Abstract
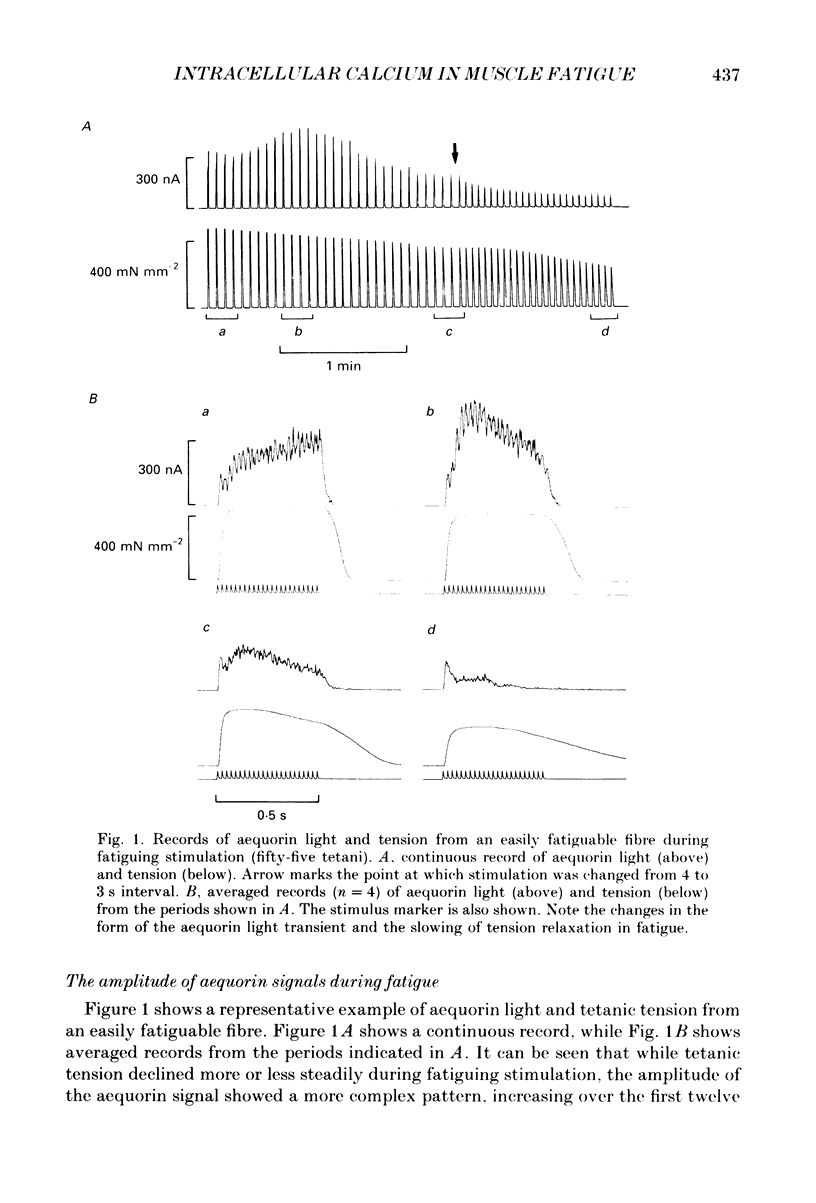
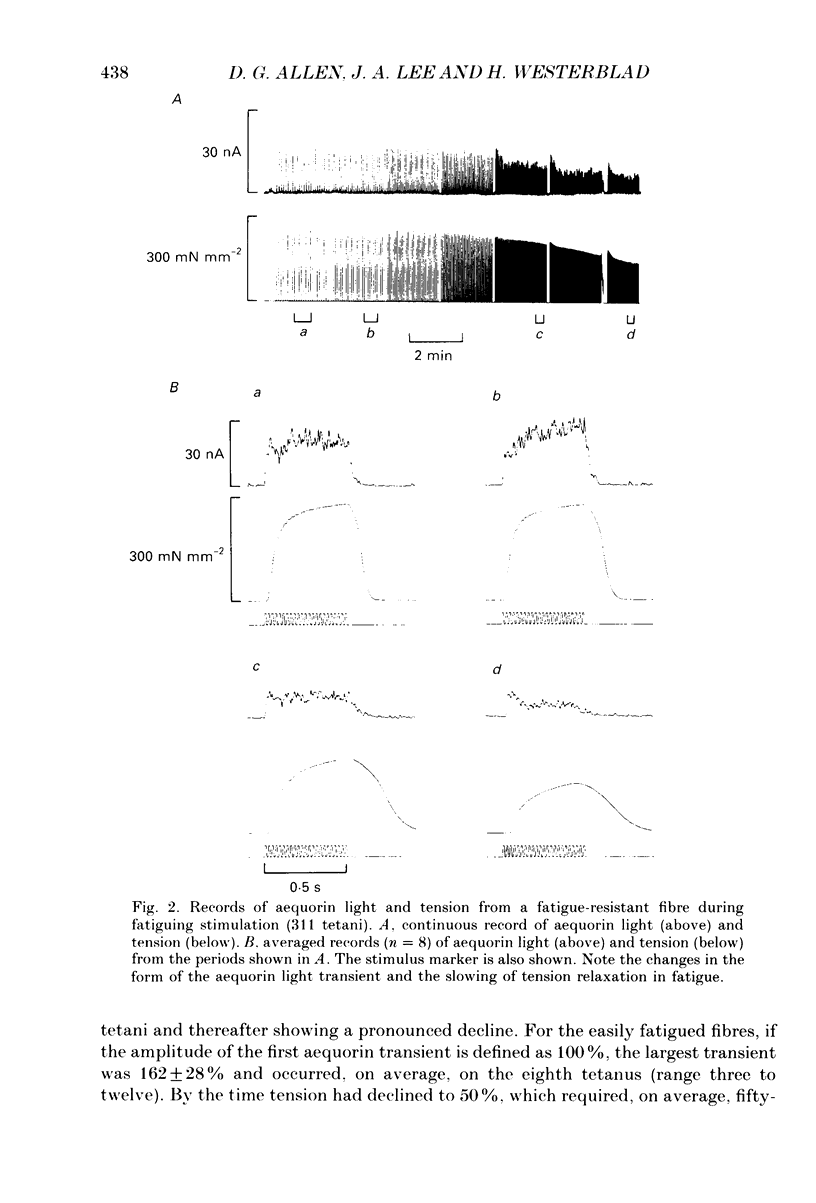
1. Single muscle fibres were dissected from Xenopus lumbrical muscles and microinjected with the photoprotein aequorin in order to measure the myoplasmic free calcium concentration ([Ca2+]i). Fatigue was produced by repeated intermittent tetanic stimulation continued until tension had declined to approximately 50% of the initial level. Fibres were then allowed to recover by giving tetani at less frequent intervals. Aequorin light (a measure of [Ca2+]i) and tension were measured during fatiguing stimulation and recovery. 2. During fatiguing stimulation, tetanic tension declined steadily, but peak aequorin light first increased before declining substantially. The largest light signal was about 155% of initial control while at the end of fatiguing stimulation the tetanic light fell to about 14% of control. 