ABSTRACT
Introduction
Richter's transformation (RT) from chronic lymphocytic leukemia (CLL) to lymphoma carries poor prognosis. This case series examines the efficacy of lisocabtagene maraleucel (liso‐cel) in six RT patients, highlighting the impact of concurrent ibrutinib therapy.
Methods
Six patients were with RT who received liso‐cel from were included in this single institution case series. Clinical data related to efficacy, safety, CAR‐T expansion kinetics, and measurable residual disease were collected.
Results
The best overall response was 83.3%. The four patients who received ibrutinib concurrent with liso‐cel therapy continue to show MRD‐negative complete response till date. None experienced severe (grade 3+) cytokine release syndrome while 1 had severe (grade 3+) immune‐effector cell‐associated neurotoxicity syndrome (ICANS). All patients were noted to have in vivo CAR expansion.
Conclusion
This series of real cases suggests liso‐cel with concurrent ibrutinib may be a promising treatment for RT, warranting further exploration.
Keywords: CAR‐T therapy, ibrutinib, Richter's transformation
1. Introduction
Richter's transformation (RT), the transformation of chronic lymphocytic leukemia (CLL) to an aggressive lymphoma, is associated with unfavorable clinical outcomes, including a median survival of 5–12 months from diagnosis [1]. Allogeneic stem cell transplantation is an option for patients who respond to initial chemoimmunotherapy [2]. However, no effective treatment alternatives for chemoimmunotherapy‐refractory Richter's transformation currently exist. Treatment with CD19‐targeted CAR T‐cell (CAR‐T) has had tremendous success in patients with relapsed or refractory large B cell lymphoma (LBCL) [3, 4, 5, 6, 7, 8]. However, overall post‐treatment clinical outcomes have been poor in patients with CLL receiving CAR‐T, which may be explained by CLL‐induced T‐cell anergy [8, 9]. In addition, patients with RT were excluded from pivotal trials for axicabtagene cileulocel (axi‐cel) and tisagenlecleucel [5, 6].
Five patients with RT were included in the TRANSCEND NHL 001 trial for lisocabtagene maraleucel (liso‐cel), yet there remains limited knowledge on the efficacy and safety of CAR‐T in RT treatment [7]. Kittai et al. reported the clinical outcomes in 9 RT patients treated with axi‐cel, as an off‐label use. Another study by Kittai et al. showed outcomes of 69 patients with RT across nine centers receiving various anti‐CD19 directed CAR‐T [4]. Further, strategies like adding ibrutinib have been used to improve CAR‐T function and proliferation in CLL and refractory NHL [3, 10, 11], but its concurrent use with CAR‐T therapy in RT has only minimally been explored [12].
2. Methods
All patients consented to a Stanford University Institutional Review Board‐approved data collection protocol. All patients with RT who received liso‐cel between September 2021 and November 2023 were included in this series (Table 1). Five patients received fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) on Day –5, Day –4, and Day –3 as lymphodepleting (LD) therapy, while one received bendamustine (90 mg/m2) on Day –4 and Day –3 as an alternative therapy due to the nationwide fludarabine shortage in 2022. Toxicities were classified and managed as per the American Society of Transplantation and Cellular Therapy grading and Common Terminology Criteria for Adverse Effects 2017 [13]. The response was assessed using the Lugano 2014 criteria, and measurable residual disease (MRD) was measured using ClonoSEQ™, based on next‐generation sequencing of CLL clonal sequences from peripheral blood samples [14, 15]. CD19 CAR‐T expansion was measured by real‐time flow cytometry with anti‐idiotype‐FMC63 conjugated to Dylight 65013 and calculated by adding the absolute values of CD4 and CD8 CAR‐T cells [16].
TABLE 1.
