Abstract
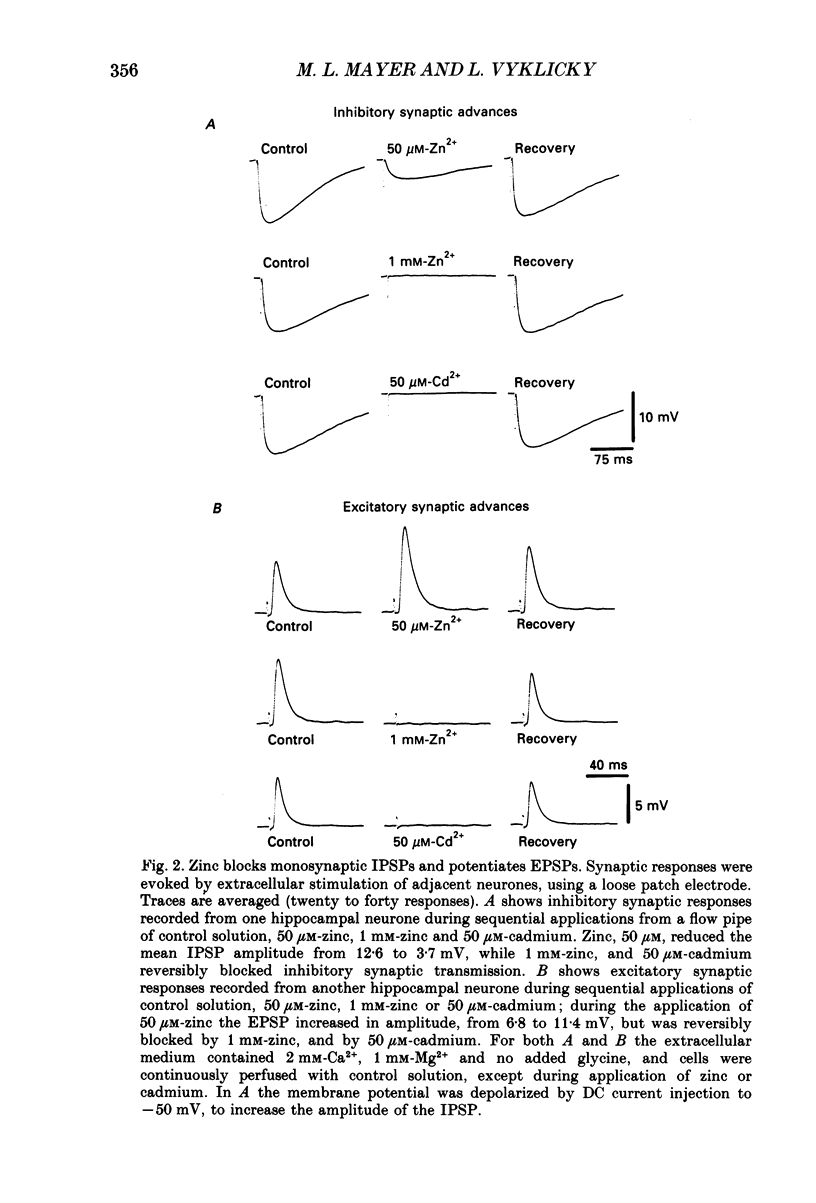
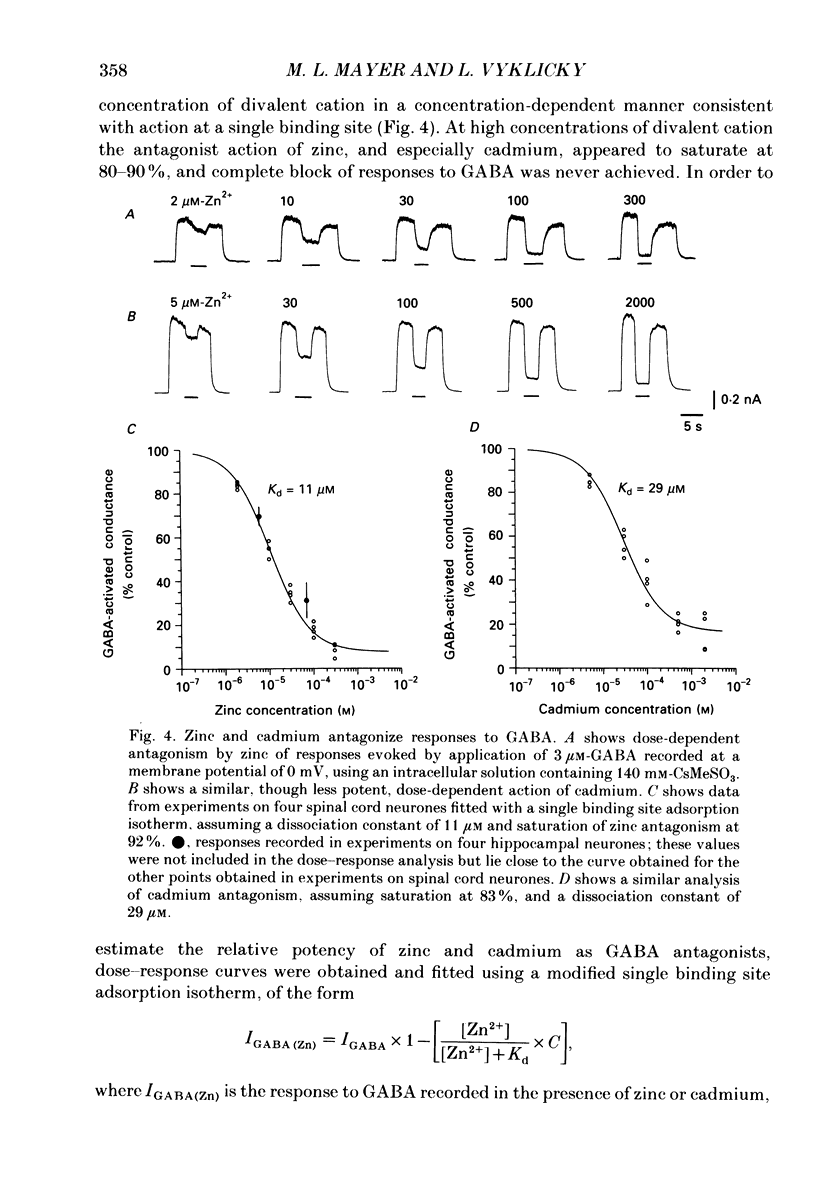
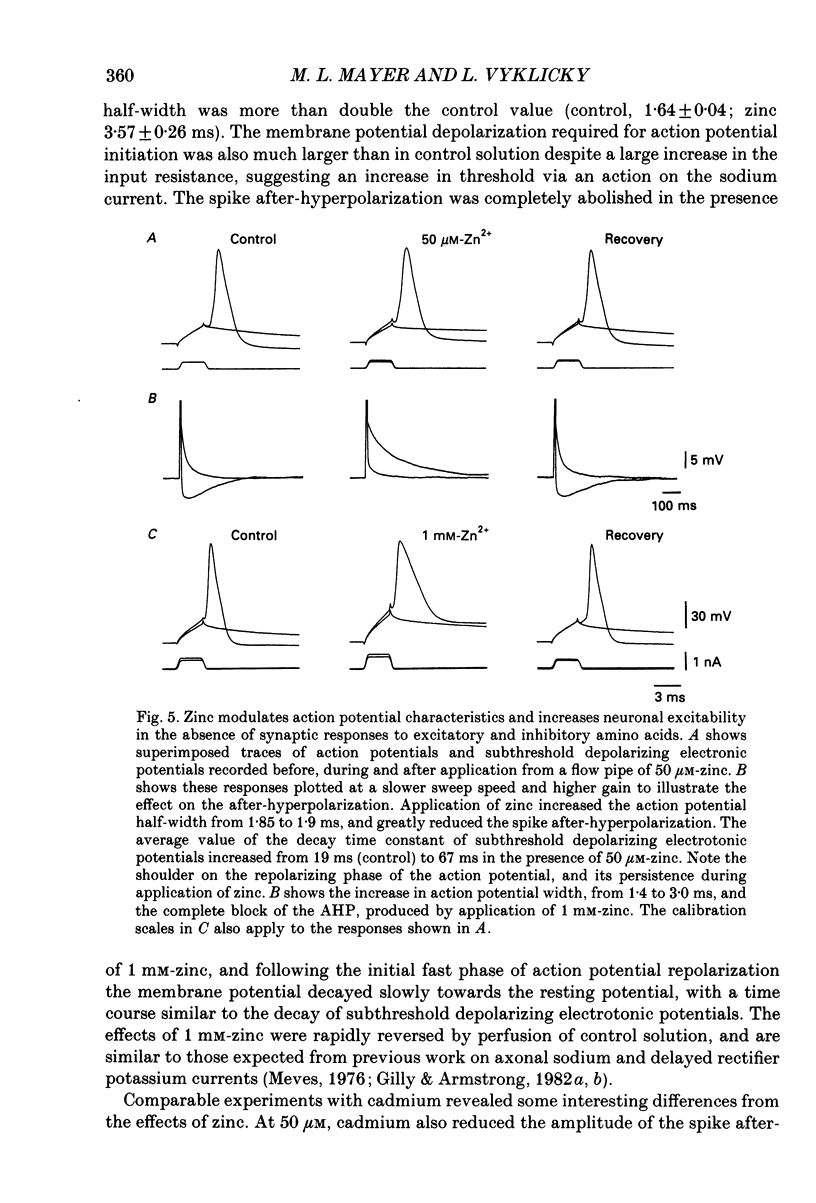
1. The whole-cell configuration of the patch clamp method was used to record from hippocampal neurones in cell culture. Synaptic responses were evoked by loose patch stimulation of adjacent presynaptic neurones in low-density cultures. Agonists and antagonists were applied rapidly, using an array of flow pipes each of diameter 250 microns, positioned within 100 microns of the postsynaptic neurone. 2. Bath application of 50 microM-zinc produced prolonged periods of synaptic barrage and action potential discharge. Flow pipe application of 50 microM-zinc, in glycine-free solution with 1 mM-Mg2+, produced on average a 75% reduction of IPSP amplitude, but increased the average EPSP amplitude to 171% of control. However, after block of gamma-aminobutyric acid (GABA) receptors with bicuculline, zinc had no effect on EPSP amplitude, suggesting that potentiation recorded in control solutions reflects block of polysynaptic IPSPs. 3. Consistent with the block of IPSPs postsynaptic responses to flow pipe applications of GABA were blocked by zinc, with fast-on, fast-off kinetics. The equilibrium dissociation constant (Kd) for zinc block of GABA responses, estimated from fit of a single binding site adsorption isotherm, was 11 microM and sufficient