Abstract
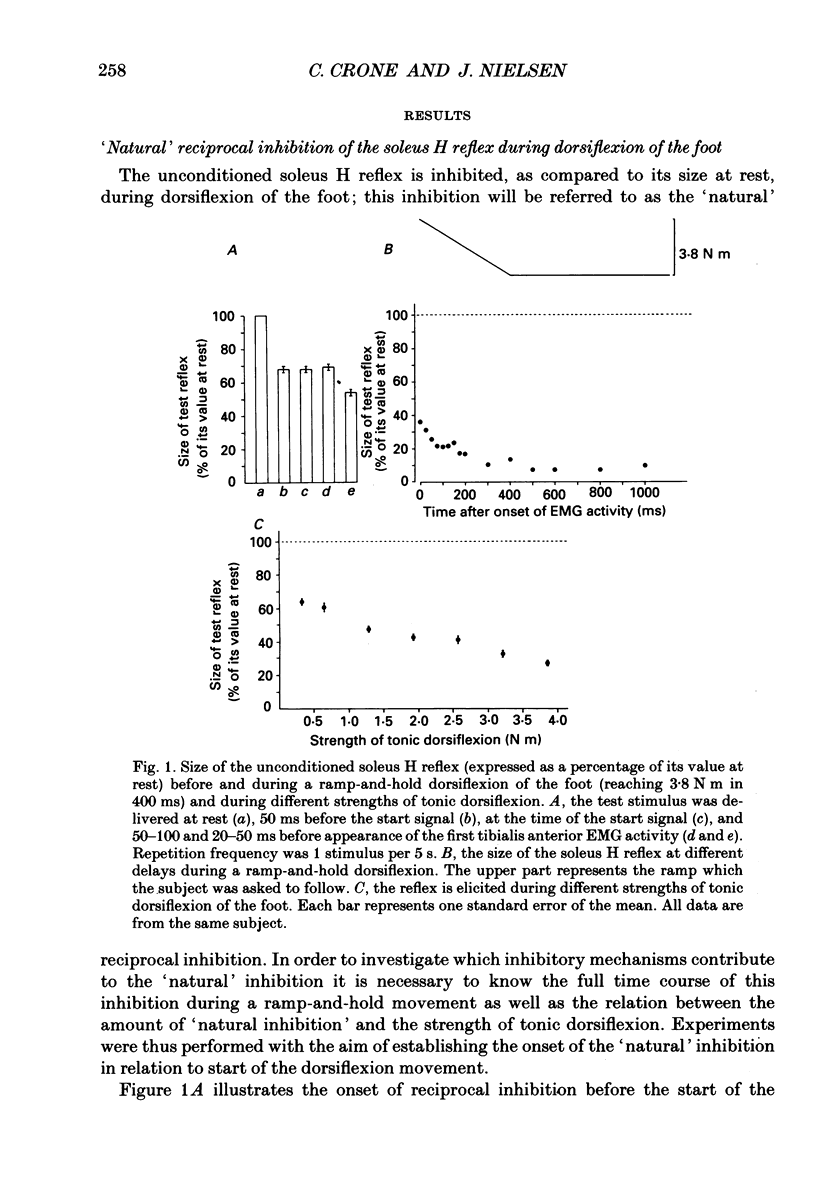
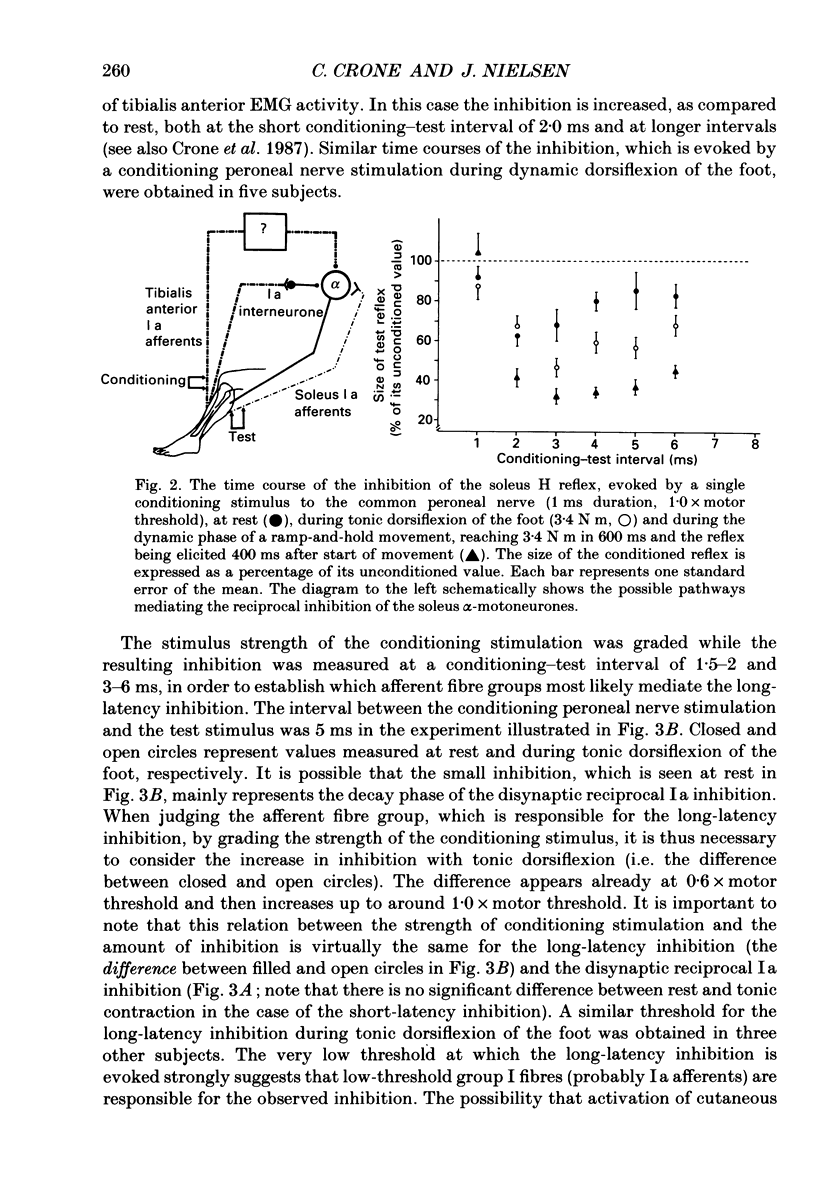
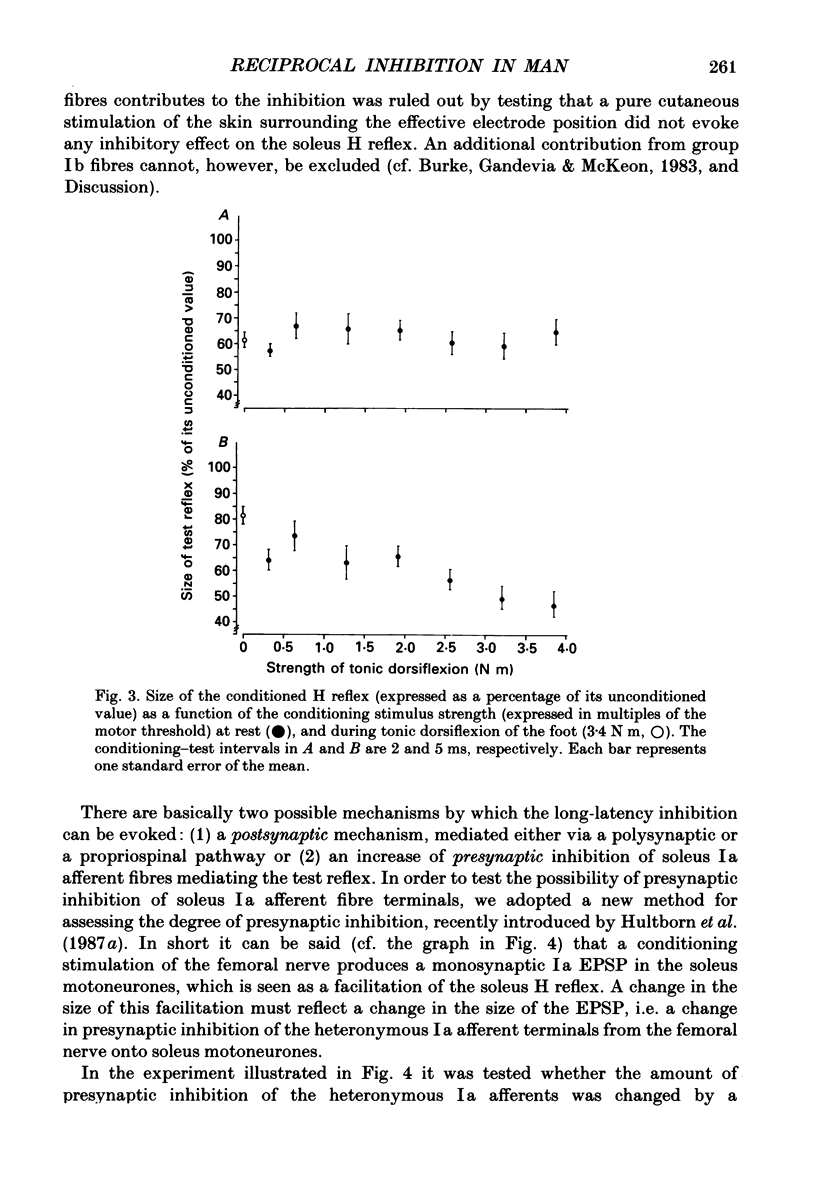
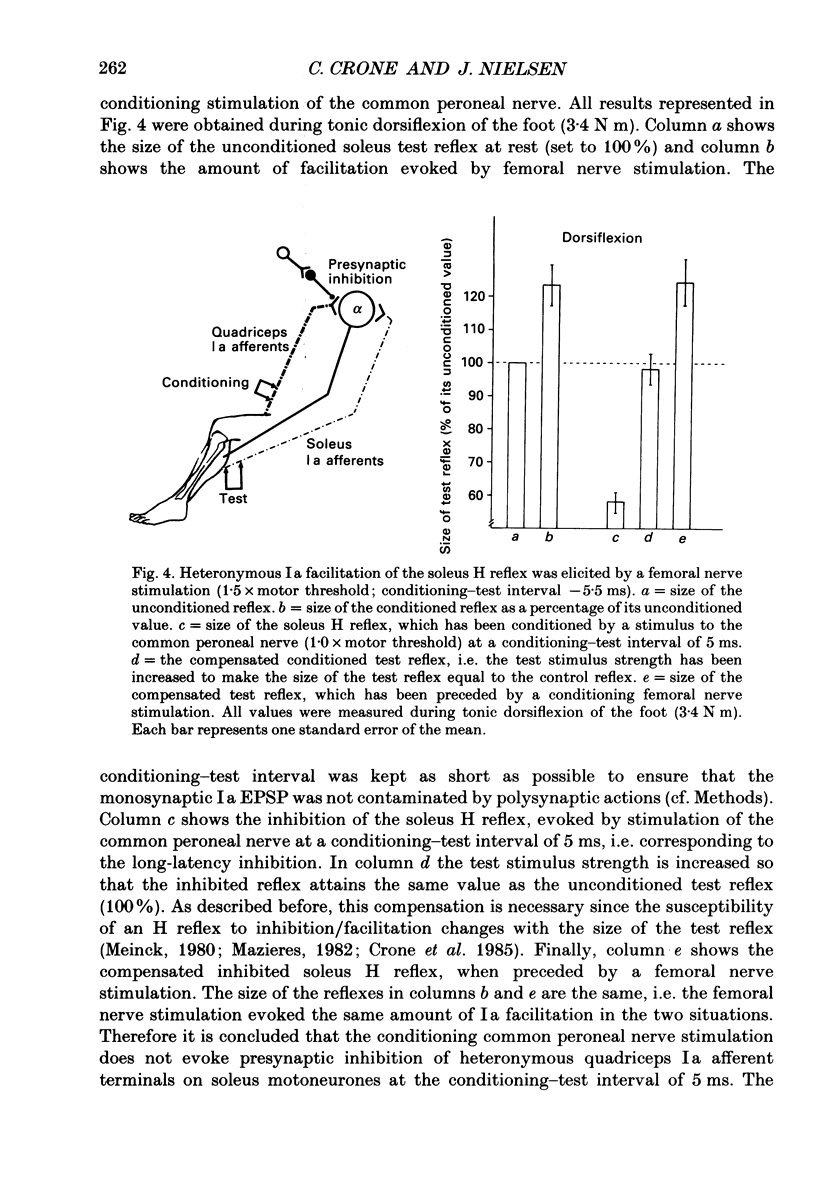
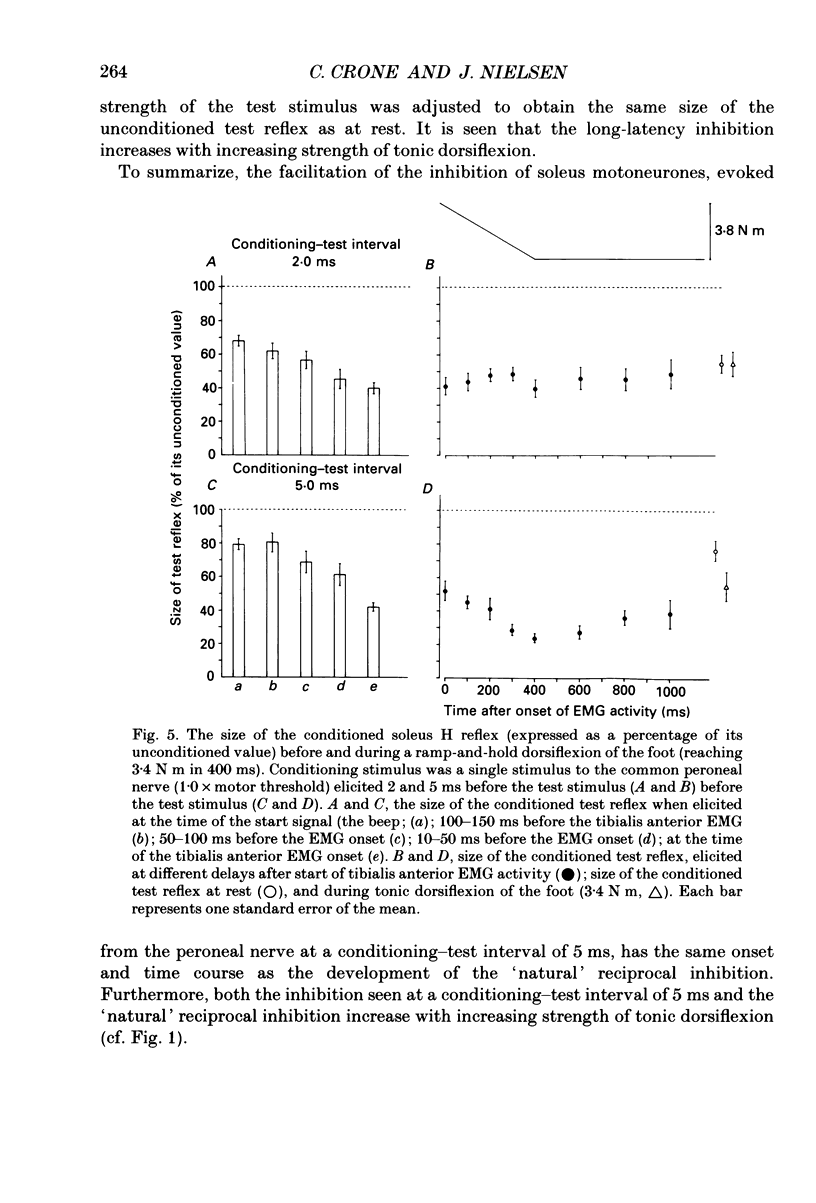
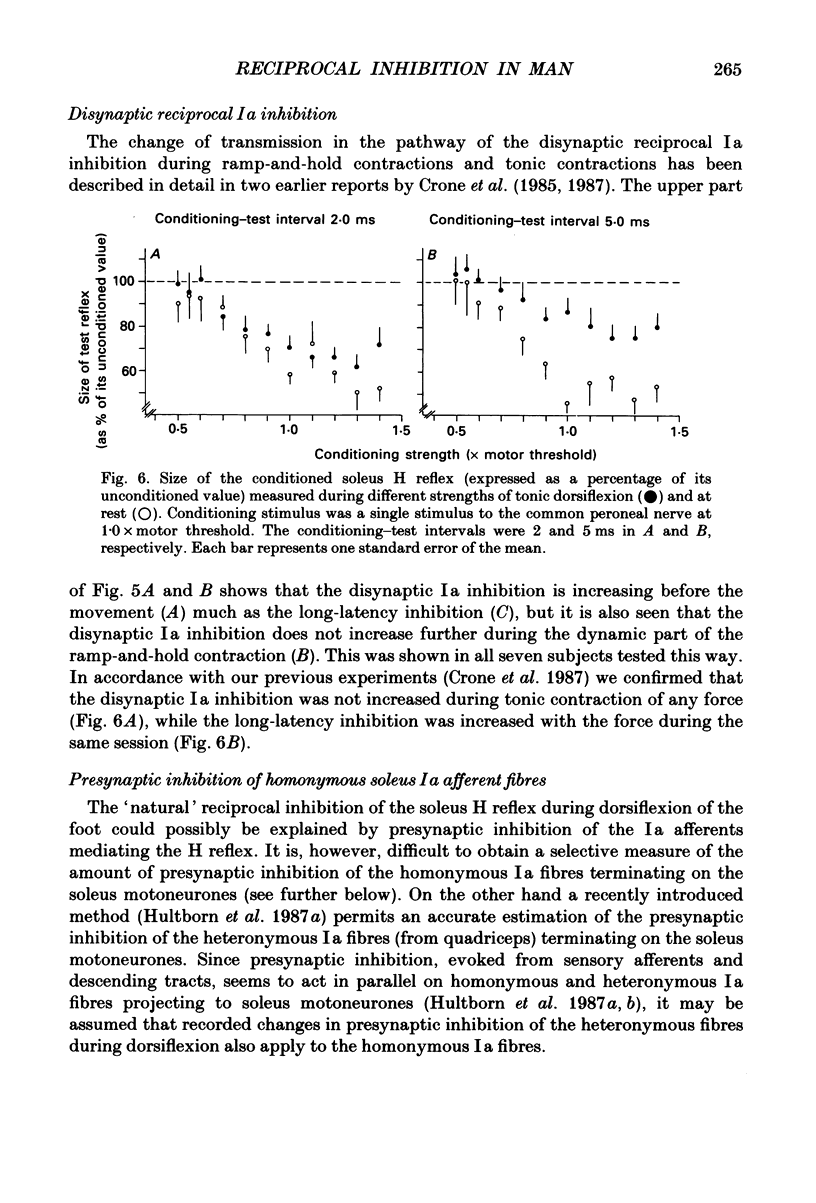
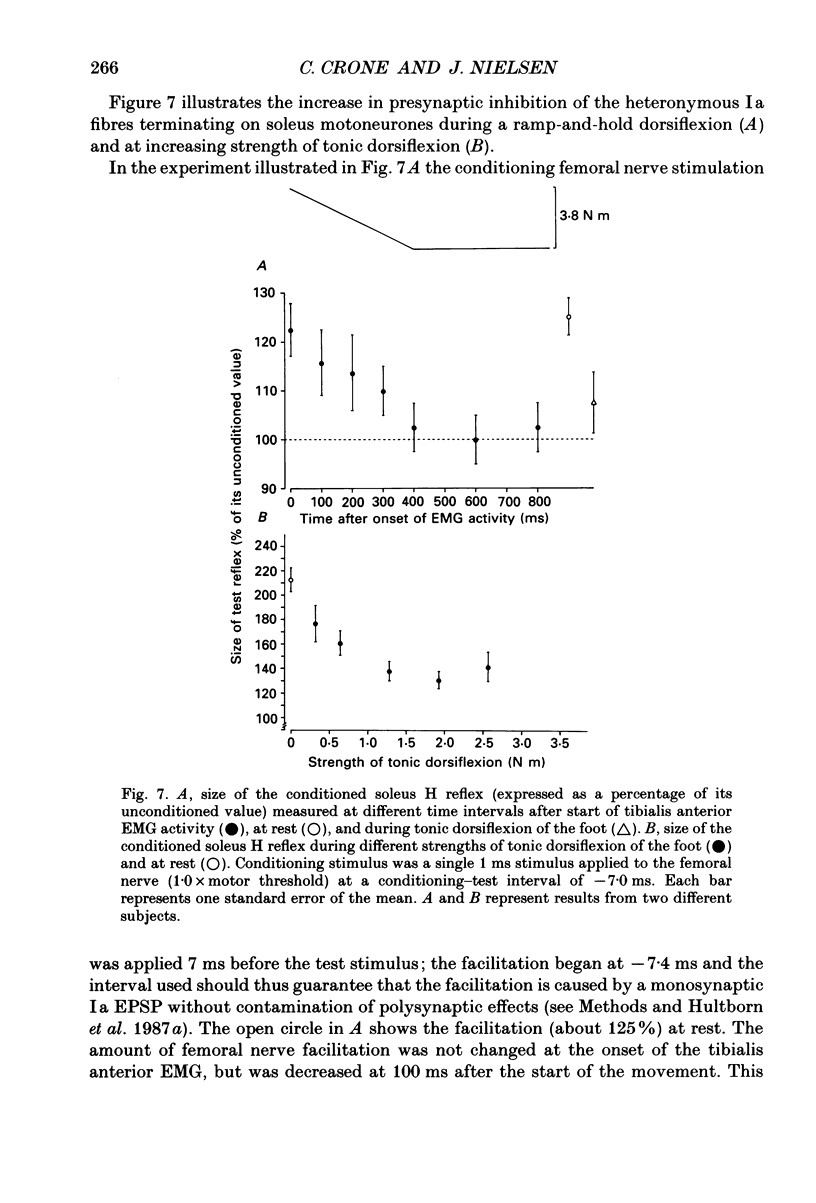
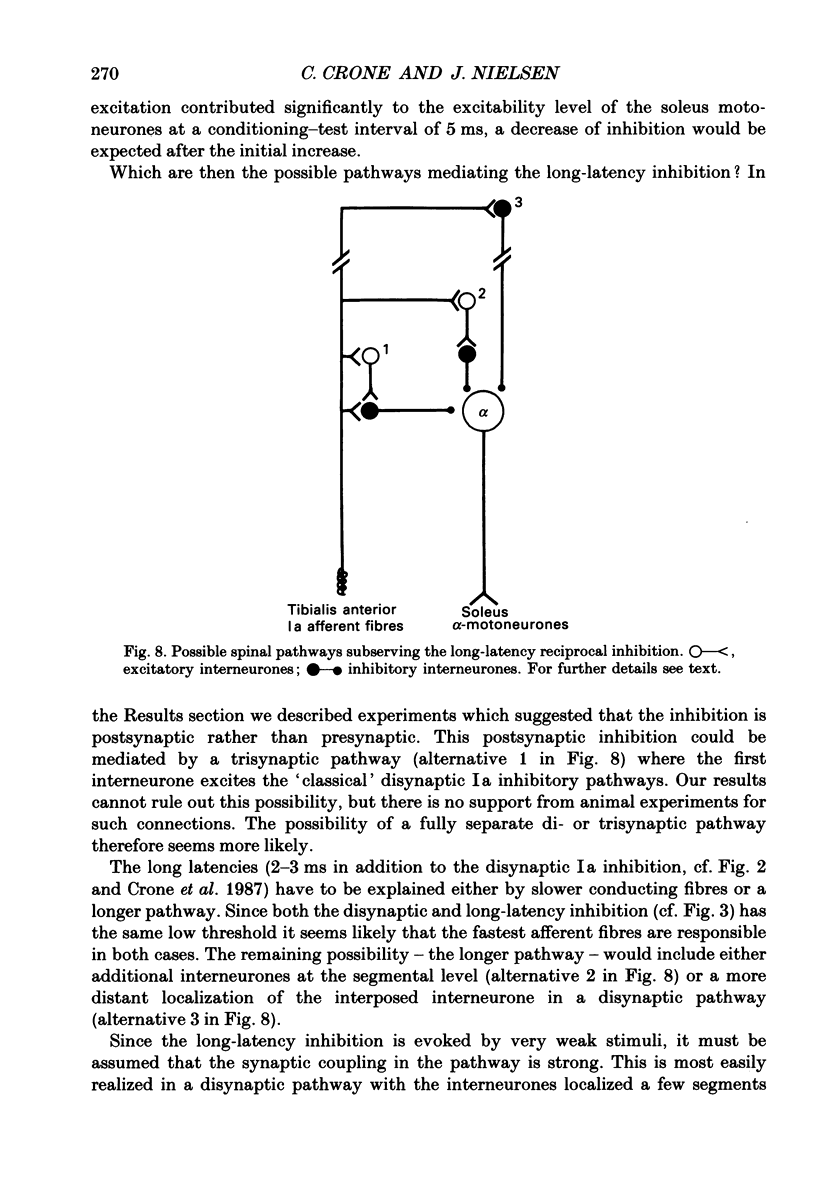
1. The inhibition of the soleus Hoffmann reflex (H reflex) during voluntary dorsiflexion of the foot--henceforth referred to as 'natural' reciprocal inhibition--was found to be initiated 50 ms before the onset of the EMG activity in the tibialis anterior muscle and to increase gradually during a ramp-and-hold dorsiflexion. There was a positive correlation between strength of tonic dorsiflexion and amount of 'natural' reciprocal inhibition. 