Patient characteristics including baseline demographics, prior treatment history, infusion details, and safety and efficacy outcomes.
| Patient ID | Age | Sex | Del 17p |
RT presentation at CAR‐T |
Clonally related |
Number of prior lines of therapy pre‐RT |
Prior lines of therapy pre‐RT | Number of prior lines of therapy post‐RT | Prior lines of therapy post‐RT | Concurrent Ibrutinib therapy |
Days of ibrutinib Before apheresis |
History of allogenic transplant prior to liso‐cel | Lympho‐depletion | CRS maximum grade |
ICANS maximum grade |
Current status |
Days to last follow‐up |
Best response |
Response at last contact | MRD at last contact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 59 | M | No | PR | Yes | 1 | BR | 1 | RCEOP | Yes | 14 | No | Bendamustine | 0 | 0 | Alive | 756 | CR | CR | 0 |
| B | 61 | F | Yes | PR | Yes | 7 |
FCR CFAR PCI trial with Ibrutinib Ofatumumab Obinutuzumab Idelalisib with Obinutuzumab |
5 |
DA‐EPOCH‐R Venetoclax Venetoclax + Ibrutinib Allotransplant RCHOP |
Yes | 7 | Yes | FC | 1 | 1 | Alive | 589 | CR | CR | 0 |
| C | 63 | F | No | PD | Yes | 9 |
Rituxan RCVP Fludarabine Rituximab RCVP BR Rituximab Ibrutinib Venetoclax‐Rituximab Obinutuzumab |
2 | RCHOP+Ibrutinib | No | 27 | No | FC | 2 | 3 | Dead | 124 | PR | PD | 64 |
| D | 68 | M | No | PD | No | 0 | NA | 3 |
RCHOP RT RT |
Yes | 10 | No | FC | 1 | 0 | Alive | 379 | PR | CR | 0 |
| E | 65 | F | Yes | PD | Yes | 1 | FCR | 1 | RCHOP | Yes | 54 | No | FC | 0 | 0 | Alive | 294 | CR | CR | 0 |
| F | 56 | M | No | PD | Yes | 4 |
FCR Ibrutinib Venetoclax zanubrutinib |
1 | DA‐EPOCH‐R + venetoclax | No | NA | No | FC | 1 | 2 | Dead | 22 | NA | PD | NA |
Del 17p, deletion 17p; RT, Richter's transformation; FCR, fludarabine cytoxan rituximab; CFAR, cyclophosphamide fludarabine alemtuzumab rituximab; DA‐EPOCH‐R, dose‐adjusted etoposide prednisone vincristine cyclophosphamide doxorubicin rituximab; RCEOP, rituximab, cyclophosphamide, etoposide, vincristine and oral prednisolone; RCHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; FC, fludarabine and cytoxan; liso‐cel, lisocabtagene maraleucel; CRS, cytokine release syndrome; ICANS, immune effector cell associated neurotoxicity syndrome; CR, complete response; PR, partial response; PD, progressive disease; NA, not applicable.
3. Results
Among the six patients, the median age was 62 years (range, 56–68), with three male and three female patients (Table 1). The mean lines of therapy for CLL prior to RT was 2.5 (range 0–9) and post‐RT prior to CART was 1.5 (range 1–5). All patients had progressive disease prior to CART except Patient B who had stable disease. All patients had clonally related disease except Patient D. Only Patient B had an allogeneic transplant prior to CART. Three patients (Patients B, C, and F) had previously received BTKi and/or BCL2i therapy for CLL. Median baseline level prior to CART for CRP was 2.65 mg/L (range 0.3–25.2), median baseline LDH was 188.5 U/L (range 117–1397), and median baseline ferritin was 442.5 ng/mL (range 104–6023). Two patient's diseases had both a 17p and TP53 deletion (B and E), and two (B and D) had trisomy 12. The median vein to vein time was 34 days (range 30–40). Two patients received bridging chemotherapy, all others had no bridging chemotherapy. Four of the six patients received concurrent ibrutinib with CAR‐T therapy with a median of 14 days on ibrutinib prior to apheresis (range, 7–54). Ibrutinib was continued through apheresis with LD regimen, and patients continued to receive ibrutinib beyond day +28 post‐infusion. There were no reported side effects attributable to ibrutinib.
