Abstract
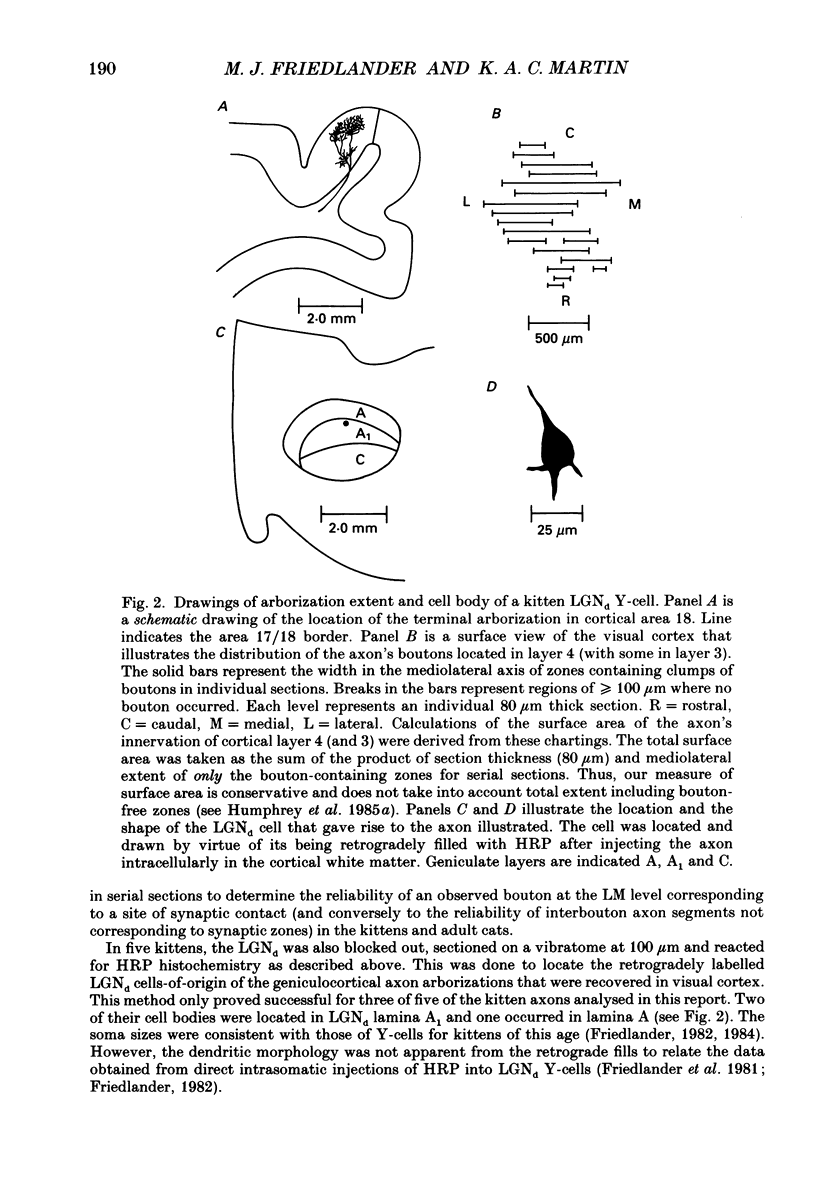
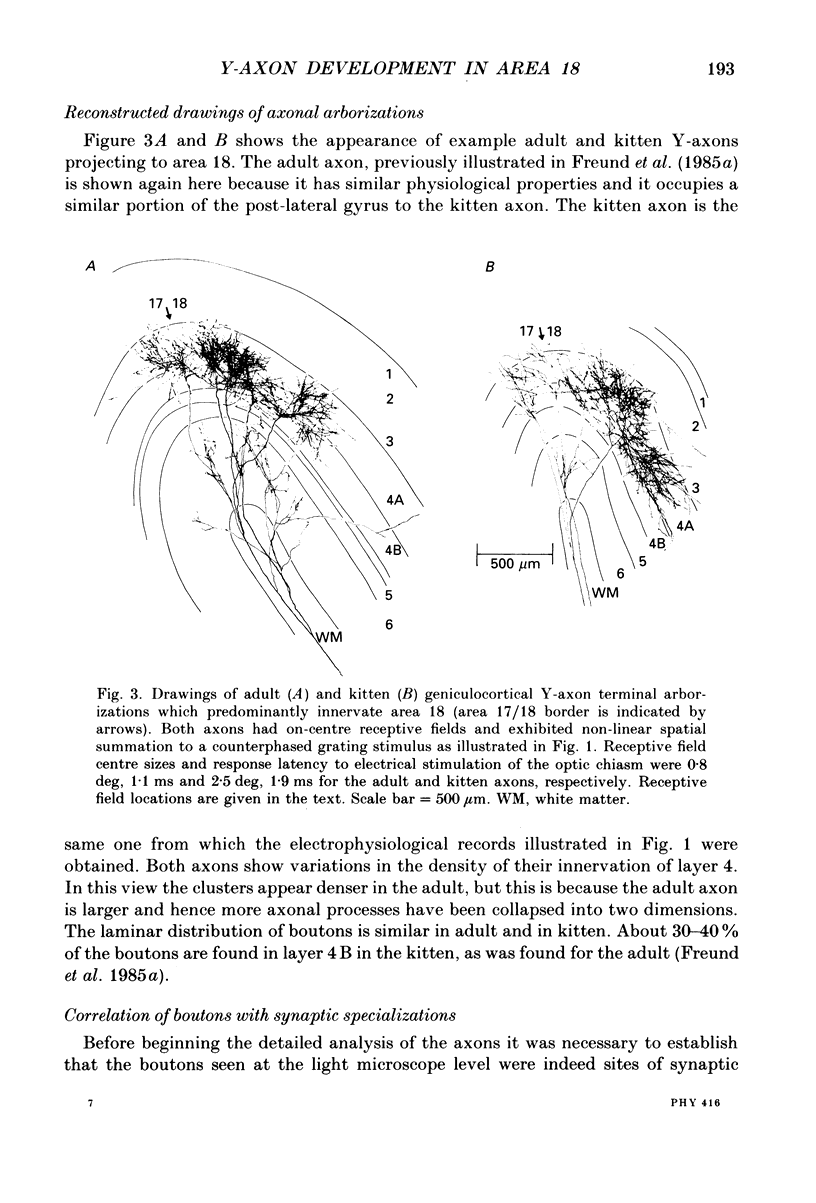
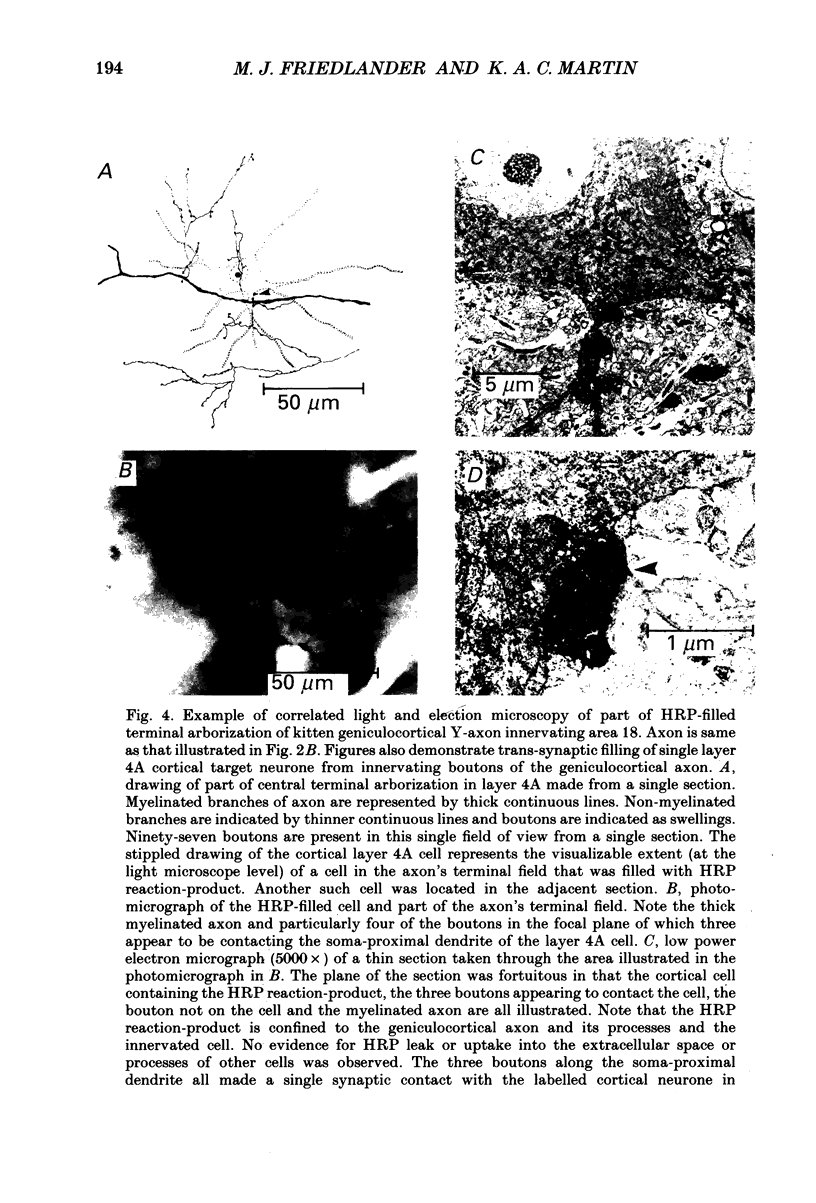
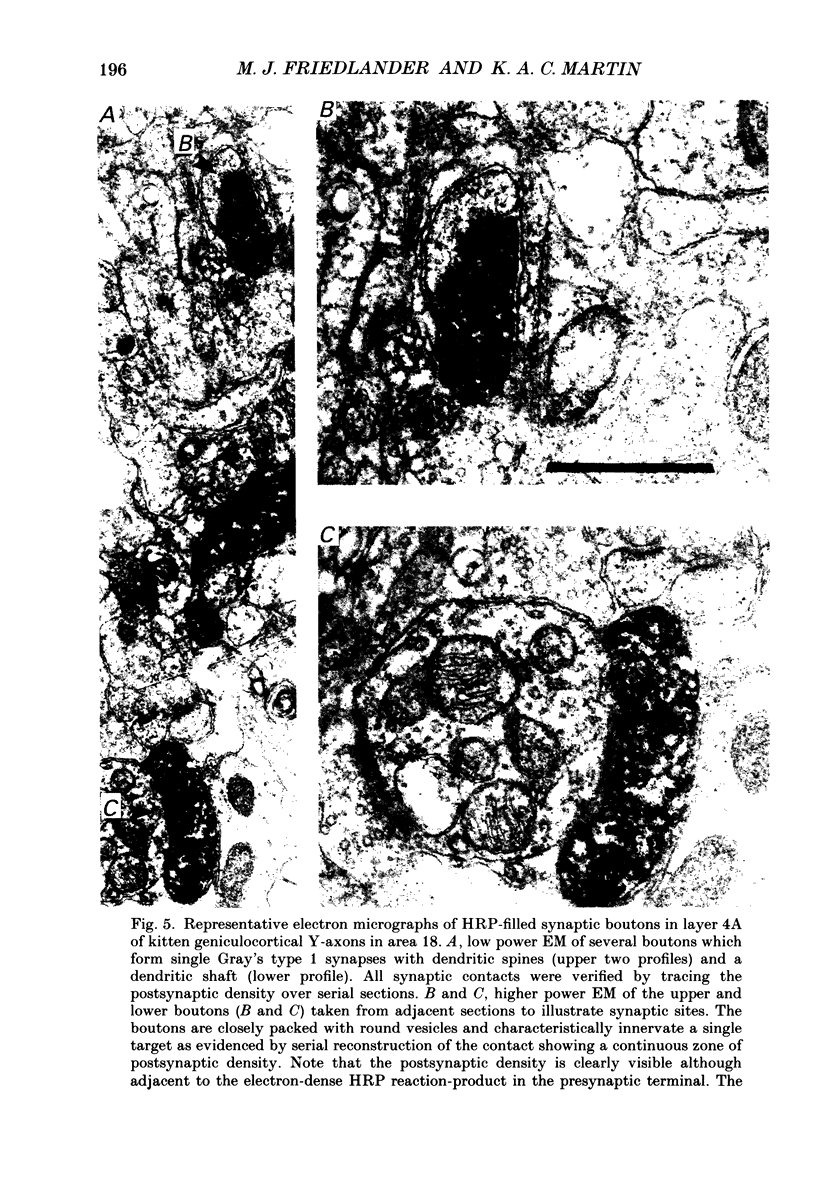
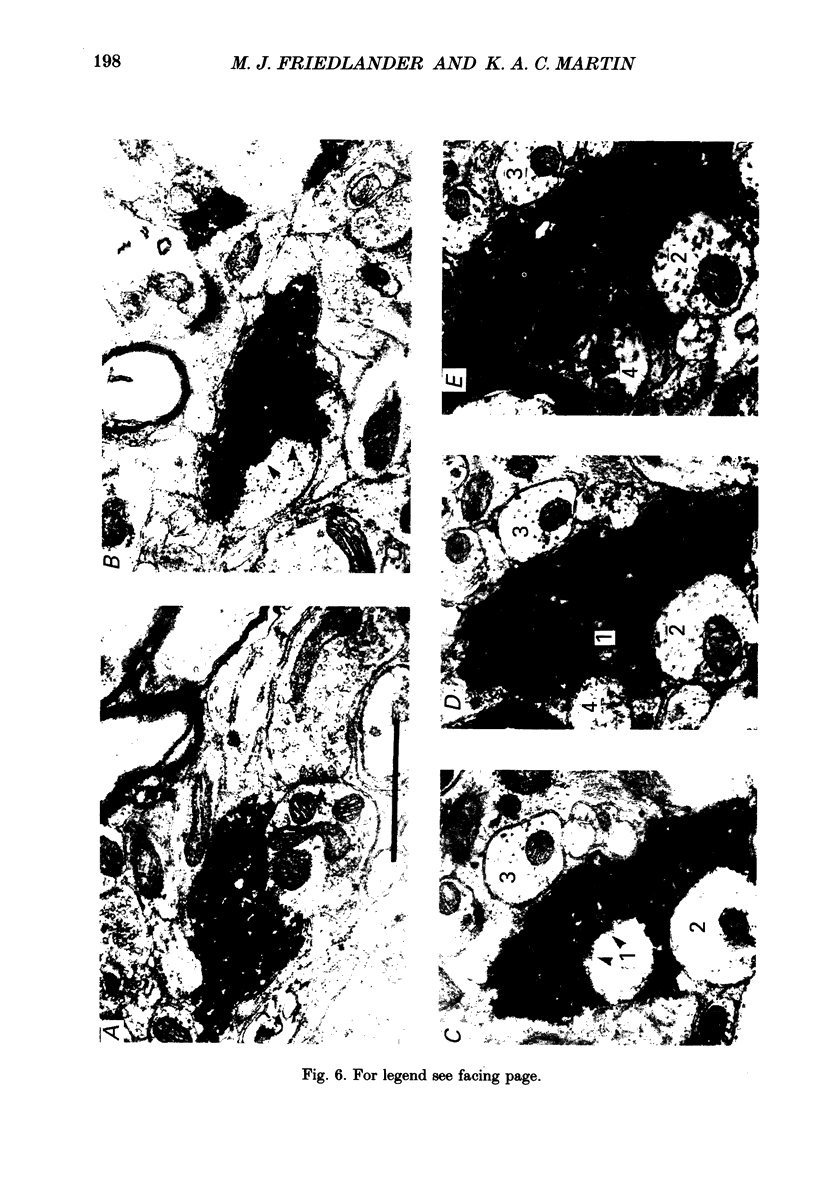
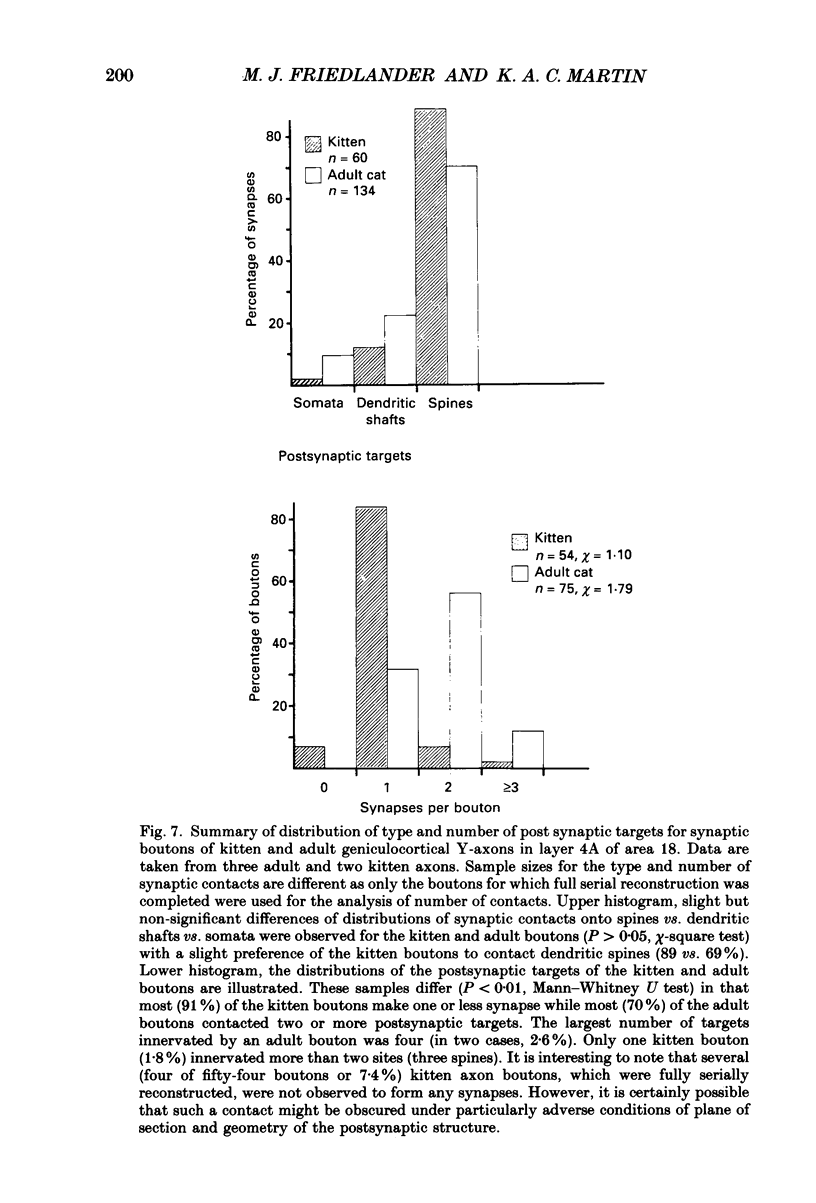
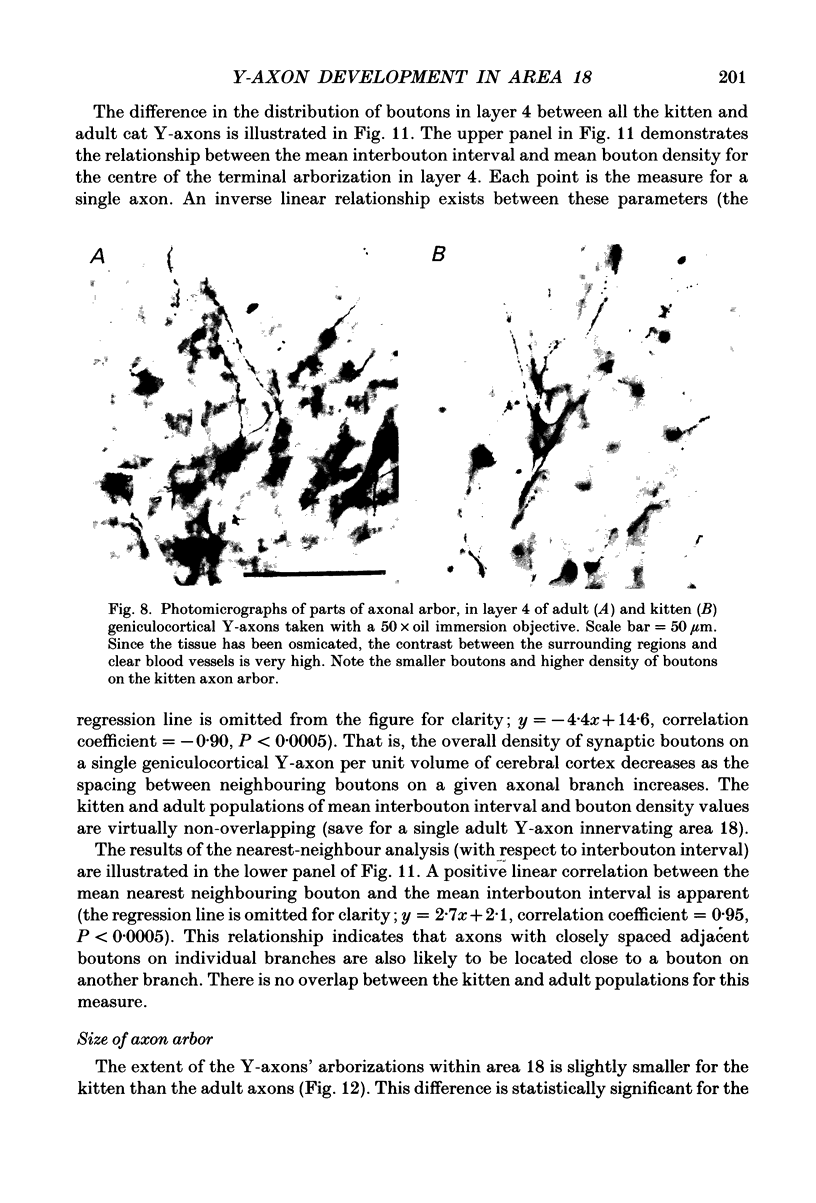
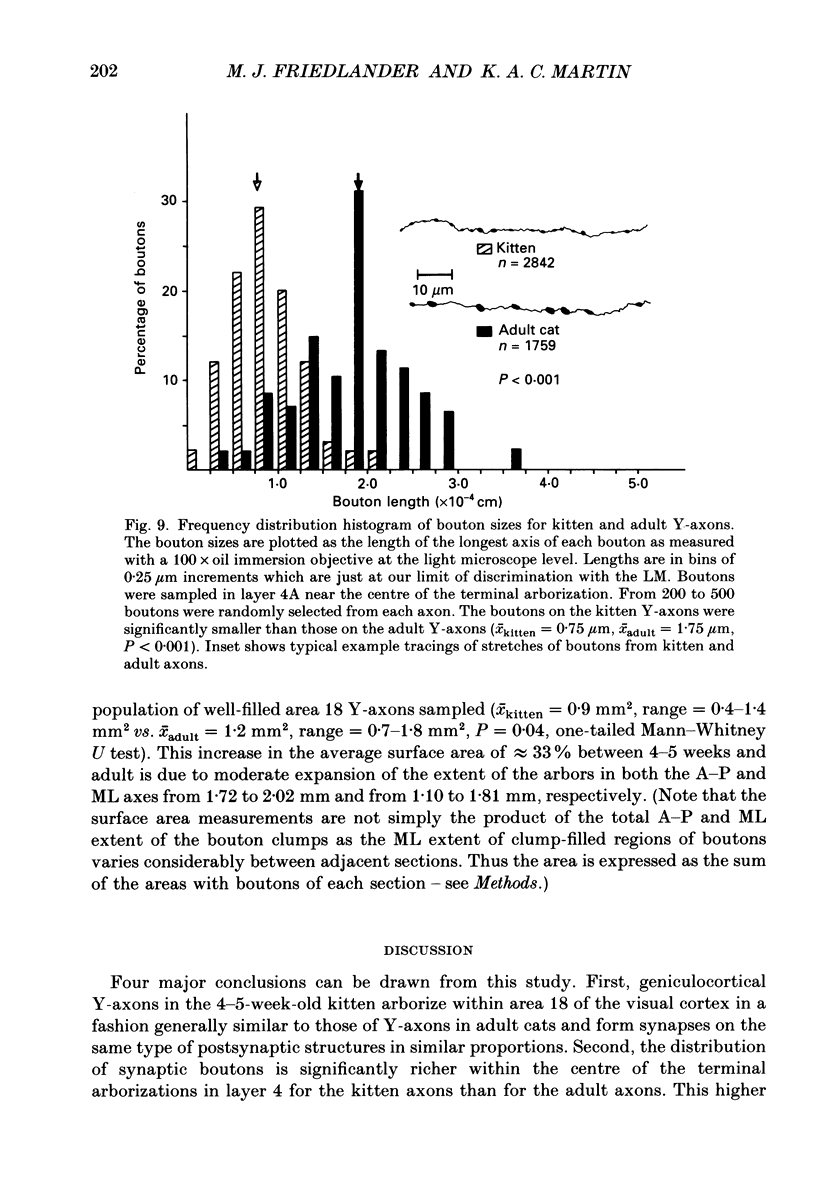
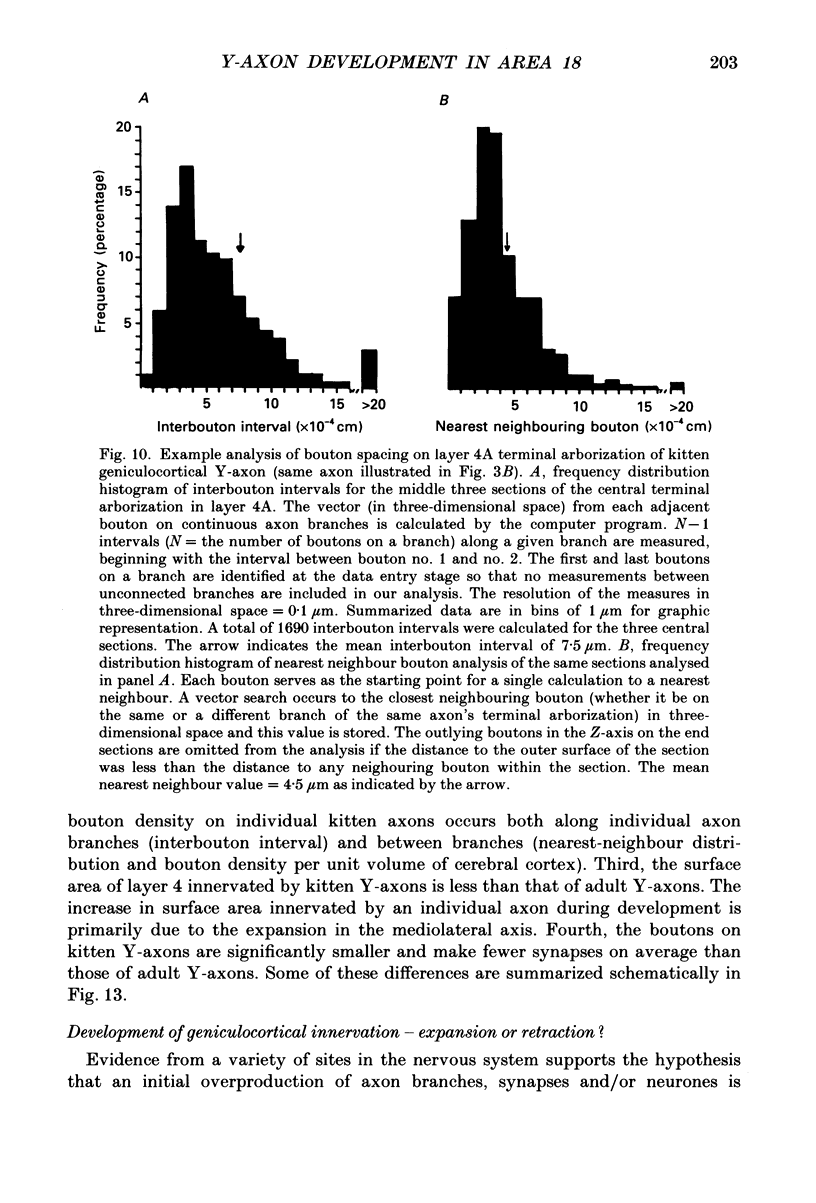
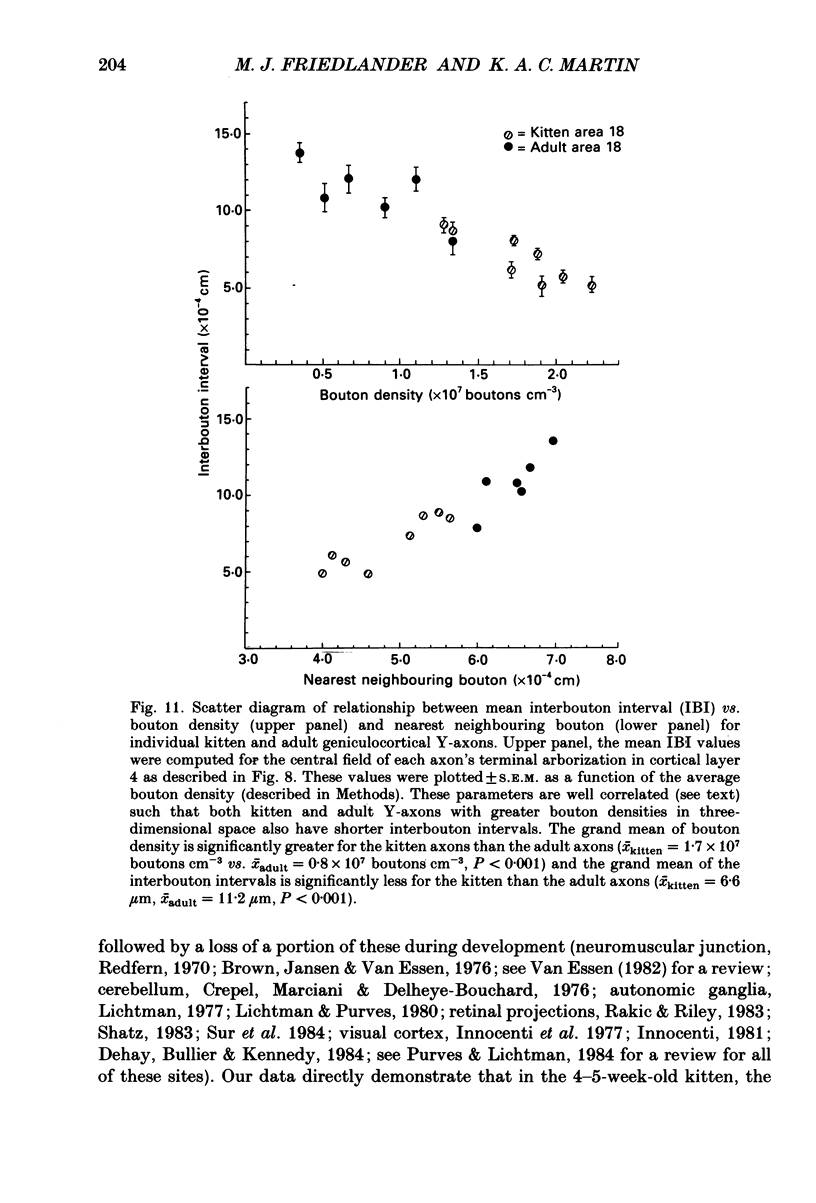
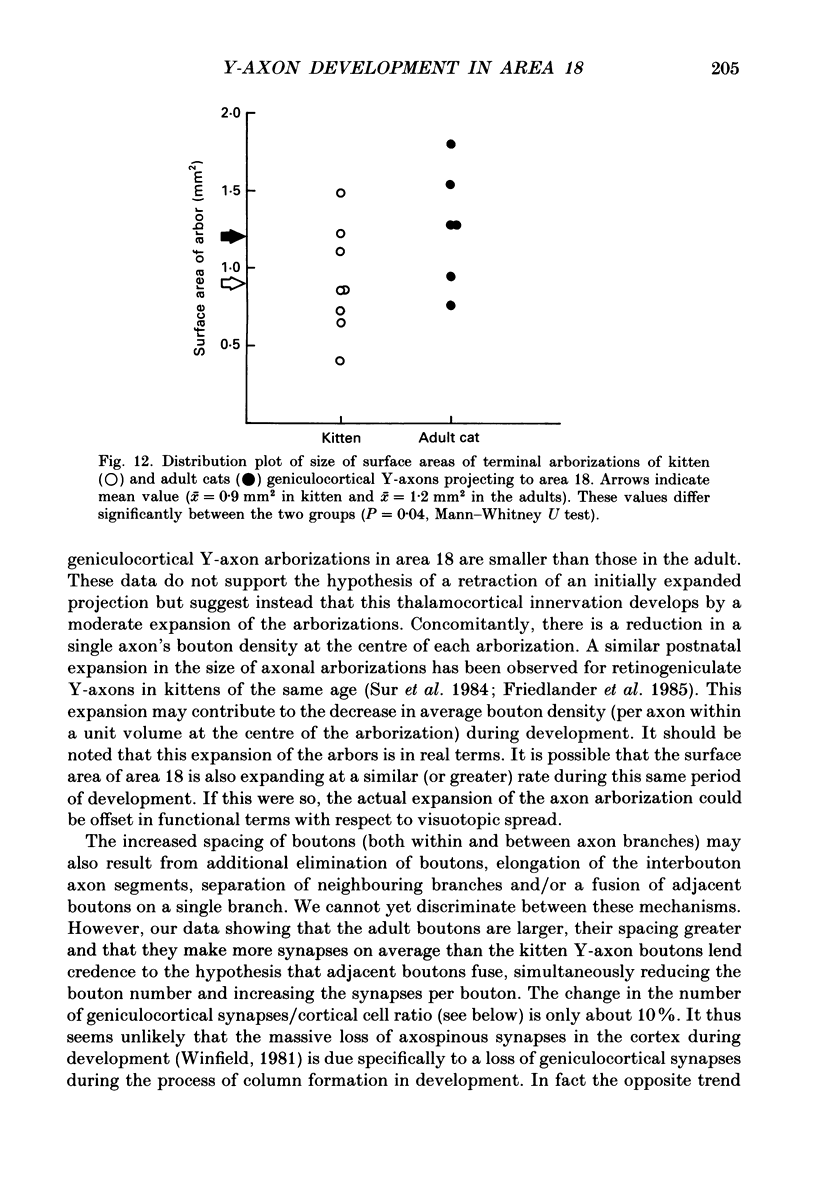
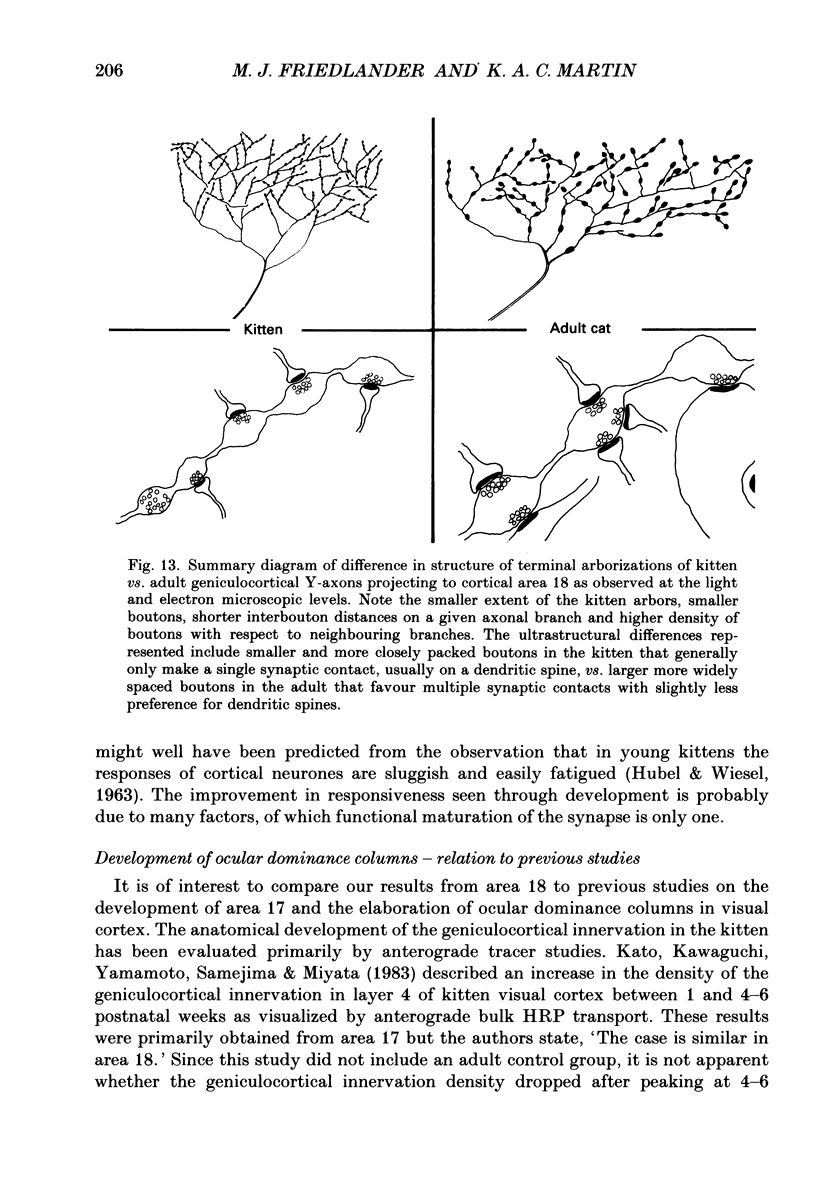
1. Geniculocortical Y-axons (n = 38) in the optic radiations of 4-5-week-old kittens (n = 20) and adult cats (n = 18) were studied both physiologically and morphologically. Axons were recorded from intracellularly and subsequently filled ionophoretically with horseradish peroxidase (HRP). The HRP filled the axons' terminal arborizations in visual cortex (particularly well for those innervating area 18). Fourteen axons appeared to be completely filled with HRP (n = 8 in kitten, n = 6 in adult) and served as the basis for the quantitative analysis of the terminal arborizations reported in this study. 2. The distribution and correspondence of the axonal boutons to presynaptic elements in cortical layer 4A was analysed at both the light and electron microscope level using computerized three-dimensional analysis and serial section reconstruction, respectively. Compared