2. The change of activity in the disynaptic and a long-latency group Ia inhibitory pathway and the change in presynaptic inhibition of the Ia fibres mediating the soleus H reflex were tested separately during ramp-and-hold dorsiflexion as well as during tonic dorsiflexion of the foot, and the results were compared with the development of the 'natural' reciprocal inhibition of the unconditioned soleus H reflex. 3. The disynaptic group I inhibition of soleus motoneurones was increased, as compared to rest, during the dynamic phase of a ramp-and-hold dorsiflexion movement, but the inhibition generally did not increase during tonic dorsiflexion of the foot. 4. The long-latency group I inhibition was seen only during dorsiflexion of the foot. It appeared around 50 ms before tibial anterior EMG activity and there was a positive correlation between strength of tonic dorsiflexion and amount of this long-latency inhibition. 5. Presynaptic inhibition of Ia afferents terminating on soleus motoneurones was estimated by an indirect method. The increase of presynaptic inhibition started soon after the onset of the ramp-and-hold dorsiflexion, and gradually became more pronounced during the ramp phase. The amount of presynaptic inhibition was positively correlated with strength of tonic dorsiflexion. 6. It is concluded that all investigated mechanisms may contribute to the 'natural' reciprocal inhibition and it seems that the different pathways are used differentially during different types of movement.