All patients successfully manufactured CAR‐T, and absolute CAR‐T expansion was noted at day 7 with a median of 0.06 cells/µL (range, 0.01–26.8). Four patients received outpatient therapy while two were inpatient. While four patients developed cytokine release syndrome (CRS), none developed CRS grade ≥3. The median time to onset of CRS was 1 day (range, 0–10) and median CRS duration was 4 days (range, 1–4). Three of these patients also developed immune‐effector cell‐associated neurotoxicity syndrome (ICANS), but only one of these patients developed grade ≥3. Median onset of ICANS was 5 days (range, 4–9) post‐infusion, and median ICANS duration was 6 days (range, 1–7). There was one patient (Patient B) who experienced prolonged cytopenia, and Patient B was also the only patient to experience a Grade 3/4 infection in the first year after CAR‐T.
DLBCL in Patient A had a Day 28 complete response, which currently persists at 756 days post‐infusion. At Day 28, DLBCL in patient B showed partial response, which improved to complete response at 3 months and persists 589 days post‐infusion. Patient C's disease progressed at month 3 after a Day 28 partial response. They died 124 days post‐infusion due to disease progression. Patient D's DLBCL achieved a partial response at Day 28 and improved to complete response at month 3 (currently maintained at post‐infusion day 379). Patient E's disease showed complete response at Day 28 (which has persisted through day 294), while Patient F experienced biopsy‐proven progression prior to death 24 days after infusion. The best overall response was 83.3%, and all 4 patients who are still alive received concurrent ibrutinib (Figure 1).
FIGURE 1.
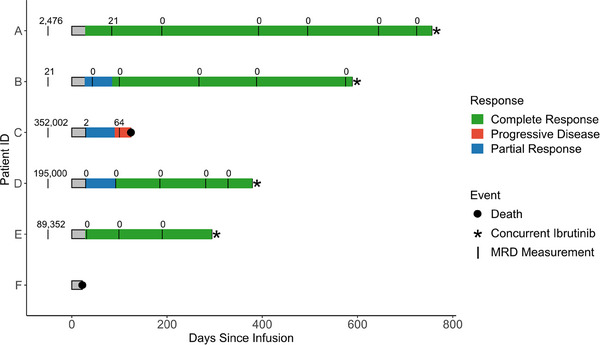
Swimmer's plot depicting response and duration of follow‐up for six patients after Lisocabtagene maraleucel for Richter's transformation, with numbers indicating minimal residual disease measurement (MRD) with CLL clones per million WBC at various time points throughout each patient's follow‐up. The first MRD measurement corresponds to the latest measurement before infusion and was set at Day –50 for all participants for visualization purposes. Patient F did not have any ClonoSEQ samples reported.
ClonoSEQ MRD assessments demonstrated that all patients with available data had high levels of MRD prior to infusion (Figure 2). Patient A had 21 clones per million white blood cell (WBC) genomes 3 months post‐infusion. They reached no detectable clones at 6 months which has persisted as of last measurement. Patients B, D, and E have had no detectable clonal cells post‐infusion. Patient C had 64 detectable clones at time of progression.
FIGURE 2.
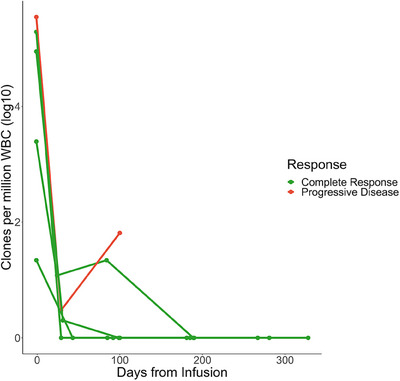
Clones per million WBC for each patient for all collected samples from the most recent sample before infusion onward. Only five patients are shown because Patient F did not have any ClonoSEQ samples reported. Data are truncated at 1‐year post‐infusion for visualization purposes.
