Abstract
The EFSA Panel on Food Additive and Flavourings (FAF Panel) provides a scientific opinion on the safety assessment of the proposed use of pea fibre concentrate (FIPEA) as a food additive. FIPEA is a powder consisting mainly of dietary fibres (i.e. pectin and hemicellulose), and low amounts of protein, derived from yellow pea (P. sativum). The manufacturing process includes extensive heat treatments, (e.g. > 100°C for more than 40 min), conditions which lead to inactivation of lectins, that in FIPEA do not pose a safety concern. A specific α‐amylase is used in the manufacturing, and this should be included in the definition of the proposed specifications. The Panel considered that the additional contribution of FIPEA to the total fibre intake in adults and toddlers would be acceptable considering the levels that are considered adequate by the NDA Panel. The Panel recommended to lower the specification limits proposed for the toxic elements. The solubility test indicates that the material does not require specific assessment at the nanoscale. No toxicological data have been submitted on FIPEA. The Panel considered that, similarly to water‐soluble soybean polysaccharides, FIPEA is not absorbed intact but undergoes extensive fermentation by the intestinal microbiota in humans and is not of genotoxic concern. Dry peas (raw material) are a staple food, with a very long history of safe use in the EU. FIPEA is extracted with hot water from the insoluble fibrous material of dehulled yellow peas, therefore the structure of the fibres is not chemically modified, and no new by‐products or components of toxicological concern are expected from the manufacturing process. The Panel concluded that there was no need for a numerical acceptable daily intake (ADI) and that pea fibre concentrate (FIPEA) as a new food additive does not raise a safety concern at the proposed use and use levels.
Keywords: dietary fibres, food additive, lectins, pea fibre, pectins, Pisum sativum L, polysaccharides
SUMMARY
The European Commission requests EFSA to perform a safety assessment to provide a scientific opinion on the safety of the proposed use of pea fibre concentrate (FIPEA) as a food additive, in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
The proposed food additive is a powder consisting mainly of dietary fibres (i.e. pectin and hemicellulose), along with low amounts of protein. The source material is yellow pea (P. sativum).
The manufacturing process begins with hot water extraction (> 100°C) from of the insoluble fibrous material of yellow peas (side stream by‐product that remains after separation of starch during pea processing in the food industry), followed by further separation, pH adjustment, hydrolysis of starch by commercially available α‐amylase enzyme, purification, sterilisation and spray‐drying. The Panel noted that the enzyme used in the manufacturing process is an α‐amylase (4‐α‐d‐glucan glucanohydrolase; EC 3.2.1.1) from the non‐genetically modified Bacillus amyloliquefaciens strain NZYM‐WR (EFSA CEP Panel, 2023) and this should be included in the definition of the proposed specifications.
The applicant provided analytical data on five batches of the proposed food additive, showing that pea fibre concentrate (FIPEA) is produced according to the proposed specifications. The Panel considered the specifications provided by the applicant sufficient to properly characterise the proposed food additive.
Considering the highest value of 68.4% of dietary fibre in pea fibre concentrate (FIPEA) and the maximum dietary exposure estimates of FIPEA (Table 4), the corresponding estimated dietary intake of fibres from pea fibre concentrate (FIPEA) in adults (body weight 70 kg) would be 2.8 g/day at the mean and 8.3 g/day at the 95th percentile. In toddlers (body weight 12 kg), the estimated dietary intake of fibres from pea fibre concentrate (FIPEA) would be 2.2 g/day at the mean and 5.9 g/day at the 95th percentile. The NDA Panel considered dietary fibre intakes of 25 g/day and 10 g/day to be adequate for normal bowel function in adults and toddlers, respectively (EFSA NDA Panel, 2010). Taking into account the overestimation of the exposure to pea fibre concentrate (FIPEA) at the maximum/typical proposed use levels, the Panel considered that the additional contribution of pea fibre concentrate (FIPEA) to the total fibre intake in adults and toddlers would be acceptable in view of the levels that are considered to be adequate for normal bowel function by the NDA Panel, also noting the health benefit of a diet rich in fibre (EFSA NDA Panel, 2010).