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B., Lundberg A., Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 10. Inhibitory pathways to forelimb motoneurones via C3-C4 propriospinal neurones. Exp Brain Res. 1984;56(2):279–292. doi: 10.1007/BF00236284. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983 Jun;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Jespersen B., Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987 Aug;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Jespersen B. Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res. 1985;59(2):418–422. doi: 10.1007/BF00230924. [DOI] [PubMed] [Google Scholar]
- Fedina L., Hultborn H. Facilitation from ipsilateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurones. Acta Physiol Scand. 1972 Sep;86(1):59–81. doi: 10.1111/j.1748-1716.1972.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Fournier E., Katz R., Pierrot-Deseilligny E. A re-evaluation of the pattern of group I fibre projections in the human lower limb on using randomly alternated stimulations. Exp Brain Res. 1984;56(1):193–195. doi: 10.1007/BF00237457. [DOI] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C., Stark L. Interactions between voluntary and postural mechanisms of thehuman motor system. J Neurophysiol. 1970 May;33(3):365–381. doi: 10.1152/jn.1970.33.3.365. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Morin C., Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987 Aug;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Pierrot-Deseilligny E., Shindo M. Changes in polysynaptic Ia excitation to quadriceps motoneurones during voluntary contraction in man. Exp Brain Res. 1986;63(2):436–438. doi: 10.1007/BF00236863. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Pierrot-Deseilligny E., Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987 Aug;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Udo M. Recurrent depression from motor axon collaterals of supraspinal inhibition in motoneurones. Acta Physiol Scand. 1972 May;85(1):44–57. doi: 10.1111/j.1748-1716.1972.tb05234.x. [DOI] [PubMed] [Google Scholar]