to adult axons, the boutons of the kitten axons were smaller (means = 0.75 vs. 1.75 microns length, P less than 0.001) and more densely spaced both along individual axon branches (means = 6.60 vs. 11.20 microns interbouton interval, P less than 0.001) and between neighbouring branches of the same axon (means = 4.7 vs. 6.4 microns nearest-neighbour distance, P less than 0.01). 3. Most kitten boutons made a single Gray's type 1 synapse on a cortical neurone, unlike adult boutons which usually contacted two or more postsynaptic targets. Both kitten and adult axons had dendritic spines as their major target. Occasionally, HRP reaction-product was observed in cortical neurones postsynaptic to the labelled geniculocortical axon, which gave some estimate of the number of synaptic contacts between a single geniculocortical axon and target cell (about five). 4. The kitten Y-axons innervated the visual cortex in a pattern similar to that of the adult, with the richest terminal branching and bouton density in layer 4A with some additional boutons distributed in layers 3, 4B and 6. The extent of the terminal arborizations primarily in layer 4A (as measured in surface views) of kitten Y-axons in area 18 was significantly less than that of adult Y-axons in area 18 (means = 0.9 mm2 vs. means = 1.2 mm2, P = 0.04). 5. We conclude that between 4 and 5 postnatal weeks and 1 year, geniculocortical Y-axons projecting to cortical area 18 undergo four major changes. These include a reduction in synaptic bouton density (both in three-dimensional space and along individual branches), a concomitant moderate expansion in the surface area of cortex innervated, an increase in bouton size and an increase in the number of synaptic contacts made by each bouton. A general proportional growth of the individual axons' terminal arborization together with fusion and/or separation of neighbouring boutons is sufficient to explain this development.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Albus K., Wolf W. Early post-natal development of neuronal function in the kitten's visual cortex: a laminar analysis. J Physiol. 1984 Mar;348:153–185. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Price D. J. The organization and post-natal development of area 18 of the cat's visual cortex. J Physiol. 1987 Mar;384:263–292. doi: 10.1113/jphysiol.1987.sp016454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe R. G., Greenough W. T., Lund J. S., Wrege K. A quantitative investigation of spine and dendrite development of neurons in visual cortex (area 17) of Macaca nemestrina monkeys. J Comp Neurol. 1979 Aug 1;186(3):473–489. doi: 10.1002/cne.901860310. [DOI] [PubMed] [Google Scholar]