3. Fibres showed a characteristic slowing of relaxation in the fatigued state. This was associated with a slowing of the rate of decline of the aequorin light signal. 4. Intracellular acidosis produced by equilibrating the Ringer solution with either 5 or 15% CO2 caused an increase in the light signal associated with a tetanus. Carbon dioxide also caused a reduction of tension and a slowing of relaxation. 5. In vivo pCa-tension curves were constructed by exposing the fibres to a series of K+ concentrations which produced contractures of different sizes. Light and tension were measured during periods when both were relatively stable and the light signal was subsequently converted to pCa. 6. Exposure of fibres to 5 or 15% CO2 caused the pCa-tension curve to be shifted to the right of the control curve. This indicates a reduced Ca2+ sensitivity of the contractile proteins, which is in agreement with results from skinned fibre studies. 7. The pCa-tension points obtained from tetani during the early part of fatiguing stimulation also deviated to the right of the control pCa-tension curve, suggesting a reduced Ca2+ sensitivity of the contractile proteins. At the end of fatiguing stimulation, however, pCa-tension points did not differ greatly from the control pCa-tension curve, suggesting that Ca2+ sensitivity was approximately normal. Thus the reduced [Ca2+]i during tetani at the end of fatiguing stimulation (when tension was reduced to approximately 50%) could explain all of the reduction in tension. 