Abstract
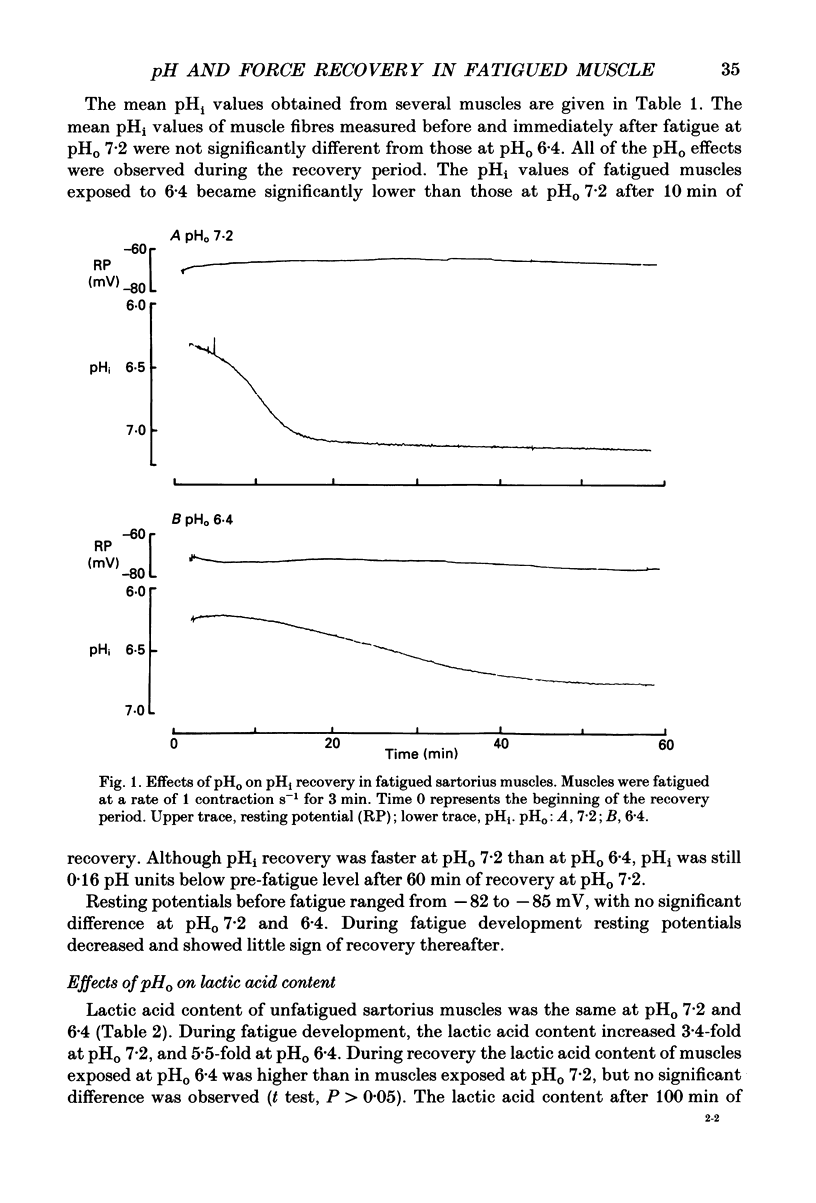
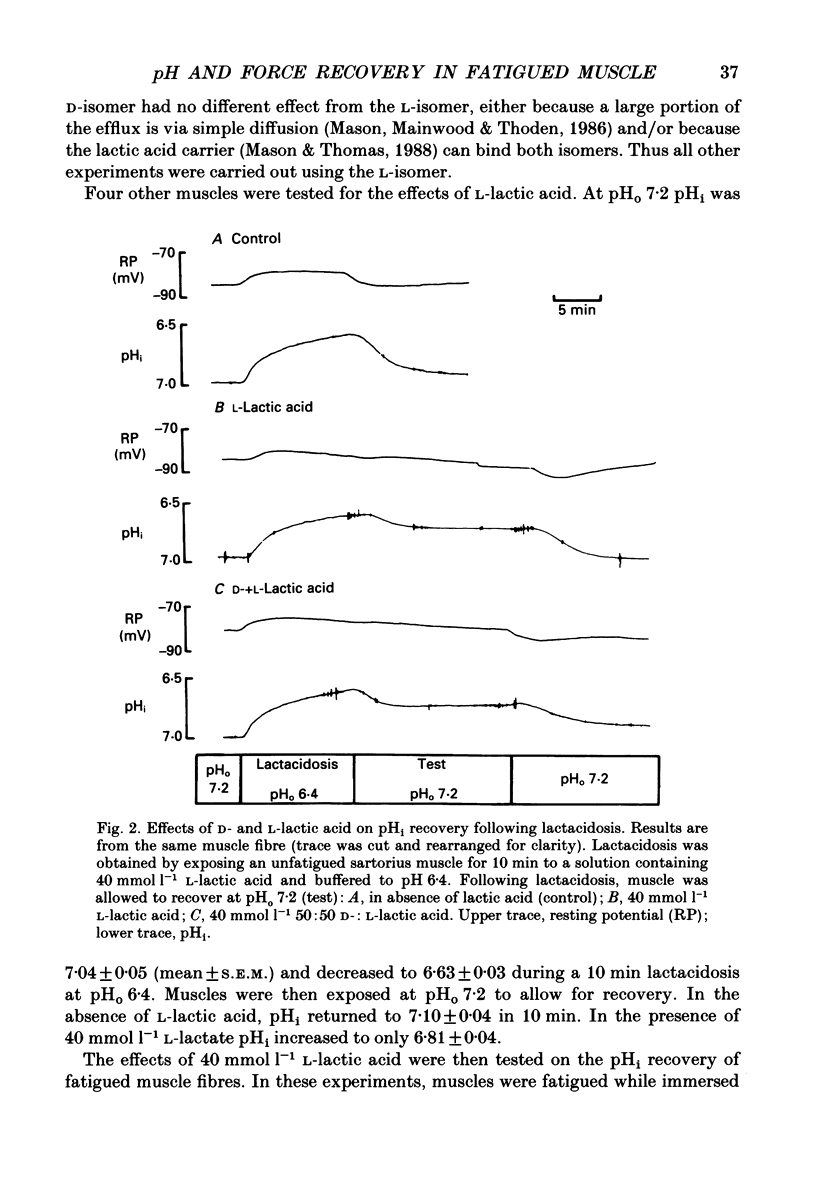
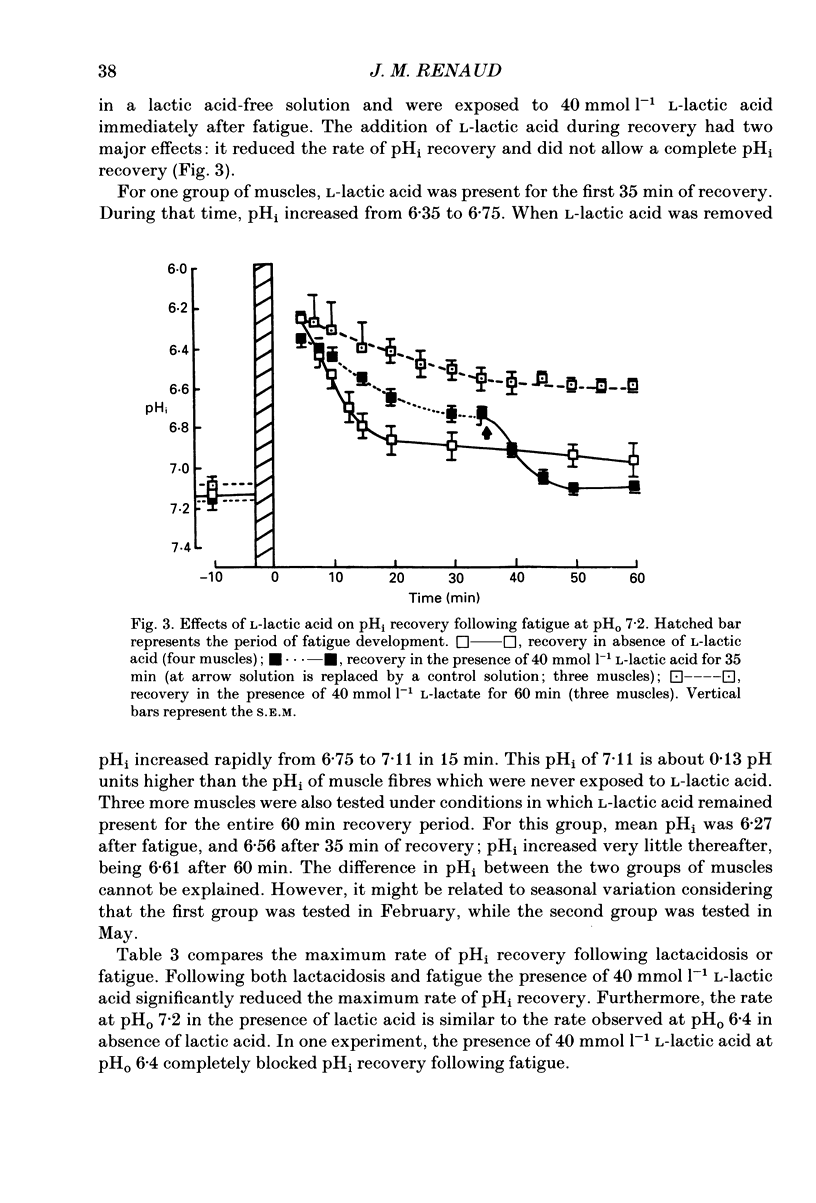
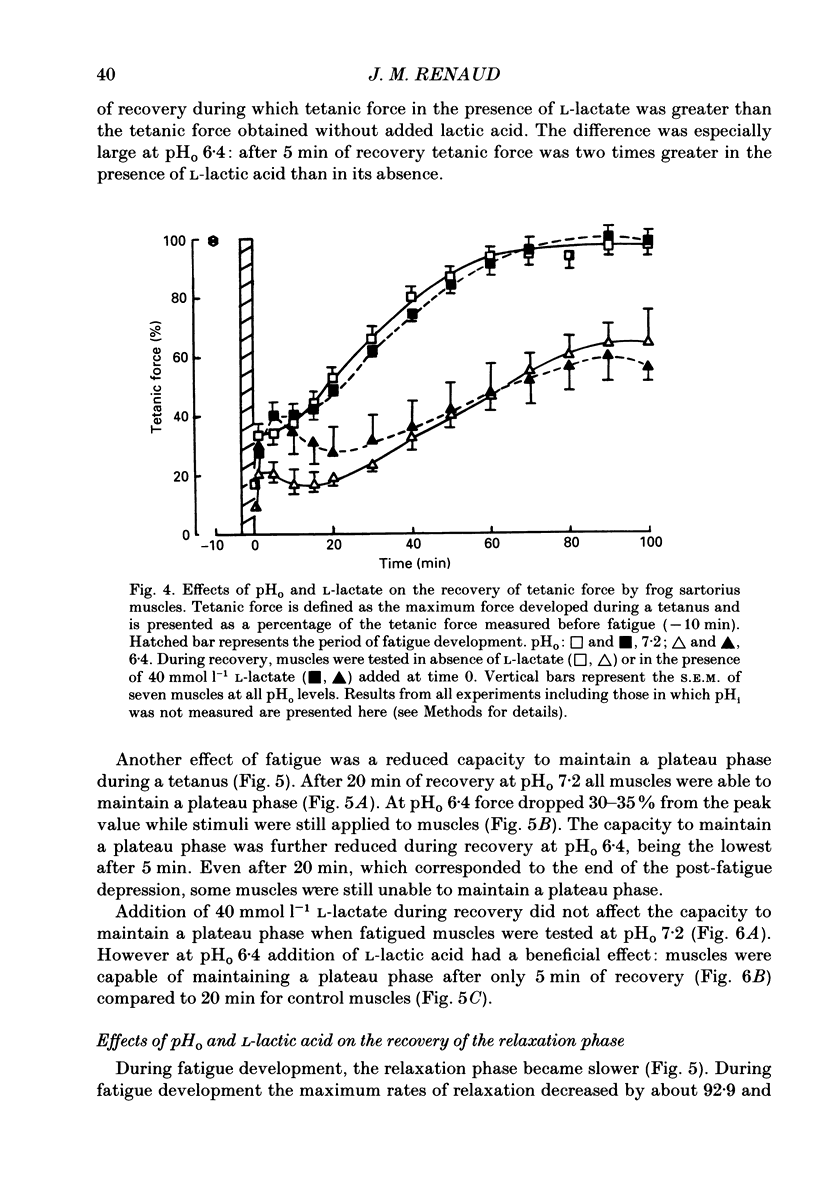
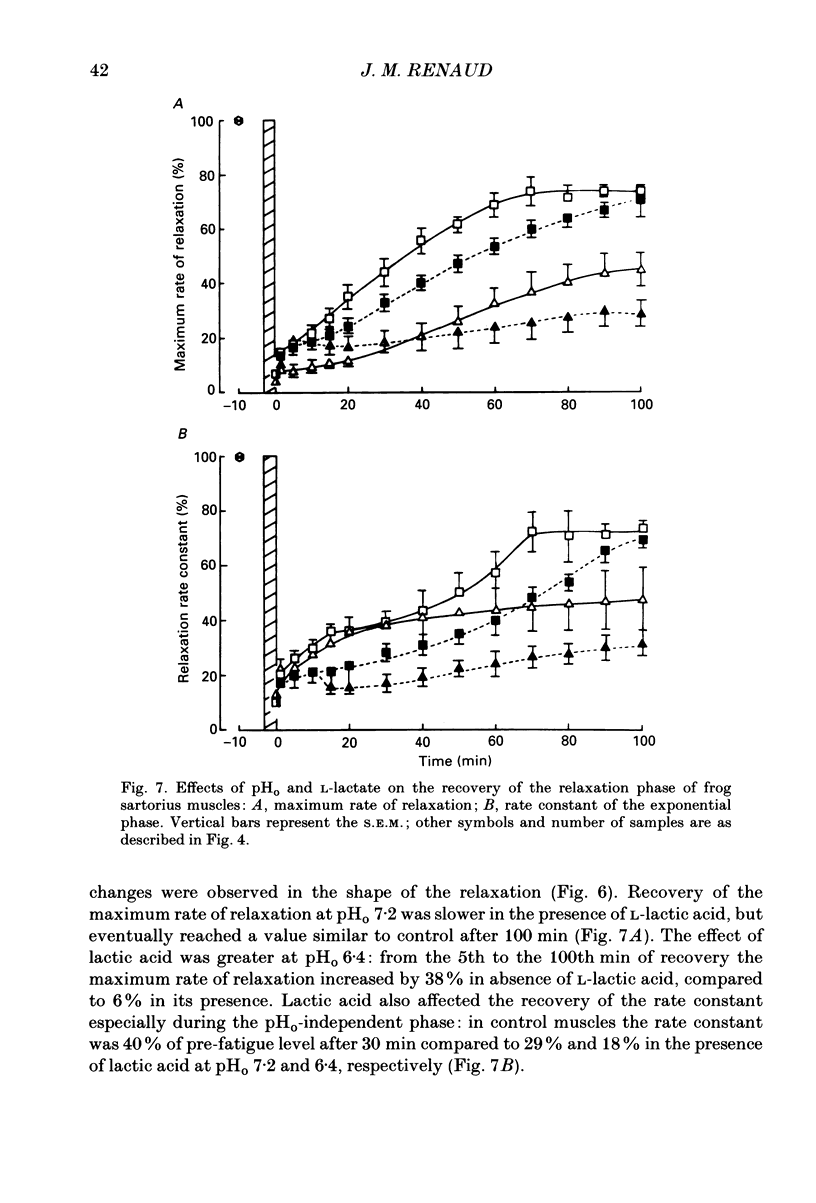
1. The effects of pHo (extracellular pH) and lactic acid on pHi (intracellular pH) and tetanic force were examined in frog sartorius muscle. Ion-selective microelectrodes were used to measure pHi. Tetanic force was elicited by field stimulation. Experiments were performed in HEPES-buffered solution equilibrated with 100% O2. 2. Mean pHi values (+/- S.E.M.) of unfatigued frog sartorius muscles were 7.14 +/- 0.02 and 7.05 +/- 0.09 at pHo 7.2 and 6.4, respectively. 3. A stimulation at a rate of one 100 ms tetanic contraction per second for 3 min reduced pHi to 6.21 +/- 0.09 and 6.20 +/- 0.04 at pHo 7.2 and 6.4, respectively. Meanwhile at pHo 7.2, the tetanic force (defined as the maximum force developed during a tetanus) decreased by 82.9 +/- 2.6%, the maximum rate of relaxation decreased by 92.9 +/- 0.9%, and the rate constant of the relaxation decreased by 88.5 +/- 1.6%. At pHo 6.4, the decrease in tetanic force, maximum rate of relaxation and rate constant were 90.6 +/- 1.8%, 93.8 +/- 0.5 and 87.5 +/- 2.7%, respectively. 