- Braastad B. O., Heggelund P. Development of spatial receptive-field organization and orientation selectivity in kitten striate cortex. J Neurophysiol. 1985 May;53(5):1158–1178. doi: 10.1152/jn.1985.53.5.1158. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Dehay C., Bullier J., Kennedy H. Transient projections from the fronto-parietal and temporal cortex to areas 17, 18 and 19 in the kitten. Exp Brain Res. 1984;57(1):208–212. doi: 10.1007/BF00231149. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. The development of spatial-frequency selectivity in kitten striate cortex. J Physiol. 1981 Jul;316:1–10. doi: 10.1113/jphysiol.1981.sp013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T. F., Martin K. A., Somogyi P., Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J Comp Neurol. 1985 Dec 8;242(2):275–291. doi: 10.1002/cne.902420209. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Martin K. A., Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. I. Arborization patterns and quantitative distribution of postsynaptic elements. J Comp Neurol. 1985 Dec 8;242(2):263–274. doi: 10.1002/cne.902420208. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Martin K. A., Vahle-Hinz C. The structure of the terminal arborizations of physiologically identified retinal ganglion cell Y axons in the kitten. J Physiol. 1985 Feb;359:293–313. doi: 10.1113/jphysiol.1985.sp015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R. Effects of monocular deprivation on the distribution of cell types in the LGNd: a sampling study with fine-tipped micropipettes. Exp Brain Res. 1984;53(2):451–461. doi: 10.1007/BF00238175. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R., Sherman S. M. Effects of monocular deprivation on the structure-function relationship of individual neurons in the cat's lateral geniculate nucleus. J Neurosci. 1982 Mar;2(3):321–330. doi: 10.1523/JNEUROSCI.02-03-00321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. J. Structure of physiologically classified neurones in the kitten dorsal lateral geniculate nucleus. Nature. 1982 Nov 11;300(5888):180–183. doi: 10.1038/300180a0. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Geisert E. E. The projection of the lateral geniculate nucleus to area 18. J Comp Neurol. 1985 Aug 1;238(1):101–106. doi: 10.1002/cne.902380109. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Henderson Z. An anatomical investigation of projections from lateral geniculate nucleus to visual cortical areas 17 and 18 in newborn kitten. Exp Brain Res. 1982;46(2):177–185. doi: 10.1007/BF00237174. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985 Mar 8;233(2):159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Termination patterns of individual X- and Y-cell axons in the visual cortex of the cat: projections to area 18, to the 17/18 border region, and to both areas 17 and 18. J Comp Neurol. 