4. Conclusion
To our knowledge, this is the first case series focusing on the real‐world experience of liso‐cel as treatment for RT. None of the patients developed grade ≥3 CRS, and only one developed ≥3 ICANS. The best overall response was achieved at 83.3% (n = 5/6). This correlated with MRD‐negative response, highlighting the strong response to CAR‐T. The four patients who received ibrutinib concurrent with CAR‐T therapy continue to show MRD‐negative complete response today.
While our sample size is limited, it supports further exploration to determine whether combining ibrutinib with liso‐cel can improve response in RT. Ibrutinib inhibits both Bruton's tyrosine kinase and interleukin‐2 inducible kinase (ITK). ITK plays a key role in the activation of both Th1 and Th2 cells. In Th1 cells, ITK signaling is supportive but not critical to redundant resting lymphocyte kinase signaling, whereas in Th2 cells, ITK signaling is essential to activation [17]. Given the significant influence of T‐cell anergy on CAR‐T efficacy in RT, concurrent ibrutinib with CAR‐T therapy may be an impactful strategy to overcome this challenge.
Author Contributions
SB, CS, and AG designed the study. SB, CS, AG, and BS collected the data. AG, CS, DL, and SB analyzed the data and wrote the original manuscript. All authors performed critical reviews of the manuscript and had full access to all the data in the study. All authors contributed to the final version of the manuscript.
Ethics Statement
This study obtained Stanford Institutional Review Board approval under application number 43375. All patients provided written informed consent prior to enrollment in the study.
Patient Consent Statement
All patients provided written informed consent prior to enrollment in the study.
Conflicts of Interest
SD has served on the scientific advisory board for Kite/Gilead and has received research funding from Kite/Gilead. DBM: Honoraria from Janssen, Fosun Kite Biotechnology; Consulting or Advisory Role with Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics LLC, an AbbVie Company, Janssen, Research Funding from Pharmacyclics LLC, an AbbVie Company, Novartis, Roche/Genentech, Kite, a Gilead Company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio. SB has served on the scientific advisory board for Allogene.
Clinical Trial Registration
The authors have confirmed clinical trial registration is not needed for this submission.
Funding: No external funding was obtained for this study.
Courtney J. Smith and Anmol Goyal are joint first authors.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Al‐Sawaf O., Robrecht S., Bahlo J., et al., “Richter Transformation in Chronic Lymphocytic Leukemia (CLL)—A Pooled Analysis of German CLL Study Group (GCLLSG) Front Line Treatment Trials,” Leukemia 35, no. 1 (2021): 169–176, 10.1038/s41375-020-0797-x. [DOI] [PubMed] [Google Scholar]
- 2. Puckrin R., Owen C., Fontaine A., Peters A., Stewart D., and Shafey M., “Allogeneic Hematopoietic Cell Transplantation for Richter Transformation of Chronic Lymphocytic Leukemia: An Intention‐to‐Transplant Analysis,” Bone Marrow Transplantation 58, no. 7 (2023): 817–819, 10.1038/s41409-023-01978-6. [DOI] [PubMed] [Google Scholar]
- 3. Gill S., Vides V., Frey N. V., et al., “Anti‐CD19 CAR T Cells in Combination With Ibrutinib for the Treatment of Chronic Lymphocytic Leukemia,” Blood Advances 6, no. 21 (2022): 5774–5785, 10.1182/bloodadvances.2022007317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kittai A. S., Bond D., Huang Y., et al., “Anti‐CD19 Chimeric Antigen Receptor T‐Cell Therapy for Richter Transformation: An International, Multicenter, Retrospective Study,” Journal of Clinical Oncology. Official Journal of the American Society of Clinical Oncology 42, no. 17 (2024): 2071–2079, 10.1200/JCO.24.00033. [DOI] [PubMed] [Google Scholar]