4. The maximum rates of recovery of pHi following fatigue were 0.068 +/- 0.05 and 0.025 +/- 0.05 pH units min-1 at pHo 7.2 and 6.4, respectively. Recovery of normal tetanic force and relaxation rate was also slower at acidic pHo than at neutral pHo. 5. In the presence of 40 mmol l-1 L-lactic acid at pHo 7.2, the maximum rate of pHi recovery following fatigue was only 0.027 +/- 0.03 pH units min-1 at pHo 7.2. The presence of lactic acid also reduced the recovery of the relaxation phase, but not the recovery of tetanic force. 6. It is suggested that pHi recovery is not a limiting factor for tetanic force recovery and that the extracellular H+ inhibits tetanic force recovery by acting at a site located on the outer surface of the sarcolemma. The recovery of the relaxation phase is believed to be pHi dependent.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Benadé A. J., Heisler N. Comparison of efflux rates of hydrogen and lactate ions from isolated muscles in vitro. Respir Physiol. 1978 Mar;32(3):369–380. doi: 10.1016/0034-5687(78)90124-x. [DOI] [PubMed] [Google Scholar]
- Bountra C., Kaila K., Vaughan-Jones R. D. Effect of repetitive activity upon intracellular pH, sodium and contraction in sheep cardiac Purkinje fibres. J Physiol. 1988 Apr;398:341–360. doi: 10.1113/jphysiol.1988.sp017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Buffer power and intracellular pH of frog sartorius muscle. Biophys J. 1986 Nov;50(5):837–841. doi: 10.1016/S0006-3495(86)83524-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. J Muscle Res Cell Motil. 