Abstract
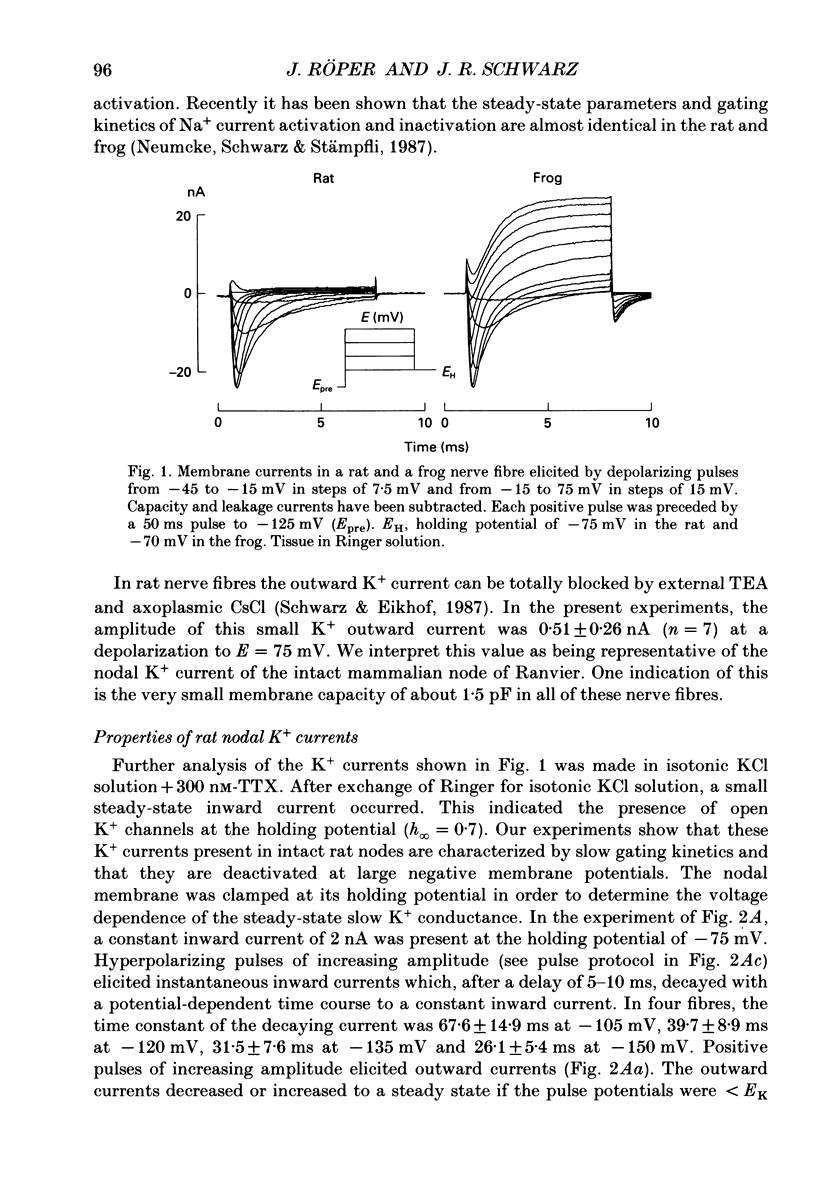
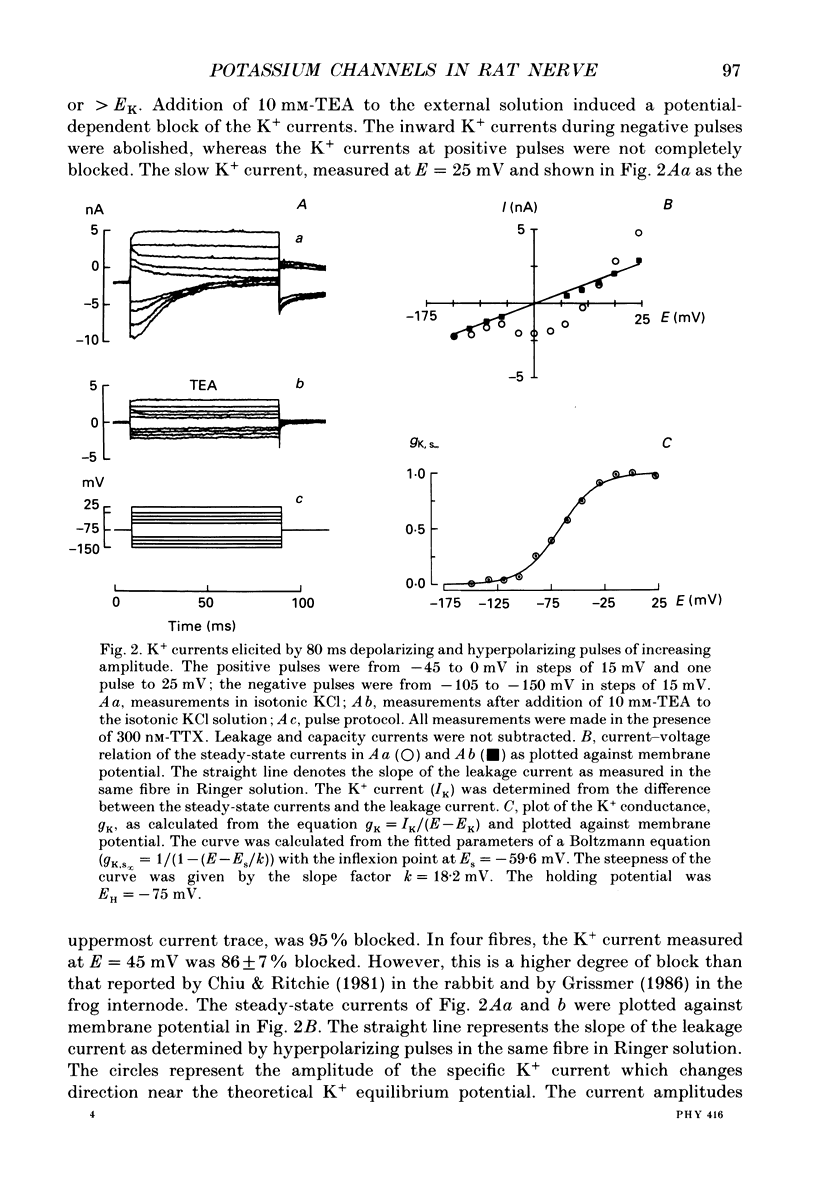
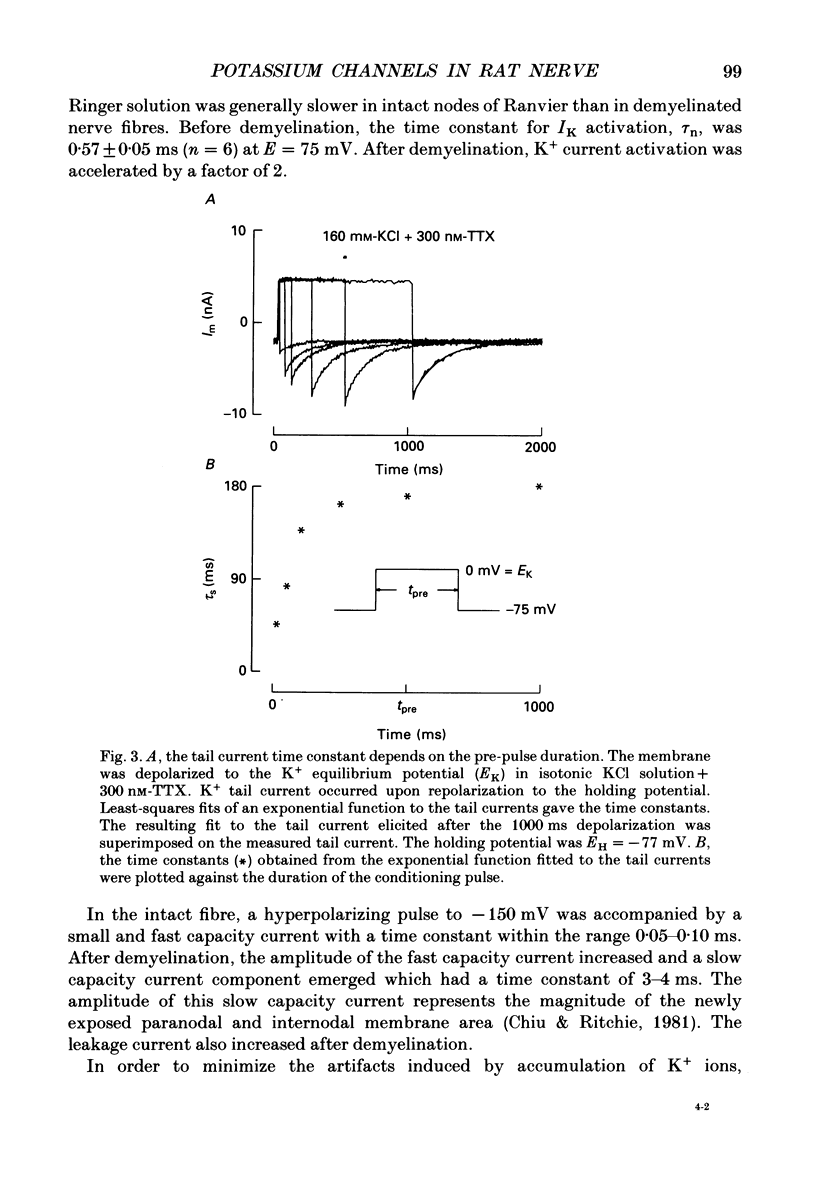
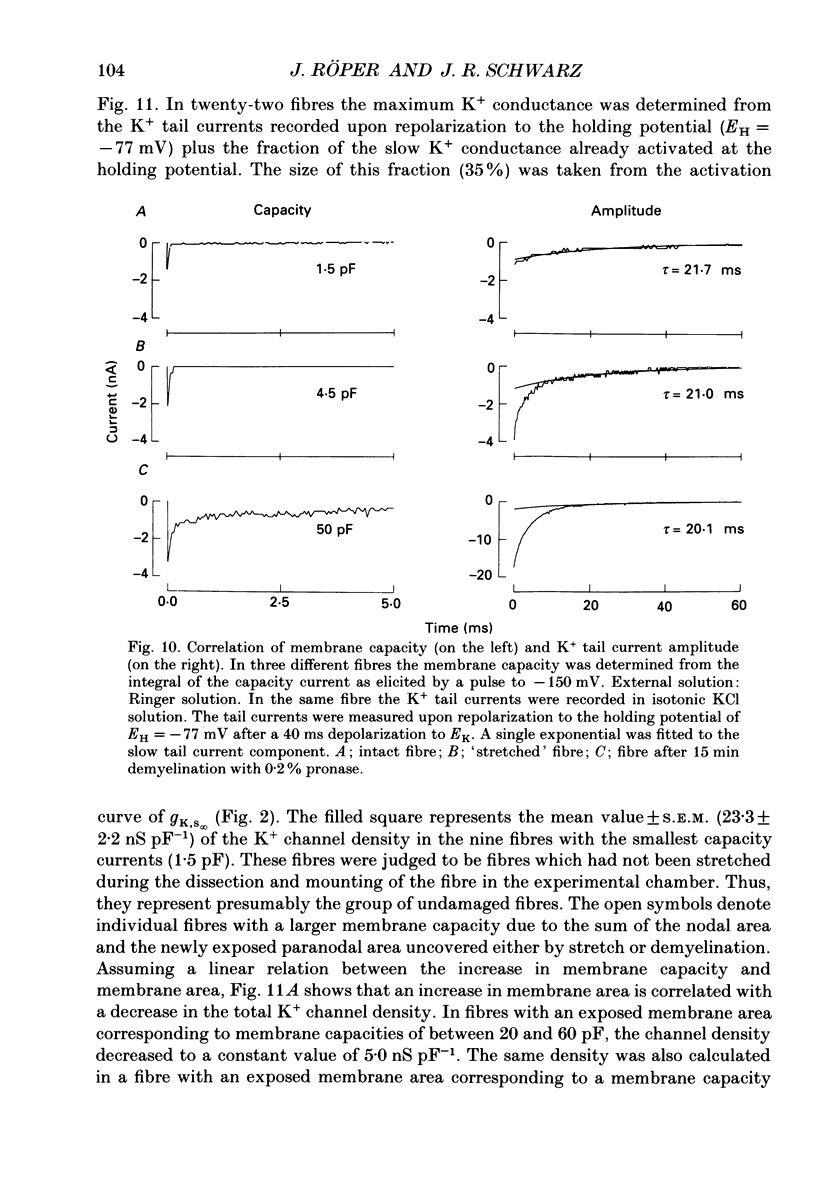
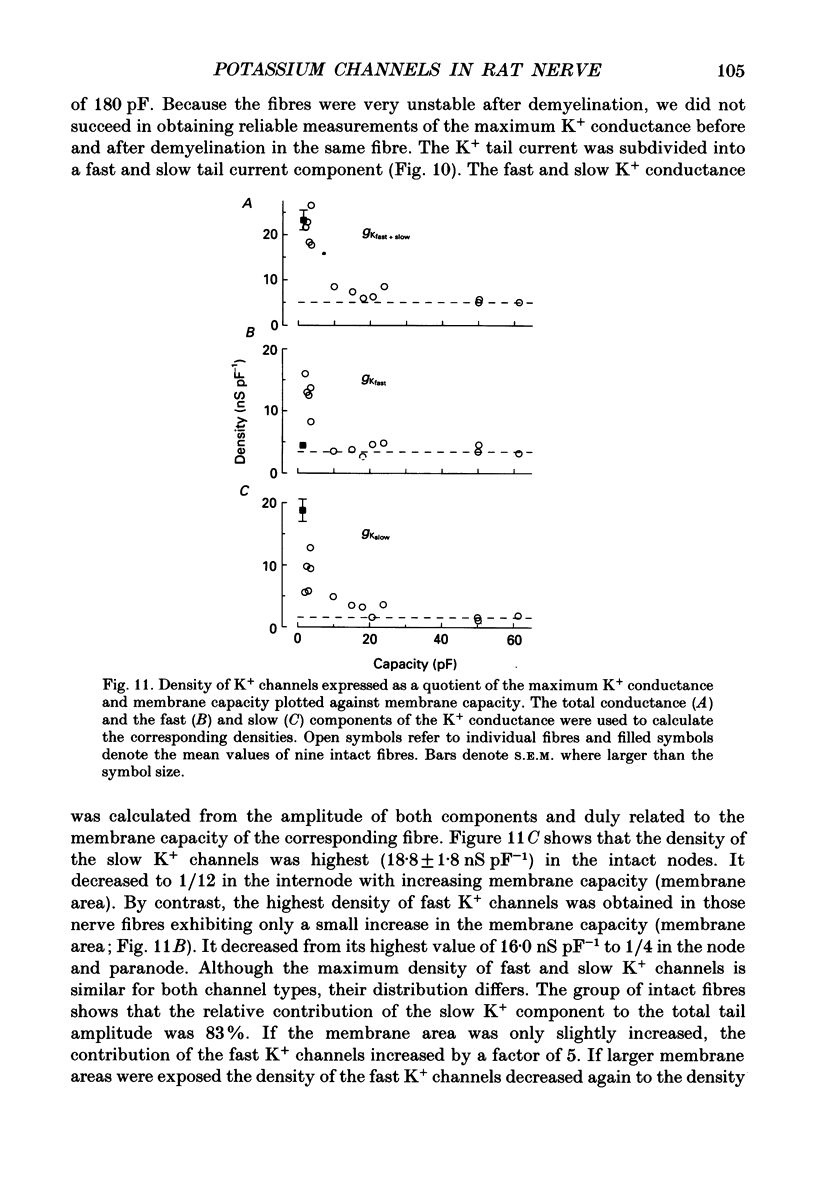
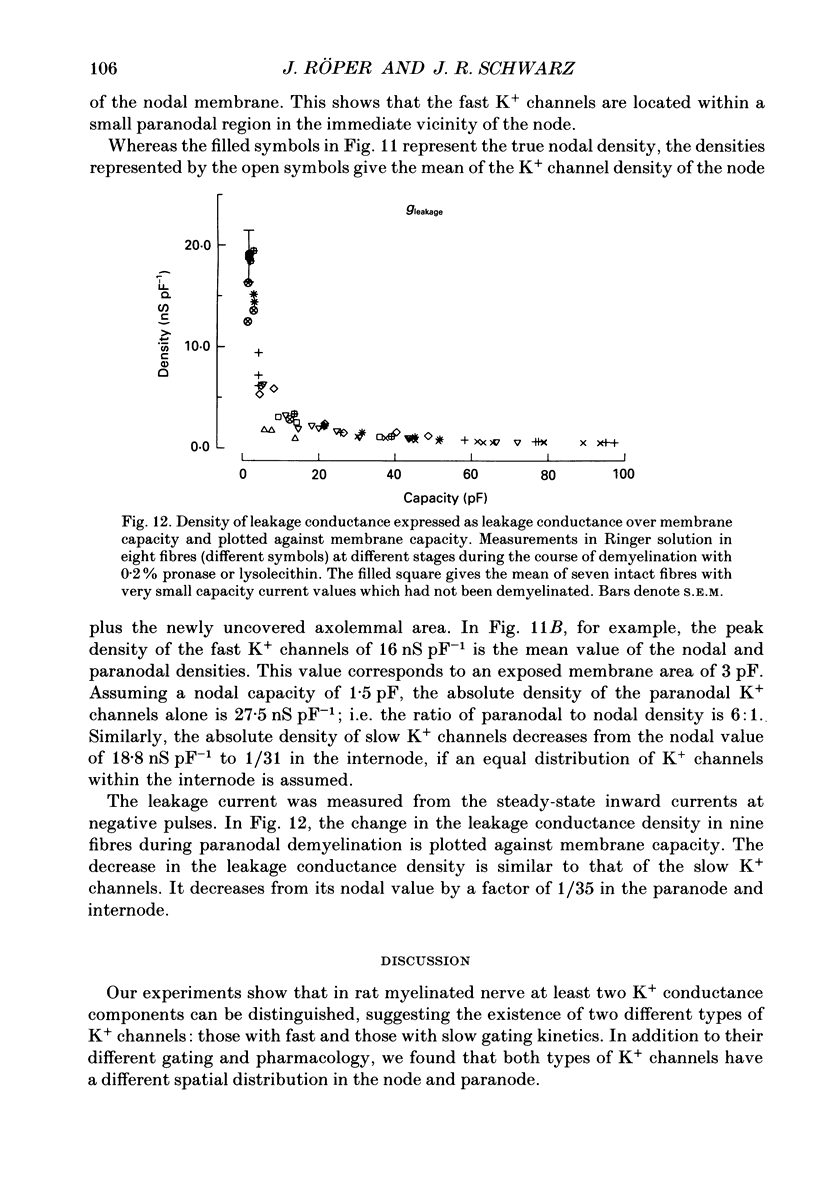
1. Potassium currents were measured in voltage-clamped single myelinated rat nerve fibres before and after paranodal demyelination with 0.2% pronase or 