to explain the degree of reduction of IPSPs by 50 microM-zinc. Zinc antagonism of responses to GABA was essentially independent of membrane potential over the range -60 to +60 mV. 4. With bicuculline methiodide and glycine added to a magnesium-free extracellular solution, to allow the study of synaptic responses mediated by N-methyl-D-aspartic acid (NMDA) receptors, zinc reduced the amplitude of EPSPs to 50% of control, and decreased the decay time constant of the EPSP, suggesting that zinc blocks synaptic activation of NMDA receptors. 5. Under conditions where synaptic transmission was completely blocked with postsynaptic receptor antagonists (1-3 mM-kynurenic acid and 10-20 microM-bicuculline methiodide) 50 microM-zinc decreased the amplitude of the spike after-hyperpolarization (AHP), but did not produce large changes in action potential amplitude or half-width. Under these conditions 50 microM-zinc also decreased the current threshold required to trigger action potential discharge, and blocked accommodation so that repetitive firing replaced single action potential responses to prolonged current pulses.
Full text
PDF




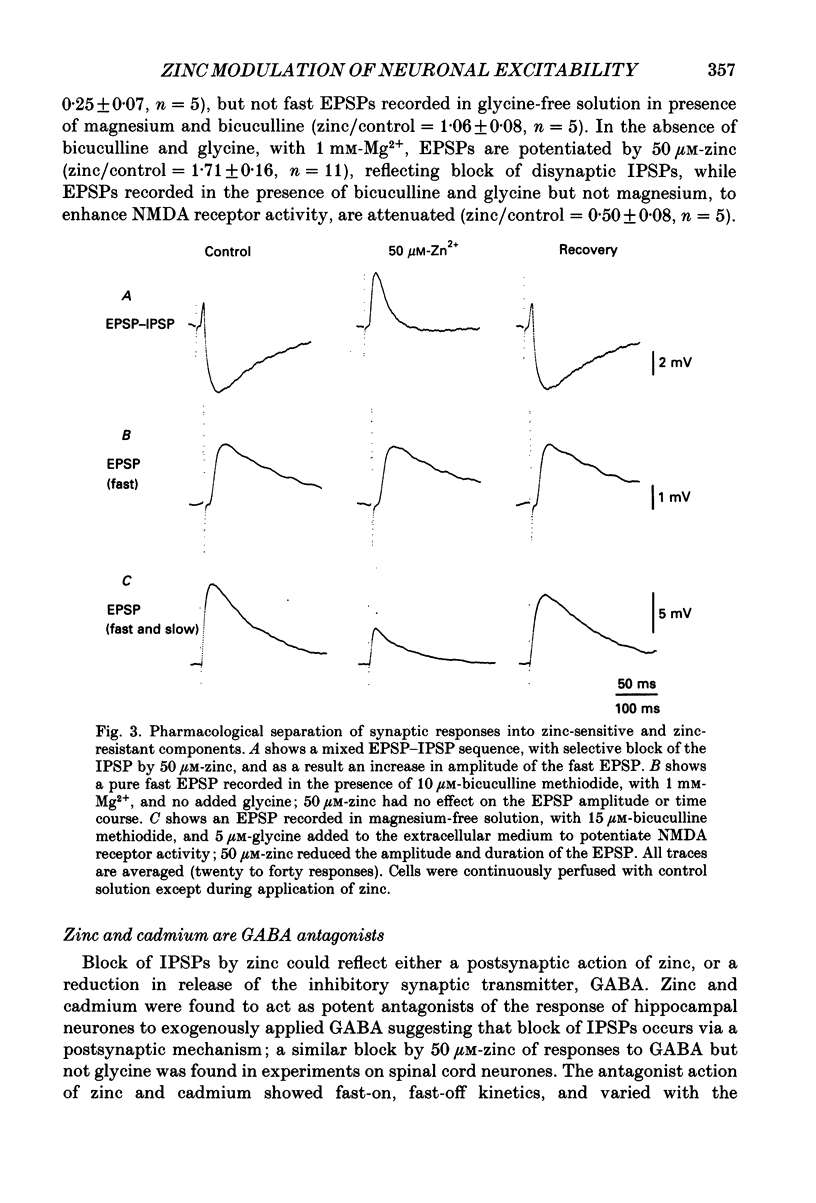