1986 Jun;7(3):269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Flitney F. W. Laser diffraction studies of sarcomere dynamics during 'isometric' relaxation in isolated muscle fibres of the frog. J Physiol. 1982 Aug;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulks J. G., Perry F. A. Effects of pH on excitation and contraction in frog twitch muscle. Can J Physiol Pharmacol. 1977 Jun;55(3):709–723. doi: 10.1139/y77-096. [DOI] [PubMed] [Google Scholar]
- Juel C. Intracellular pH recovery and lactate efflux in mouse soleus muscles stimulated in vitro: the involvement of sodium/proton exchange and a lactate carrier. Acta Physiol Scand. 1988 Mar;132(3):363–371. doi: 10.1111/j.1748-1716.1988.tb08340.x. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Cechetto D. The effect of bicarbonate concentration on fatigue and recovery in isolated rat diaphragm muscle. Can J Physiol Pharmacol. 1980 Jun;58(6):624–632. doi: 10.1139/y80-103. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Lucier G. E. Fatigue and recovery in isolated frog sartorius muscles: the effects of bicarbonate concentration and associated potassium loss. Can J Physiol Pharmacol. 1972 Feb;50(2):132–142. doi: 10.1139/y72-020. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Renaud J. M. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol. 1985 May;63(5):403–416. doi: 10.1139/y85-072. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P., Paterson R. A. The metabolic changes in frog sartorius muscles during recovery from fatigue at different external bicarbonate concentrations. Can J Physiol Pharmacol. 1972 Feb;50(2):143–155. doi: 10.1139/y72-021. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P. The effects of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. J Physiol. 1975 Aug;250(1):1–22. doi: 10.1113/jphysiol.1975.sp011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. J., Mainwood G. W., Thoden J. S. The influence of extracellular buffer concentration and propionate on lactate efflux from frog muscle. Pflugers Arch. 1986 May;406(5):472–479. doi: 10.1007/BF00583369. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Thomas R. C. A microelectrode study of the mechanisms of L-lactate entry into and release from frog sartorius muscle. J Physiol. 1988 Jun;400:459–479. doi: 10.1113/jphysiol.1988.sp017132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Mainwood G. W. The effects of pH on the kinetics of fatigue and recovery in frog sartorius muscle. Can J Physiol Pharmacol. 1985 Nov;63(11):1435–1443. doi: 10.1139/y85-236. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Kerrick W. G. The effects of pH on Ca2+-activated force in frog skeletal muscle fibers. Pflugers Arch. 1979 May 15;380(1):41–45. doi: 10.1007/BF00582610. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sandow A., Zeman R. J. Tetanus relaxation. Temperature effects and Arrhenius analysis. Biochim Biophys Acta. 1979 Jul 10;547(1):27–35. doi: 10.1016/0005-2728(79)90092-6. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Seo Y. Effects of extracellular pH on lactate efflux from frog sartorius muscle. Am J Physiol. 1984 Sep;247(3 Pt 1):C175–C181. doi: 10.1152/ajpcell.1984.247.3.C175. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Radda G. K. A 31P-nuclear magnetic resonance study of skeletal muscle metabolism in rats depleted of creatine with the analogue beta-guanidinopropionic acid. Biochim Biophys Acta. 1984 Sep 14;805(1):79–88. doi: 10.1016/0167-4889(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Shulman R. G. NMR spectroscopy of living cells. Sci Am. 1983 Jan;248(1):86–93. doi: 10.1038/scientificamerican0183-86. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Sejersted O. M. Biochemical correlates of fatigue. A brief review. Eur J Appl Physiol Occup Physiol. 1988;57(3):336–347. doi: 10.1007/BF00635993. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Tension restoration with caffeine in fatigued Xenopus muscle fibres of various types. Acta Physiol Scand. 1987 Jun;130(2):357–358. doi: 10.1111/j.1748-1716.1987.tb08148.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