TABLE 4.
Summary of dietary exposure to pea fibre concentrate (FIPEA) from its proposed maximum/typical use levels as a food additive in six population groups, estimated with FAIM (minimum‐maximum across the dietary surveys in mg/kg bw per day).
| Infants (12 weeks‐11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Proposed maximum use level exposure assessment scenario | ||||||
| Mean | 1.6–41 | 27–267 | 33–247 | 22–123 | 12–58.3 | 5.5–25 |
| 95th percentile | 11–191 | 95–719 | 95–530 | 71–296 | 43–174 | 25–84 |
| Proposed typical use level exposure assessment scenario | ||||||
| Mean | 0.2–4.1 | 2.7–27 | 3.3–25 | 2.1–12 | 1.2–5.8 | 0.5–2.5 |
| 95th percentile | 1.1–19 | 9.5–72 | 10–53 | 7.1–30 | 4.3–17 | 2.5–8.4 |
Upon EFSA's request, the applicant provided analytical data regarding antinutritional factors, including lectin activity in five batches of FIPEA, ranging from 3830 to 3840 hemagglutination activity unit (HAU/g). The European Commission requested EFSA to perform a risk assessment on the presence of plant lectins in food (EFSA‐Q‐2024‐00195); the assessment is ongoing. Currently, there is no health‐based guidance values (HBGV) set for lectins. The Panel noted that the haemagglutination assay used to monitor the lectin activity is not a direct measurement of their concentration; in addition, it has several drawbacks and limitations, such as low specificity and reproducibility, leading to inconsistent or misleading results. No cases of food poisoning from lectins in EU and outside are reported for peas (BfR, 2024), rather than for (red kidney) beans. 1 The Panel noted that lectins among legumes differ. According to BfR (2024), when legumes are consumed, most lectins are not expected to have any adverse effects on human health. However, ingesting certain lectins, which are mainly found in raw and insufficiently prepared legumes, can lead to adverse health effects, including symptoms such as nausea, vomiting and diarrhoea (BfR, 2024). Studies demonstrate that lectin activity is significantly reduced by heat treatment (Adamcová et al., 2021; Hernández‐Infante et al., 1998; Leontowicz et al., 1998; Shi et al., 2018). The Panel noted that the manufacturing process of FIPEA entails extensive heat treatments (e.g. > 100°C for more than 40 min), conditions which lead to inactivation of lectins. Based on the above, the Panel considered that lectins in the proposed food additive do not pose a safety concern.
Analytical data on the levels of As, Pb, Cd and Hg were provided by the applicant for five samples of the proposed food additive. The Panel assessed the risk that would result if those toxic elements were present in pea fibre concentrate (FIPEA) at two concentration scenarios (i) considering their presence at the proposed specification limits and (ii) considering the analytical data provided, which correspond to the LOQs of the toxic elements. The Panel recommended to lower the specification limits proposed by the applicant for all four toxic elements (Pb, Cd, Hg, As), taking into account (i) the results of the calculations performed by the Panel (the MOE for Pb and As are close to or lower than the MOE of 1 at which risk cannot be excluded, Table 7), (ii) the fact that the proposed food additive is not the only potential dietary source of toxic elements and that (iii) the maximum limits should be established based on actual levels in the commercial food additive.
TABLE 7.
Risk assessment for toxic elements from the use of pea fibre concentrate (FIPEA).