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J., St Pierre T., Minnich J., Barbeau A. Seizures in rats associated with divalent cation inhibition of NA + -K + -ATP'ase. Can J Biochem. 1971 Nov;49(11):1217–1224. doi: 10.1139/o71-175. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L., Mayer M. L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988 Oct;8(10):3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson C. J., Klitenick M. A., Manton W. I., Kirkpatrick J. B. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 1983 Aug 29;273(2):335–339. doi: 10.1016/0006-8993(83)90858-2. [DOI] [PubMed] [Google Scholar]
- Galvan M., Grafe P., ten Bruggencate G. Convulsant actions of 4-aminopyridine on the guinea-pig olfactory cortex slice. Brain Res. 1982 Jun 3;241(1):75–86. doi: 10.1016/0006-8993(82)91230-6. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Slowing of sodium channel opening kinetics in squid axon by extracellular zinc. J Gen Physiol. 1982 Jun;79(6):935–964. doi: 10.1085/jgp.79.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P. B., Brenneman D. E., Neale E. A. Morphological and biochemical differences expressed in separate dissociated cell cultures of dorsal and ventral halves of the mouse spinal cord. Brain Res. 1987 Sep 15;420(2):313–323. doi: 10.1016/0006-8993(87)91252-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Howell G. A., Welch M. G., Frederickson C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984 Apr 19;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B. Excitatory amino acid antagonists as novel anticonvulsants. Adv Exp Med Biol. 1986;203:321–329. doi: 10.1007/978-1-4684-7971-3_24. [DOI] [PubMed] [Google Scholar]