0.2% lysolecithin added to the external solution. Sodium currents were blocked by 300 nM-tetrodotoxin. For the purpose of comparison, intact frog nerve fibres were also investigated. 2. Our results suggest the existence of at least two distinct types of K+ channels in the intact node of Ranvier, one with slow and another with fast gating kinetics, in the ratio 4:1. 3. In the rat nodal membrane, slow K+ channels have voltage-dependent time constants of K+ deactivation with tau n = 68 ms at E = -105 mV and tau n = 26 ms at E = -150 mV at 20 degrees C. The activation curve of the slow K+ conductance is sigmoid with an inflexion point at -60 mV. This means that about 35% of the slow K+ channels are in the open state at the resting potential of -77 mV. Slow K+ channels could be blocked by 10 mM-tetraethylammonium chloride, but were insensitive to 4-aminopyridine. 4. After paranodal demyelination the ratio of fast to slow K+ channels increased from 17 to 83%. As in the frog (Dubois, 1981 alpha), the population of fast K+ channels in the rat may consist of two different subgroups, both of which can be blocked by 4-aminopyridine. 5. Demyelination was accompanied by an increase in the capacity current which was used to estimate the exposed membrane area. The density of slow and fast K+ channels was calculated from the quotient of the steady-state K+ conductance to membrane area. The density of the slow K+ channels is maximal in the nodal membrane and decreases to 1/31 in the internode. By contrast, the distribution of the fast K+ channels differs, their density being maximal in the paranode and decreasing to one-sixth in the node and internode.