- Iles J. F. Reciprocal inhibition during agonist and antagonist contraction. Exp Brain Res. 1986;62(1):212–214. doi: 10.1007/BF00237419. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res. 1977 Sep 28;29(3-4):323–346. doi: 10.1007/BF00236174. [DOI] [PubMed] [Google Scholar]
- Kots Ia M., Zhukov V. I. O supraspinal'nom upravlenii segmentarnymi tsentrami myshts-antagonistov u cheloveka. 3. "Nastroika" spnal'nogo apparata retsiproknogo tormozheniia v period organizatsii proizvol'nogo dvizheniia. Biofizika. 1971 Nov-Dec;16(6):1085–1092. [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of human wrist flexor motoneurones, possibly via propriospinal neurones. J Physiol. 1988 Nov;405:747–764. doi: 10.1113/jphysiol.1988.sp017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck H. M. Facilitation and inhibition of the human H reflex as a function of the amplitude of the control reflex. Electroencephalogr Clin Neurophysiol. 1980 Feb;48(2):203–211. doi: 10.1016/0013-4694(80)90305-3. [DOI] [PubMed] [Google Scholar]
- Shindo M., Harayama H., Kondo K., Yanagisawa N., Tanaka R. Changes in reciprocal Ia inhibition during voluntary contraction in man. Exp Brain Res. 1984;53(2):400–408. doi: 10.1007/BF00238170. [DOI] [PubMed] [Google Scholar]
- Simoyama M., Tanaka R. Reciprocal La inhibition at the onset of voluntary movements in man. Brain Res. 1974 Dec 27;82(2):334–337. doi: 10.1016/0006-8993(74)90615-5. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21(5):529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]