8. After fatiguing stimulation, tension and light recovered monotonically in some fibres; however, in the majority of fibres, tension and light showed a secondary decline followed by a slower recovery (post-contractile depression). 9. During post-contractile depression, caffeine contractures or tetani in the presence of caffeine gave increased aequorin light signals and the tension developed was close to that produced in an unfatigued tetanus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
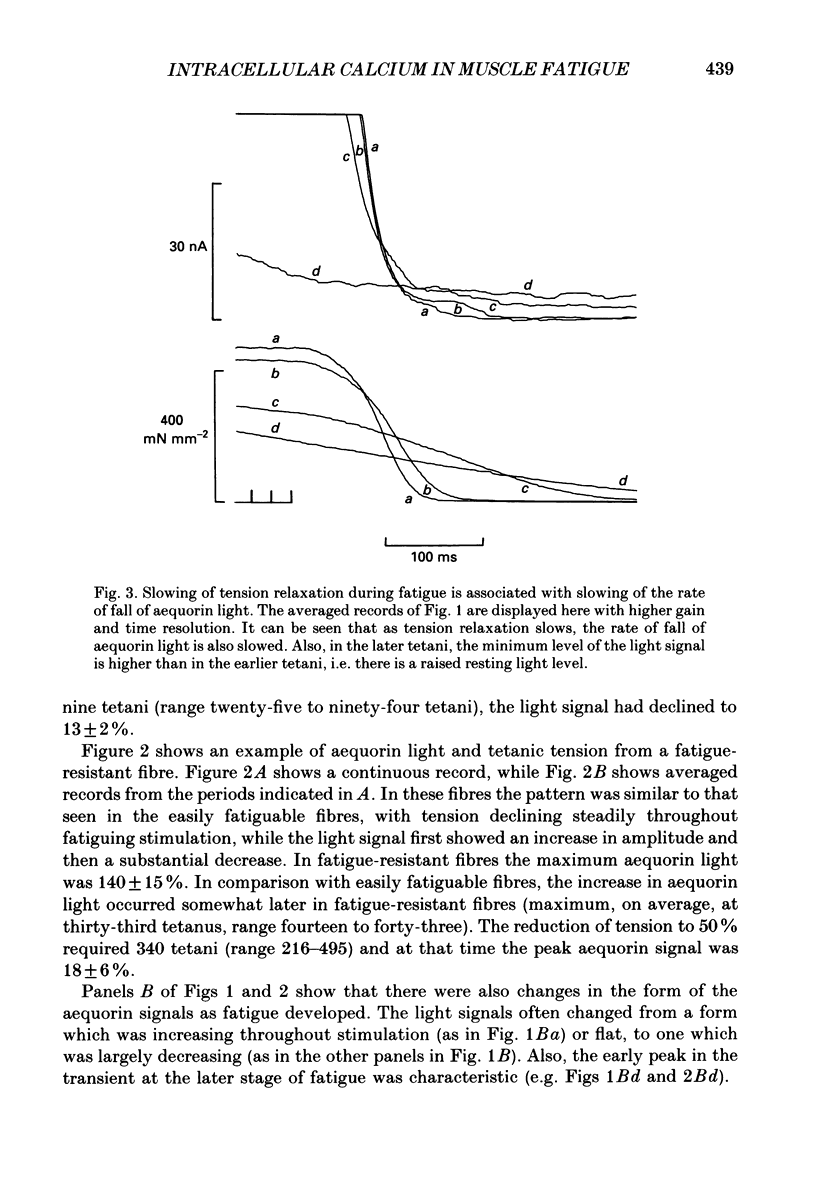
PDF




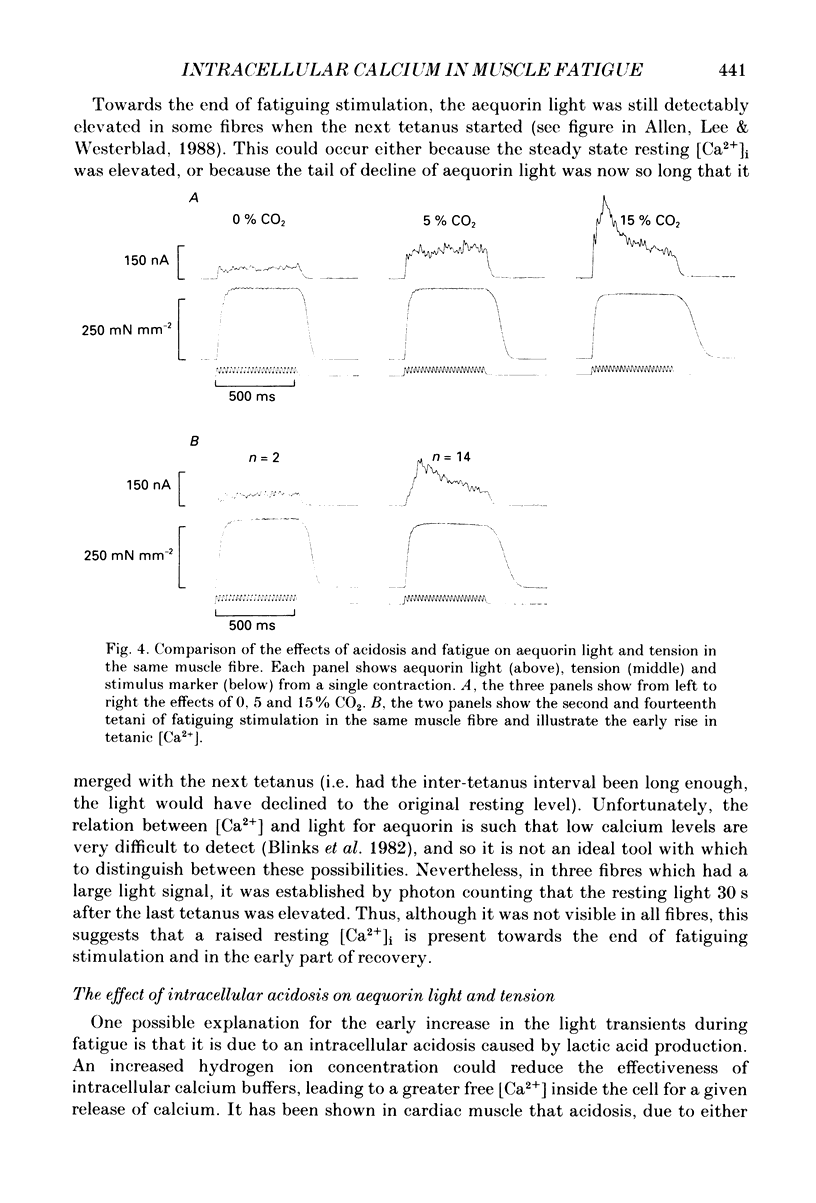









Selected References
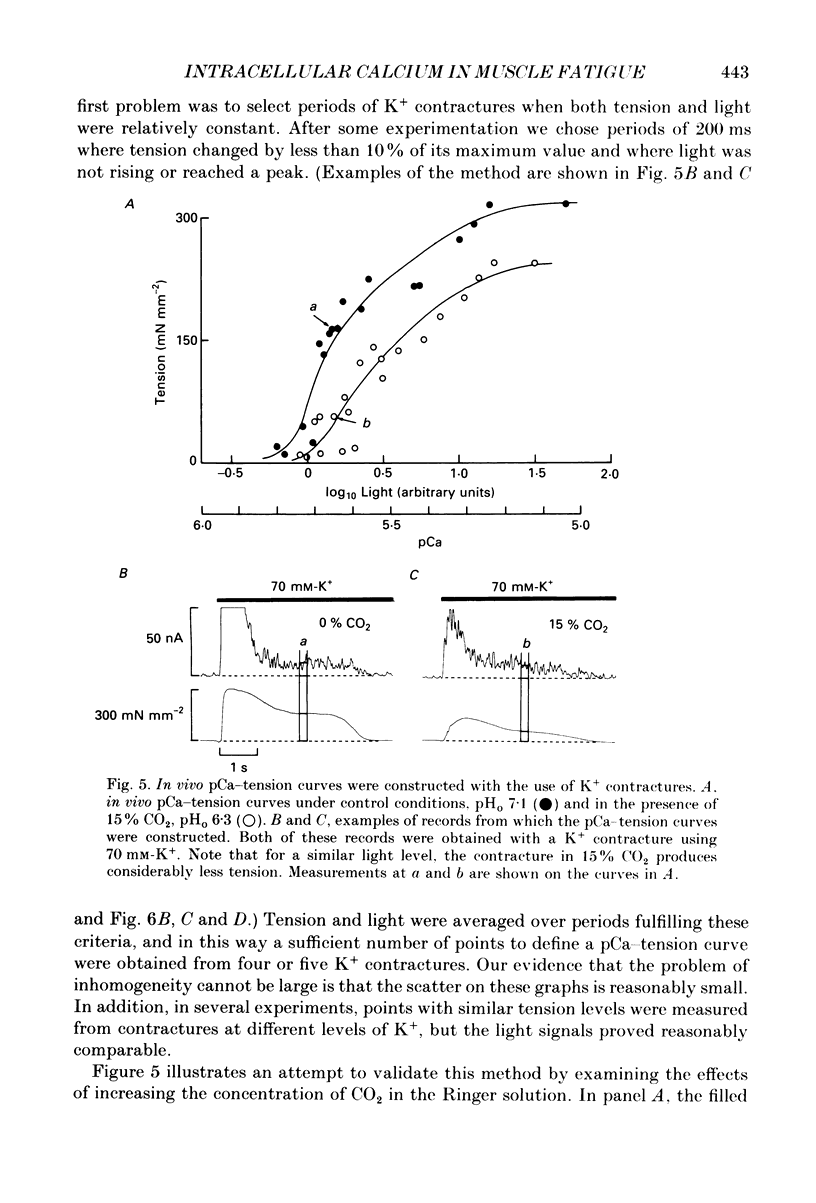
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
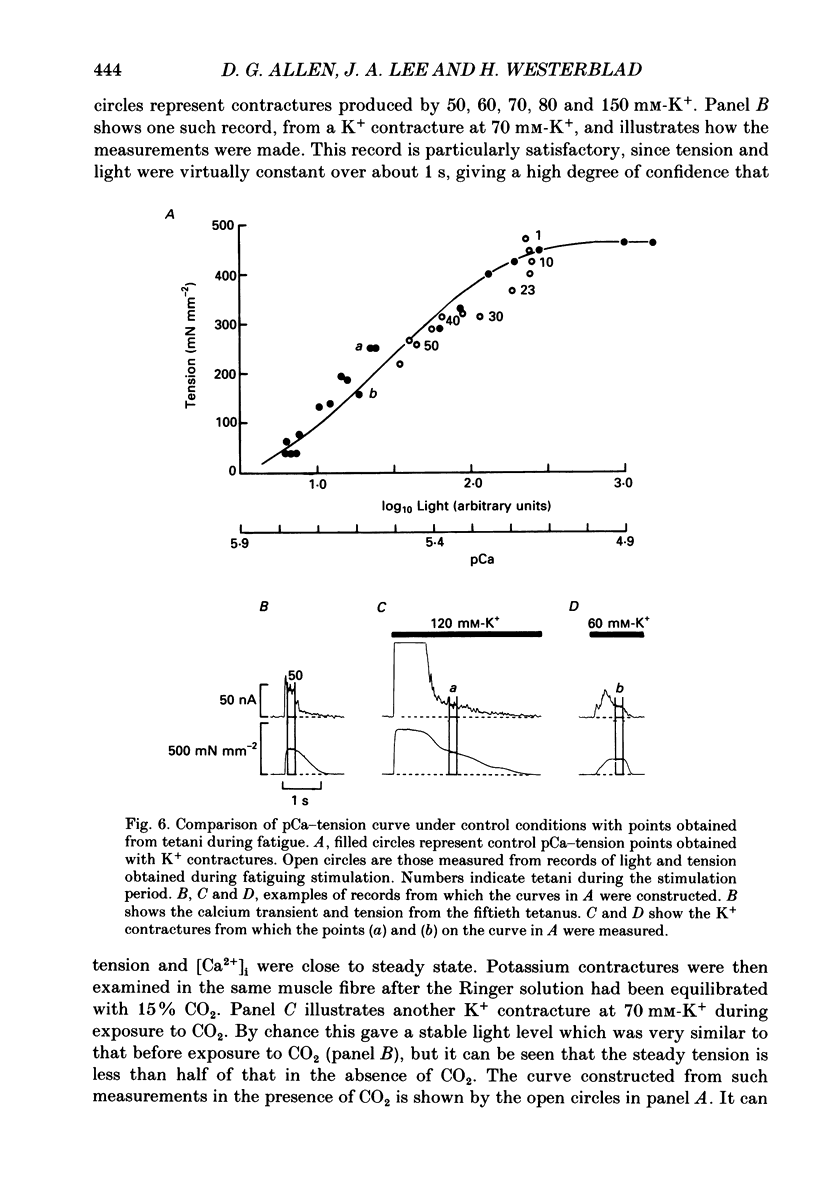
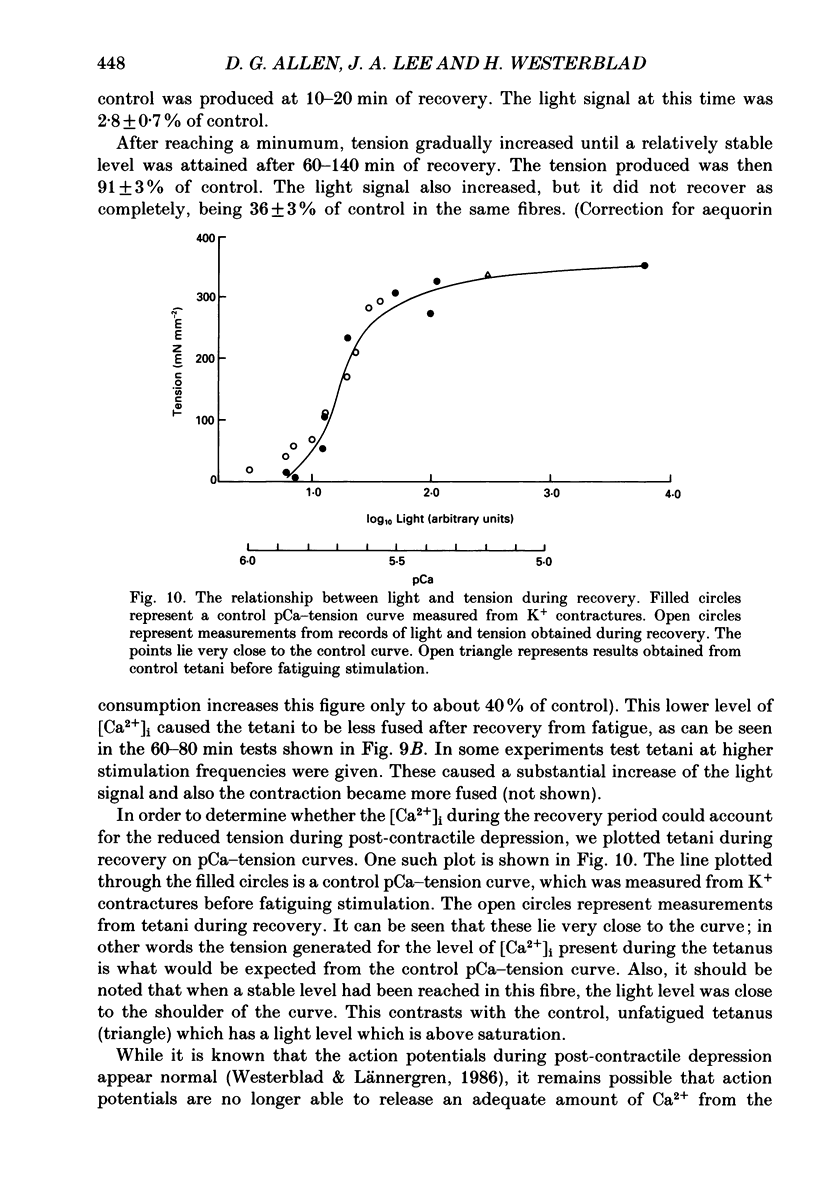
- Bigland-Ritchie B., Woods J. J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984 Nov-Dec;7(9):691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