1985 Mar 8;233(2):190–212. doi: 10.1002/cne.902330204. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M. Growth and reshaping of axons in the establishment of visual callosal connections. Science. 1981 May 15;212(4496):824–827. doi: 10.1126/science.7221566. [DOI] [PubMed] [Google Scholar]
- Kato N., Kawaguchi S., Yamamoto T., Samejima A., Miyata H. Postnatal development of the geniculocortical projection in the cat: electrophysiological and morphological studies. Exp Brain Res. 1983;51(1):65–72. doi: 10.1007/BF00236803. [DOI] [PubMed] [Google Scholar]
- Koppel H., Innocenti G. M. Is there a genuine exuberancy of callosal projections in development? A quantitative electron microscopic study in the cat. Neurosci Lett. 1983 Oct 31;41(1-2):33–40. doi: 10.1016/0304-3940(83)90219-7. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Mangel S. C., Wilson J. R., Sherman S. M. Development of neuronal response properties in the cat dorsal lateral geniculate nucleus during monocular deprivation. J Neurophysiol. 1983 Jul;50(1):240–264. doi: 10.1152/jn.1983.50.1.240. [DOI] [PubMed] [Google Scholar]
- Milleret C., Gary-Bobo E., Buisseret P. Comparative development of cell properties in cortical area 18 of normal and dark-reared kittens. Exp Brain Res. 1988;71(1):8–20. doi: 10.1007/BF00247518. [DOI] [PubMed] [Google Scholar]