Full text
PDF



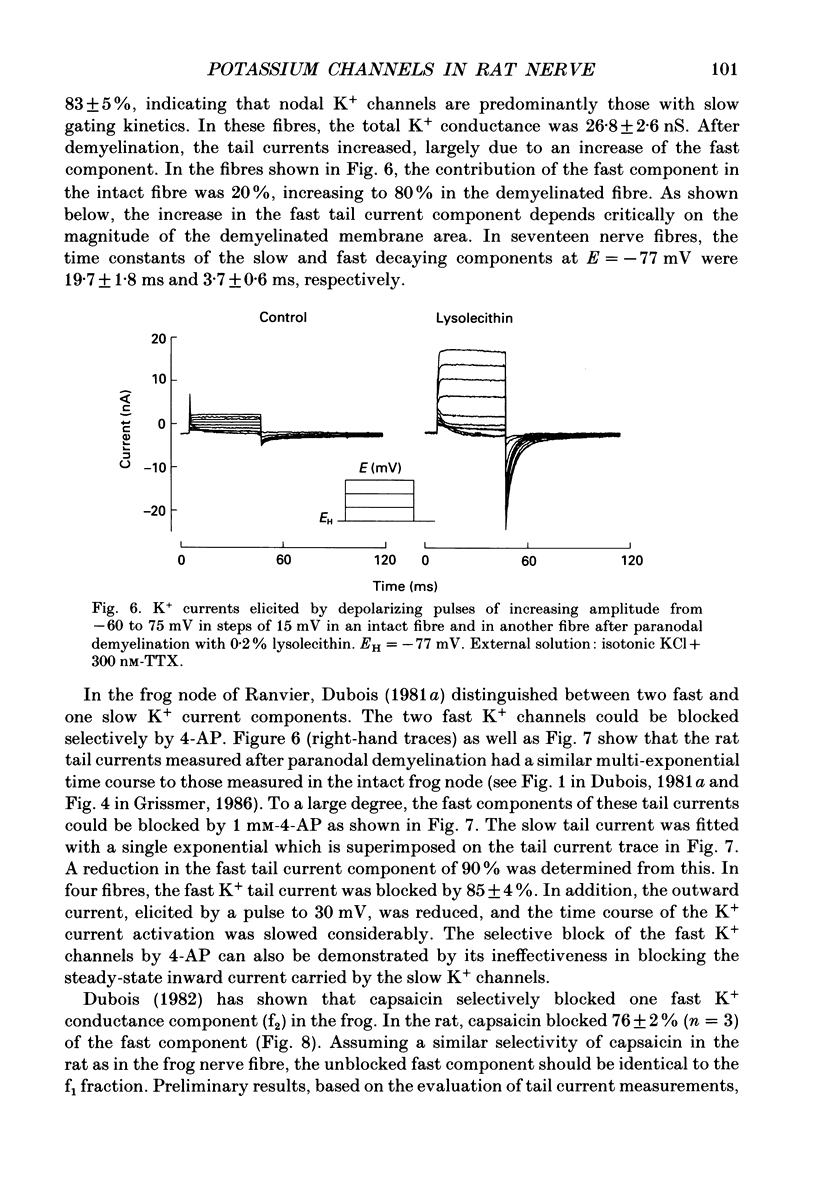
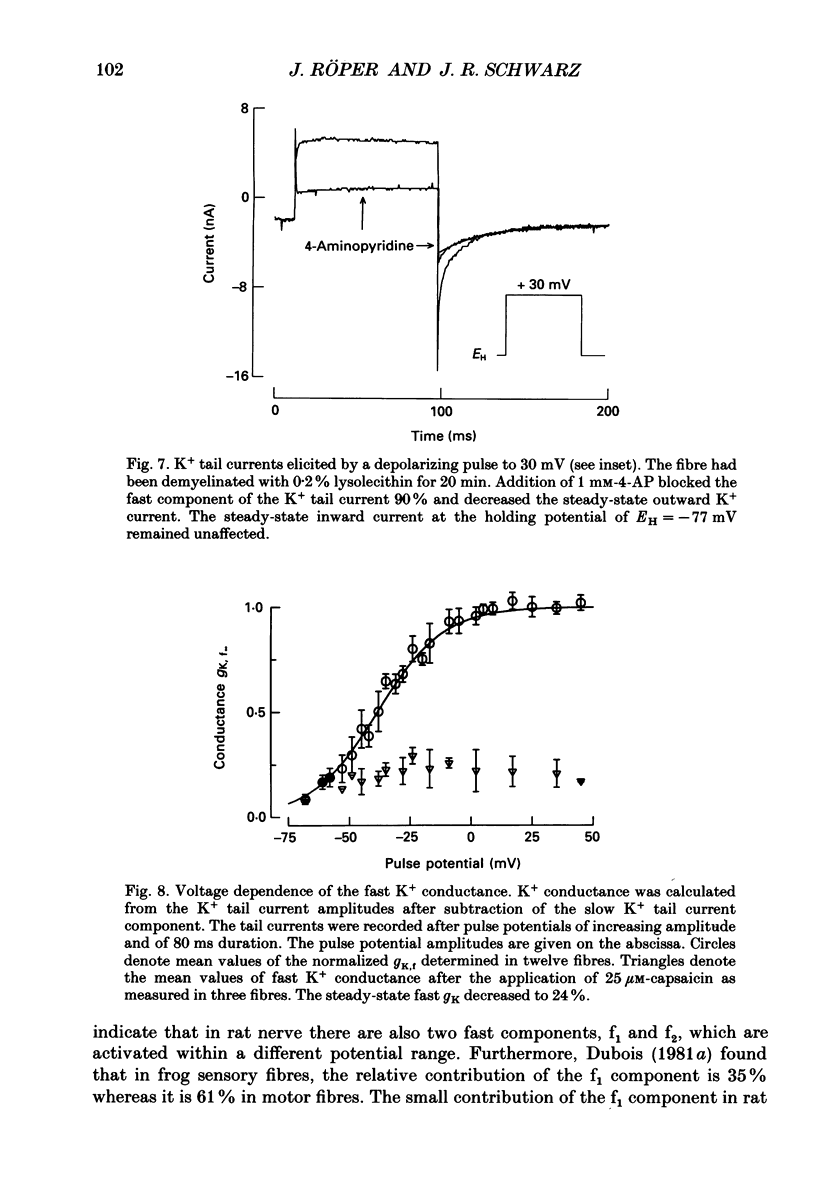
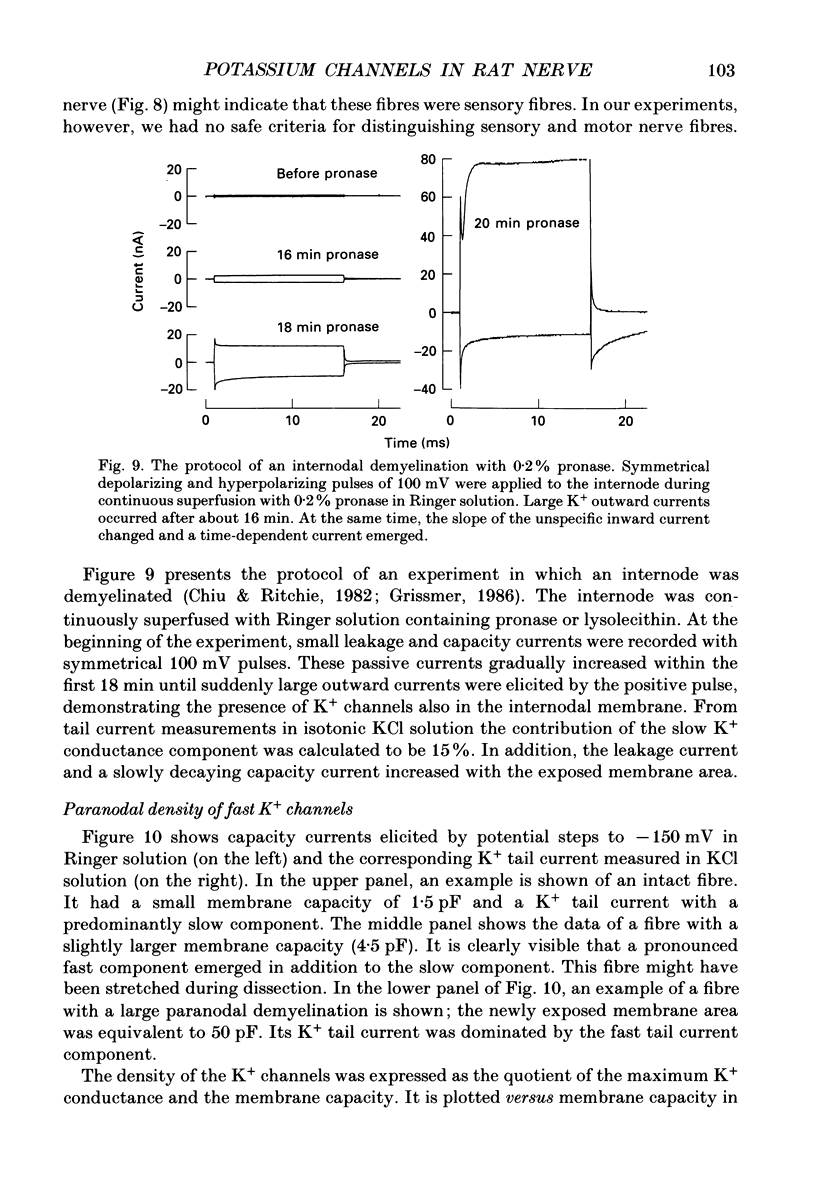




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987 Feb;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold C. H., Rydmark M. Electrophysiology and morphology of myelinated nerve fibers. VI. Anatomy of the paranode-node-paranode region in the cat. Experientia. 1983 Sep 15;39(9):964–976. doi: 10.1007/BF01989761. [DOI] [PubMed] [Google Scholar]
- Binah O., Palti Y. Potassium channels in the nodal membrane of rat myelinated fibres. Nature. 1981 Apr 16;290(5807):598–600. doi: 10.1038/290598a0. [DOI] [PubMed] [Google Scholar]
- Brismar T. Electrical properties of isolated demyelinated rat nerve fibres. Acta Physiol Scand. 1981 Oct;113(2):161–166. doi: 10.1111/j.1748-1716.1981.tb06877.x. [DOI] [PubMed] [Google Scholar]
- Brismar T. Potential clamp analysis of membrane currents in rat myelinated nerve fibres. J Physiol. 1980 Jan;298:171–184. doi: 10.1113/jphysiol.1980.sp013074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Potential clamp experiments on myelinated nerve fibres from alloxan diabetic rats. Acta Physiol Scand. 1979 Mar;105(3):384–386. doi: 10.1111/j.1748-1716.1979.tb06356.x. [DOI] [PubMed] [Google Scholar]
- Brismar T., Schwarz J. R. Potassium permeability in rat myelinated nerve fibres. Acta Physiol Scand. 1985 Jun;124(2):141–148. doi: 10.1111/j.1748-1716.1985.tb07645.x. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Evidence for the presence of potassium channels in the internode of frog myelinated nerve fibres. J Physiol. 