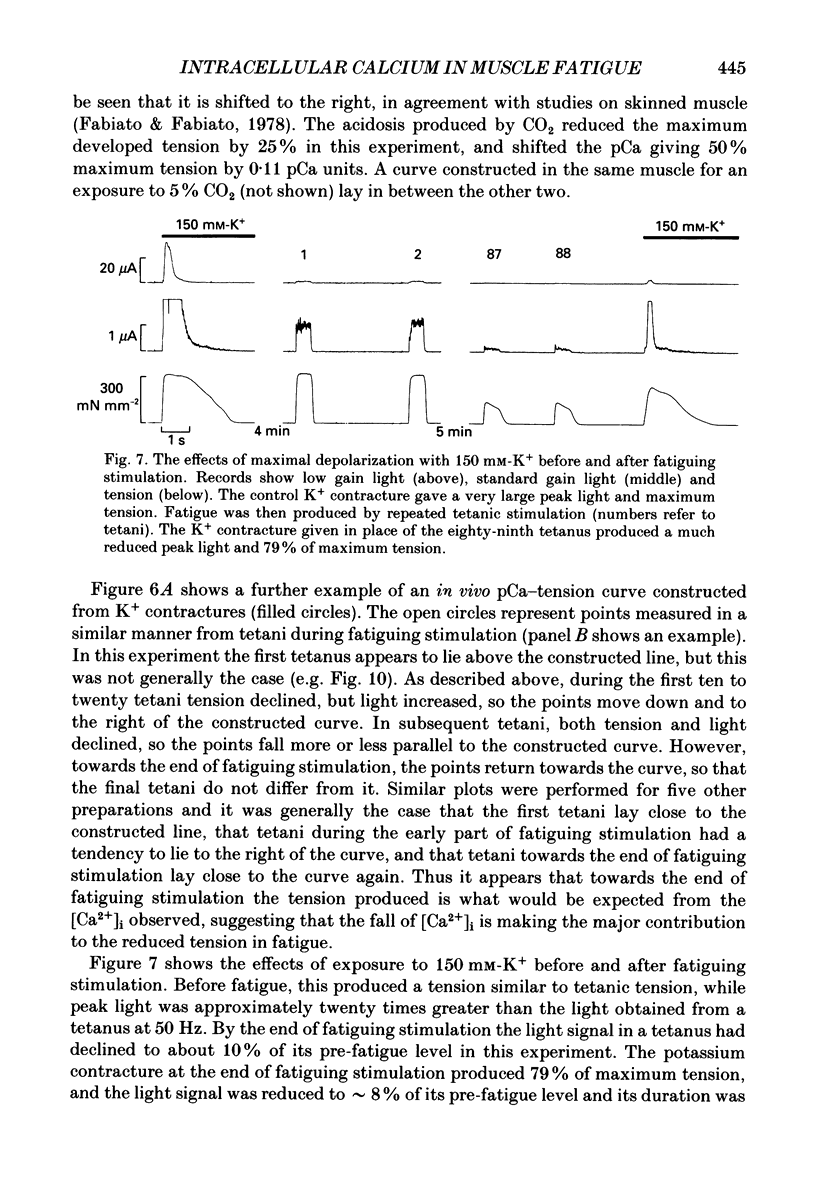
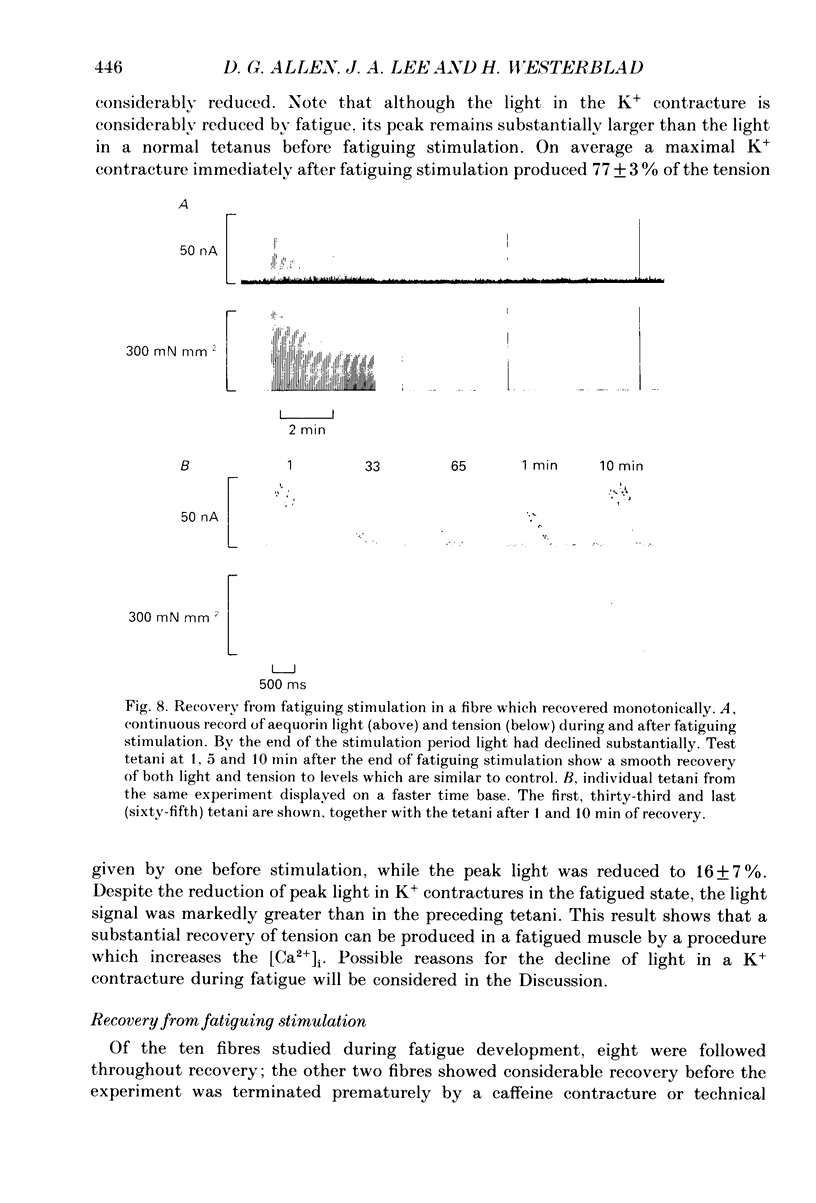
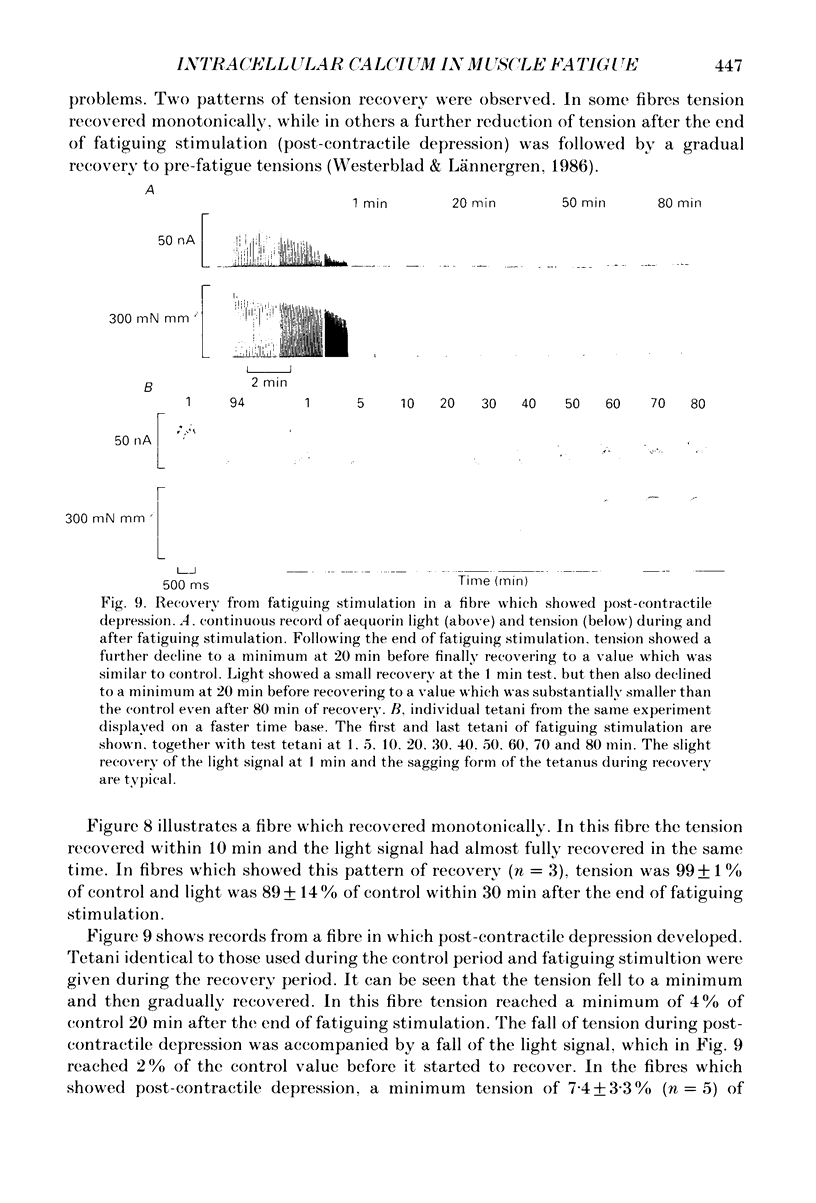
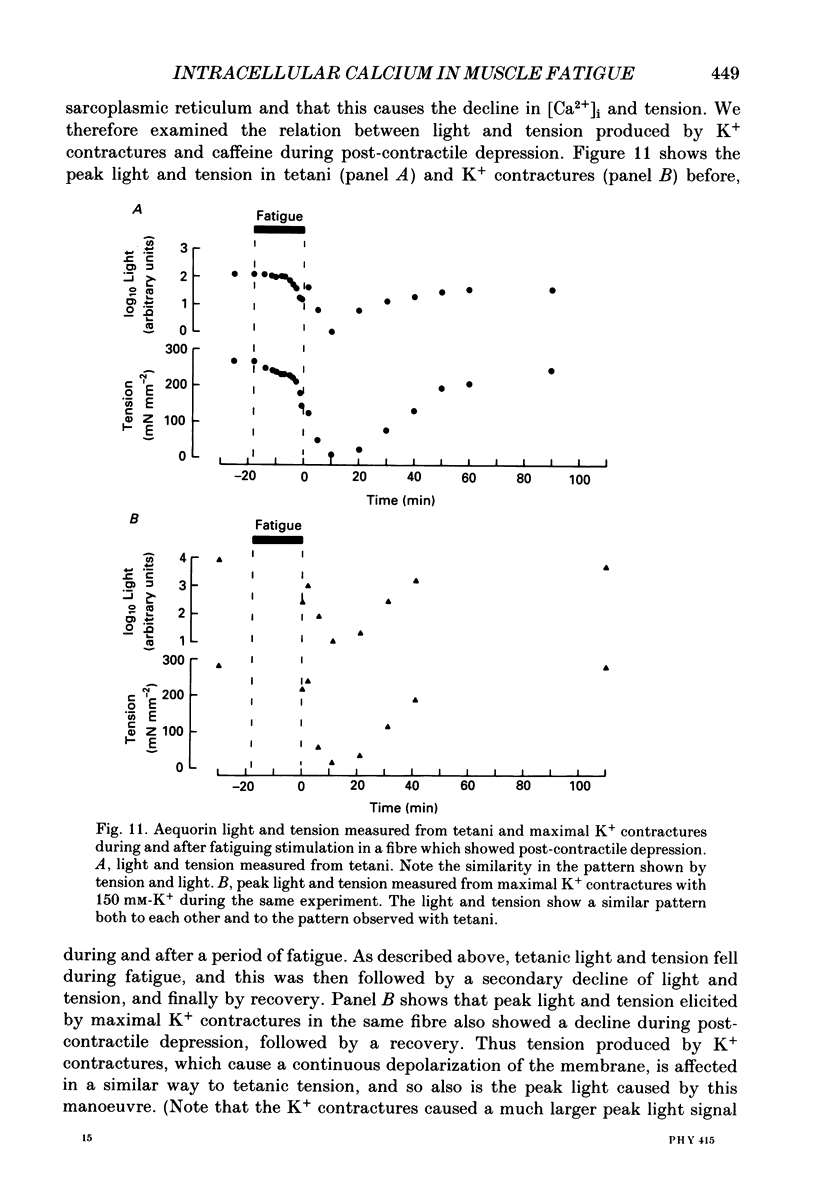
- Blanchard E. M., Pan B. S., Solaro R. J. The effect of acidic pH on the ATPase activity and troponin Ca2+ binding of rabbit skeletal myofilaments. J Biol Chem. 1984 Mar 10;259(5):3181–3186. [PubMed] [Google Scholar]
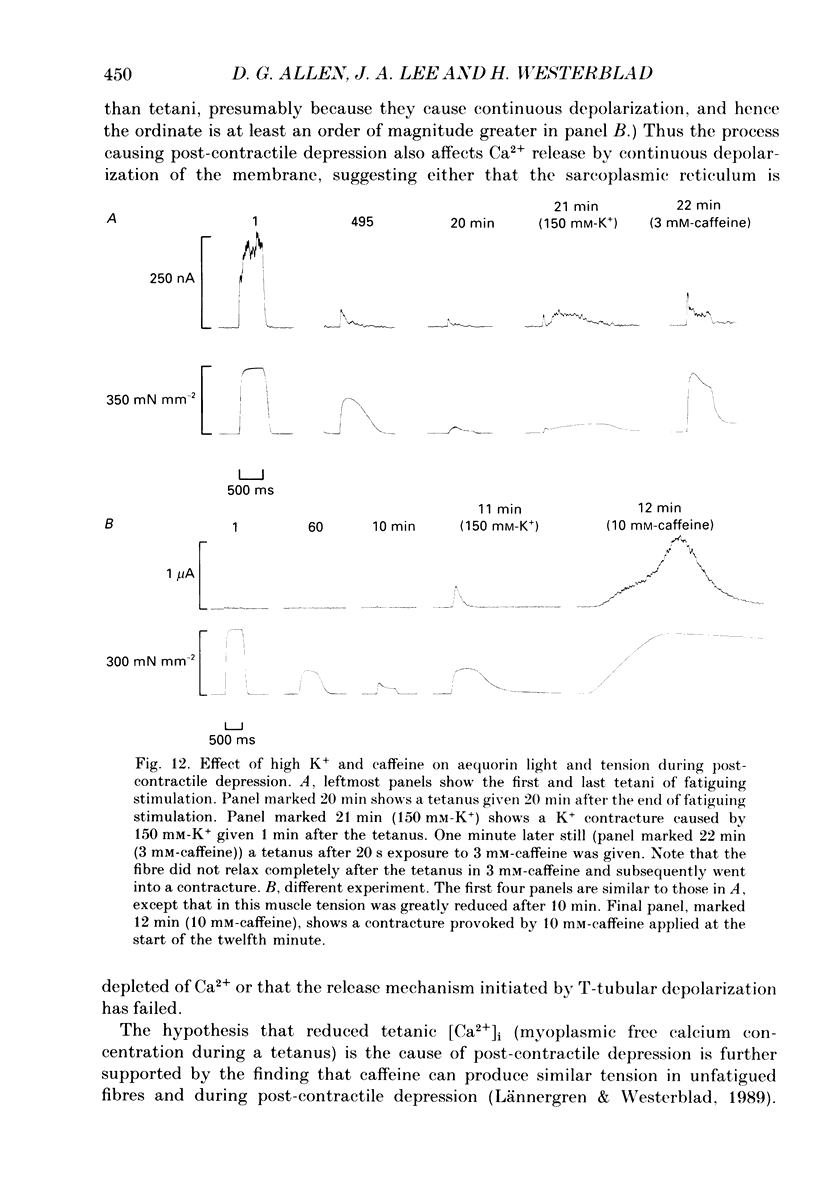
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. J Muscle Res Cell Motil. 1986 Jun;7(3):269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Curtin N. A. Intracellular pH and buffer power of type 1 and 2 fibres from skeletal muscle of Xenopus laevis. Pflugers Arch. 1987 Apr;408(4):386–389. doi: 10.1007/BF00581133. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Use of aequorin for the appraisal of the hypothesis of the release of calcium from the sarcoplasmic reticulum induced by a change of pH in skinned cardiac cells. Cell Calcium. 1985 Apr;6(1-2):95–108. doi: 10.1016/0143-4160(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Somlyo A. V., McClellan G., Shuman H., Borrero L. M., Somlyo A. P. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: electron probe analysis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Mahoney C. W., Kerrick W. G. MgADP- increases maximum tension and Ca2+ sensitivity in skinned rabbit soleus fibers. Pflugers Arch. 1987 Sep;410(1-2):30–36. doi: 10.1007/BF00581892. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Meyer R. A. Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. Am J Physiol. 1985 May;248(5 Pt 1):C542–C549. doi: 10.1152/ajpcell.1985.248.5.C542. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force and membrane potential during and after fatiguing, continuous high-frequency stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):359–368. doi: 10.1111/j.1748-1716.1986.tb07989.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Maximum tension and force-velocity properties of fatigued, single Xenopus muscle fibres studied by caffeine and high K+. J Physiol. 1989 Feb;409:473–490. doi: 10.1113/jphysiol.1989.sp017508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Vergara J. L., Rapoport S. I. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978 Nov;72(5):593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechère J. F., Derancourt J., Haiech J. The participation of parvalbumins in the activation-relaxation cycle of vertebrate fast skeletal-muscle. FEBS Lett. 1977 Mar 15;75(1):111–114. doi: 10.1016/0014-5793(77)80064-1. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Allen D. G. Effects of metabolic blockade on intracellular calcium concentration in isolated ferret ventricular muscle. Circ Res. 1988 Jun;62(6):1223–1236. doi: 10.1161/01.res.62.6.1223. [DOI] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Orozco C., Heller P. F., Froehlich J. P. Single calcium channels in native sarcoplasmic reticulum membranes from skeletal muscle. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7741–7745. doi: 10.1073/pnas.83.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Force and membrane potential during and after fatiguing, intermittent tetanic stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):369–378. doi: 10.1111/j.1748-1716.1986.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