- Price D. J., Blakemore C. The postnatal development of the association projection from visual cortical area 17 to area 18 in the cat. J Neurosci. 1985 Sep;5(9):2443–2452. doi: 10.1523/JNEUROSCI.05-09-02443.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Riley K. P. Overproduction and elimination of retinal axons in the fetal rhesus monkey. Science. 1983 Mar 25;219(4591):1441–1444. doi: 10.1126/science.6828871. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Luskin M. B. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat's primary visual cortex. J Neurosci. 1986 Dec;6(12):3655–3668. doi: 10.1523/JNEUROSCI.06-12-03655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M., Humphrey A. L., Sherman S. M. Monocular deprivation affects X- and Y-cell retinogeniculate terminations in cats. Nature. 1982 Nov 11;300(5888):183–185. doi: 10.1038/300183a0. [DOI] [PubMed] [Google Scholar]
- Sur M., Sherman S. M. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982 Oct 22;218(4570):389–389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Sur M., Weller R. E., Sherman S. M. Development of X- and Y-cell retinogeniculate terminations in kittens. Nature. 1984 Jul 19;310(5974):246–249. doi: 10.1038/310246a0. [DOI] [PubMed] [Google Scholar]
- Tootle J. S., Friedlander M. J. Postnatal development of receptive field surround inhibition in kitten dorsal lateral geniculate nucleus. J Neurophysiol. 1986 Aug;56(2):523–541. doi: 10.1152/jn.1986.56.2.523. [DOI] [PubMed] [Google Scholar]
- Tootle J. S., Friedlander M. J. Postnatal development of the spatial contrast sensitivity of X- and Y-cells in the kitten retinogeniculate pathway. J Neurosci. 1989 Apr;9(4):1325–1340. doi: 10.1523/JNEUROSCI.09-04-01325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T., Suda K. Laminar differences in development of afferent innervation to striate cortex neurones in kittens. Exp Brain Res. 1982;45(3):433–446. doi: 10.1007/BF01208604. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Tessin D. E., Sherman S. M. Development of the electrophysiological properties of Y-cells in the kitten's medial interlaminar nucleus. J Neurosci. 1982 May;2(5):562–571. doi: 10.1523/JNEUROSCI.02-05-00562.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield D. A., Powell T. P. Laminar cell counts and geniculo-cortical boutons in area 17 of cat and monkey. Brain Res. 1983 Oct 31;277(2):223–229. doi: 10.1016/0006-8993(83)90929-0. [DOI] [PubMed] [Google Scholar]
- Winfield D. A. The postnatal development of synapses in the visual cortex of the cat and the effects of eyelid closure. Brain Res. 1981 Feb 9;206(1):166–171. doi: 10.1016/0006-8993(81)90110-4. [DOI] [PubMed] [Google Scholar]