1982 Jan;322:485–501. doi: 10.1113/jphysiol.1982.sp014051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Evidence for the presence of potassium channels in the paranodal region of acutely demyelinated mammalian single nerve fibres. J Physiol. 1981;313:415–437. doi: 10.1113/jphysiol.1981.sp013674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. On the physiological role of internodal potassium channels and the security of conduction in myelinated nerve fibres. Proc R Soc Lond B Biol Sci. 1984 Feb 22;220(1221):415–422. doi: 10.1098/rspb.1984.0010. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Schwarz W. Sodium and potassium currents in acutely demyelinated internodes of rabbit sciatic nerves. J Physiol. 1987 Oct;391:631–649. doi: 10.1113/jphysiol.1987.sp016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Capsaicin blocks one class of K+ channels in the frog node of Ranvier. Brain Res. 1982 Aug 12;245(2):372–375. doi: 10.1016/0006-8993(82)90820-4. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Properties of the slow K+ current of the nodal membrane. J Physiol (Paris) 1981 May;77(9):1129–1134. [PubMed] [Google Scholar]
- Grissmer S. Properties of potassium and sodium channels in frog internode. J Physiol. 1986 Dec;381:119–134. doi: 10.1113/jphysiol.1986.sp016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Two types of potassium current in rabbit cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):19–27. doi: 10.1098/rspb.1988.0061. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Waxman S. G., Hildebrand C., Ruiz J. A. Regenerating mammalian nerve fibres: changes in action potential waveform and firing characteristics following blockage of potassium conductance. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):77–87. doi: 10.1098/rspb.1982.0095. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Waxman S. G. Ionic channel organization of normal and regenerating mammalian axons. Prog Brain Res. 1987;71:89–101. doi: 10.1016/s0079-6123(08)61816-6. [DOI] [PubMed] [Google Scholar]
- Krylov B. V., Makovsky V. S. Spike frequency adaptation in amphibian sensory fibres is probably due to slow K channels. Nature. 1978 Oct 12;275(5680):549–551. doi: 10.1038/275549a0. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Tytell M. A. Macromolecular transfer from glia to the axon. J Exp Biol. 1981 Dec;95:153–165. doi: 10.1242/jeb.95.1.153. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz J. R., Stämpfli R. A comparison of sodium currents in rat and frog myelinated nerve: normal and modified sodium inactivation. J Physiol. 1987 Jan;382:175–191. doi: 10.1113/jphysiol.1987.sp016362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J Physiol. 1982 Aug;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Eikhof G. Na currents and action potentials in rat myelinated nerve fibres at 20 and 37 degrees C. Pflugers Arch. 1987 Aug;409(6):569–577. doi: 10.1007/BF00584655. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Sherratt R. M., Bostock H., Sears T. A. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980 Feb 7;283(5747):570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]