Chem. 1987 Jan 25;262(3):1274–1281. [PubMed] [Google Scholar]
- Takaya K. Mast cells and histamine in a newt, Triturus pyrrhogaster Boié. Experientia. 1968 Oct 15;24(10):1053–1054. doi: 10.1007/BF02138743. [DOI] [PubMed] [Google Scholar]
- Tooze J., Davies H. G. Light and electron microscopic observations on the spleen and the splenic leukocytes of the newt Triturus cristatus. Am J Anat. 1968 Nov;123(3):521–556. doi: 10.1002/aja.1001230308. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Yazdanbakhsh M., Eckmann C. M., Koenderman L., Verhoeven A. J., Roos D. Eosinophils do respond to fMLP. Blood. 1987 Aug;70(2):379–383. [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]