- 5. Schuster S. J., Bishop M. R., Tam C. S., et al., “Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B‐Cell Lymphoma,” New England Journal of Medicine 380, no. 1 (2019): 45–56, 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 6. Neelapu S. S., Locke F. L., Bartlett N. L., et al., “Axicabtagene Ciloleucel CAR T‐Cell Therapy in Refractory Large B‐Cell Lymphoma,” New England Journal of Medicine 377, no. 26 (2017): 2531–2544, 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abramson J. S., Palomba M. L., Gordon L. I., et al., “Lisocabtagene Maraleucel for Patients With Relapsed or Refractory Large B‐Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study,” The Lancet 396, no. 10254 (2020): 839–852, 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 8. Benjamini O., Fried S., Shouval R., et al. Anti‐CD19 Chimeric Antigen Receptor T‐Cell Therapy Has Less Efficacy in Richter Transformation Than in De Novo Large B‐Cell Lymphoma and Transformed Low‐Grade B‐Cell Lymphoma. Haematologica. Published online June 20, 2024. 10.3324/haematol.2023.284664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Bruggen J. A. C., Martens A. W. J., Fraietta J. A., et al., “Chronic Lymphocytic Leukemia Cells Impair Mitochondrial Fitness in CD8+ T Cells and Impede CAR T‐cell Efficacy,” Blood 134, no. 1 (2019): 44–58, 10.1182/blood.2018885863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gauthier J., Hirayama A. V., Purushe J., et al., “Feasibility and Efficacy of CD19‐Targeted CAR T Cells With Concurrent Ibrutinib for CLL After Ibrutinib Failure,” Blood 135, no. 19 (2020): 1650–1660, 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu M., Deng H., Mu J., et al., “Ibrutinib Improves the Efficacy of Anti‐CD19‐CAR T‐Cell Therapy in Patients With Refractory Non‐Hodgkin Lymphoma,” Cancer Science 112, no. 7 (2021): 2642–2651, 10.1111/cas.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winter A. M., Bharadwaj S., Herrera A. F., et al., “Real‐World Outcomes of lisocabtagene maraleucel (Liso‐Cel) in Patients (pt) With Richter Transformation (RT) From the Center for International Blood and Marrow Transplant Research (CIBMTR),” Journal of Clinical Oncology. Official Journal of the American Society of Clinical Oncology 42 (2024): 7010–7010, 10.1200/JCO.2024.42.16_suppl.7010. [DOI] [Google Scholar]
- 13. Lee D. W., Santomasso B. D., Locke F. L., et al., “ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated With Immune Effector Cells,” Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 25, no. 4 (2019): 625–638, 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Heertum R. L., Scarimbolo R., Wolodzko J. G., et al., “Lugano 2014 Criteria for Assessing FDG‐PET/CT in Lymphoma: An Operational Approach for Clinical Trials,” Drug Des Devel Ther 11 (2017): 1719–1728, 10.2147/DDDT.S136988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al‐Sawaf O., Zhang C., Lu T., et al., “Minimal Residual Disease Dynamics After Venetoclax‐Obinutuzumab Treatment: Extended off‐Treatment Follow‐up from the Randomized CLL14 Study,” Journal of Clinical Oncology. Official Journal of the American Society of Clinical Oncology 39, no. 36 (2021): 4049–4060, 10.1200/JCO.21.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spiegel J. Y., Patel S., Muffly L., et al., “CAR T Cells With Dual Targeting of CD19 and CD22 in Adult Patients With Recurrent or Refractory B Cell Malignancies: A Phase 1 Trial,” Nature Medicine 27, no. 8 (2021): 1419–1431, 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan C. E., Sahaf B., Logan A. C., et al., “Ibrutinib Efficacy and Tolerability in Patients With Relapsed Chronic Lymphocytic Leukemia Following Allogeneic HCT,” Blood 128, no. 25 (2016): 2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