- Meves H. The effect of zinc on the late displacement current in squid giant axons. J Physiol. 1976 Jan;254(3):787–801. doi: 10.1113/jphysiol.1976.sp011259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. A novel effect of zinc on the lobster muscle GABA receptor. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):327–341. doi: 10.1098/rspb.1982.0045. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. Pre- and postsynaptic effects of zinc on in vitro prepyriform neurones. Neurosci Lett. 1983 Sep 30;40(2):205–211. doi: 10.1016/0304-3940(83)90303-8. [DOI] [PubMed] [Google Scholar]
- Smejda Haug F. M. Heavy metals in the brain. A light microscope study of the rat with Timm's sulphide silver method. Methodological considerations and cytological and regional staining patterns. Adv Anat Embryol Cell Biol. 1973;47(4):1–71. [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wright D. M. Effect of zinc on neuronal activity in the rat forebrain. Adv Exp Med Biol. 1986;203:599–609. doi: 10.1007/978-1-4684-7971-3_46. [DOI] [PubMed] [Google Scholar]
- Wright D. M. Zinc: effect and interaction with other cations in the cortex of the rat. Brain Res. 1984 Oct 8;311(2):343–347. doi: 10.1016/0006-8993(84)90097-0. [DOI] [PubMed] [Google Scholar]