| Exposure to pea fibre concentrate (mg/kg bw per day) | (i) Considering the presence of toxic elements at the proposed specification limits | |||
|---|---|---|---|---|
| MOE for Pb at 0.5 mg/kg | % of the TWI for iHg at 0.1 mg/kg | % of the TWI for Cd at 0.1 mg/kg | MOE for iAs at 0.2 mg/kg | |
| 267 a | 3.7 | 4.7 | 7.5 | 1.1 |
| 719 b | 1.4 | 12.6 | 20.1 | 0.4 |
| (ii) Considering the analytical data provided, which correspond to the LOQs of the toxic elements provided by the applicant | ||||
|---|---|---|---|---|
| Exposure to pea fibre concentrate (mg/kg bw per day) | MOE for Pb at 0.015 mg/kg | % of the TWI for iHg at 0.01 mg/kg | % of the TWI for Cd at 0.01 mg/kg | MOE for iAs at 0.04 mg/kg |
| 267 a | 124.8 | 0.5 | 0.7 | 5.6 |
| 719 b | 46.4 | 1.3 | 2.0 | 2.1 |
Abbreviations: As, arsenic; BW, body weight; Cd, cadmium; Hg, mercury; LOQ, limit of quantification; MOE, margin of exposure; Pb, lead; TWI, tolerable weekly intake.
Highest mean exposure among different population groups (proposed maximum use level exposure assessment scenario–toddlers [Table 4]).
Highest 95th percentile exposure among different population groups (proposed maximum use level exposure assessment scenario–toddlers [Table 4]).
The applicant provided particle size distribution for five batches of the proposed food additive using DLS, ranging from 41 to 52 μm. Based on the data provided and the method limitations, the Panel cannot confirm or exclude the presence of small particles, including nanoparticles, in the proposed food additive. The applicant also provided solubility data, tested using an unspecified, unaccredited method, showing solubility of pea fibre concentrate (FIPEA) in water ranging from 6.1 to 6.9 g/100 mL at 20°C, higher than the threshold value of 33.3 g/L as a decision criterion for demonstrating that the material does not require specific assessment at the nanoscale.
The Panel noted that the proposed food additive pea fibre concentrate (FIPEA) is extracted with hot water (> 100°C) from the insoluble fibrous material of dehulled yellow peas (P. sativum), which are commonly consumed in the human diet after boiling. The functional component is soluble dietary fibre (SDF), which is commonly known to be hydrophilic, non‐crystalline and easily wetted by aqueous gastrointestinal fluid, forming viscous colloidal dispersions or gels when hydrated. Due to these properties, SDF is widely used in the food industry to modify texture and rheology and to influence the colligative properties of food systems (Li et al., 2017). Considering the solubility of the proposed food additive (6.1–6.9 g/100 mL) and the properties of SDF, the Panel considered that the consumers will not be exposed to small particles, including nanoparticles of pea fibre concentrate (FIPEA) under the proposed conditions of use. Therefore, the Panel considered that the proposed food additive can be assessed following conventional risk assessment, i.e. Guidance for submission for food additive evaluations should be followed (EFSA ANS Panel, 2012).
The applicant conducted stability tests under normal (25°C, 60% RH, for 24 months) and accelerated conditions (40°C, 75% RH, for 6 months) on five batches of the proposed food additive. The results indicate that pea fibre concentrate (FIPEA) is stable under the tested storage conditions.
At the proposed maximum use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 1.6 mg/kg bw per day in infants to 267 mg/kg bw per day in toddlers, and the 95th percentile of exposure ranged from 11 mg/kg bw per day in infants to 719 mg/kg bw per day in toddlers. At the proposed typical use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 0.2 mg/kg bw per day in infants to 27 mg/kg bw per day in toddlers, and the 95th percentile of exposure from 1.1 mg/kg bw per day in infants to 72 mg/kg bw per day in toddlers.
The applicant did not provide specific toxicity studies on FIPEA, instead supported its assessment by reporting information on water‐soluble soybean polysaccharides from the ANS Panel 2017. The Panel considered that, similarly to water‐soluble soybean polysaccharides and other dietary polysaccharides, pea fibre concentrate (FIPEA) is most likely not absorbed intact but undergoes extensive fermentation by the intestinal microbiota in humans. There is no concern with respect to genotoxicity. Dry peas, the raw material used for the manufacture of pea fibre concentrate (FIPEA), are a staple food, with a very long history of safe use in the EU. The proposed food additive is extracted with hot water (> 100°C) from the insoluble fibrous material of dehulled yellow peas (P. sativum), therefore the structure of the fibres is not chemically modified, and no new by‐products or components of toxicological concern are expected from the manufacturing process.
The Panel concluded that that there was no need for a numerical ADI and that the use of pea fibre concentrate (FIPEA) as a new food additive does not raise a safety concern at the proposed use and use levels.
1. INTRODUCTION
The present scientific opinion deals with the safety evaluation of pea fibre concentrate (FIPEA) proposed for use as a food additive in a variety of food categories.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 2 on food additives. Only food additives that are included in the Union list, in particular in Annex II to that regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No 231/2012. 3
An application has been introduced for the authorisation of the use of pea fibre concentrate as a new food additive in several food categories of Annex II to Regulation (EC) No 1333/2008.
1.1.2. Terms of Reference
The European Commission requests the European Food Safety Authority to perform a safety assessment to provide a scientific opinion on the safety of the proposed use of pea fibre concentrate as a food additive and the assessment of possible confidentiality requests in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. 4
1.2. Information on existing authorisations and evaluations
No existing authorisations or evaluations have been reported on the proposed food additive pea fibre concentrate (FIPEA) in Europe.
The main components of pea fibre concentrate are dietary fibres (i.e. pectin and hemicellulose) along with low amounts of protein.
Pectins are included in the Community list of approved food additives in Annex II and III of Regulation (EC) No 1333/2008 under the number E 440i (pectin) and E 440ii (amidated pectin). Specifications for each are established in Regulation (EU) No 231/2012.
The safety of the food additives pectin (E 440i) and amidated pectin (E 440ii) was re‐evaluated by EFSA in 2017 and 2021 in the frame of Regulation (EU) No 257/2010. 5 The first re‐evaluation opinion issued in 2017, concluded that there is no safety concern for the use of pectin (E 440i) and amidated pectin (E 440ii) as food additives for the general population and that there is no need for a numerical acceptable daily intake (ADI). The estimated exposure to pectins from their use as food additives was up to 442 mg/kg bw per day for toddlers at the 95th percentile (brand‐loyal scenario) (EFSA ANS Panel, 2017). In a follow up opinion issued in 2021, the safety of pectins was re‐evaluated for their use as food additives in foods for infants below 16 weeks of age (EFSA FAF Panel, 2021). As for infants below 16 weeks of age, it was recommended a reduction of the maximum permitted level (MPL) of pectin (E 440i) and amidated pectin (E 440ii) in food categories (FC) 13.1.5.1 (Dietary foods for special medical purposes and special formulae for infants) and 13.1.5.2 (Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC).
Prior to the re‐evaluation of E 440i and E 440ii by EFSA, non‐amidated and amidated pectins had also been subject of evaluations by the Scientific Committee on Food (SCF) (1978) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (1974, 1981, 2015, 2016).
With respect to hemicellulose, in the Community list of approved food additives of Regulation (EC) No 1333/2008, an entry exists for soybean hemicellulose (E 426). The specifications for E 426 given in Regulation (EU) No 231/2012 describe the authorised food additive as ‘a refined water‐soluble polysaccharide obtained from strain soybean fibre by hot water extraction’.
In addition, the safety of soybean hemicellulose (E 426) was re‐evaluated by EFSA under the frame of Regulation (EU) No 257/2010 (EFSA ANS Panel, 2017). Overall, it was concluded that it is very unlikely that there is a safety concern from the current use of soybean hemicellulose (E 426) as a food additive, and that there is no need for a numerical ADI. The highest exposure estimates calculated based on the maximum permitted levels were up to 191 mg/kg bw per day for children (95th percentile). However, given the limited uses, if any, reported, the Panel considered it probable that the actual dietary exposure to soybean hemicellulose (E 426) would be negligible.
The botanical source of the proposed food additive, P. sativum, is included in the EFSA's compendium of botanicals 6 , 7 because of the presence of lectins identified as components of potential toxicological concern. Plant lectins are proteins found in certain plants, which bind to carbohydrate structures of cells in the human intestine or blood and thereby induce cell division, cause agglutination (‘clumping’) of red blood cells (haemagglutination), influence the immune system, change the permeability of the intestinal barrier and generally disrupt cell metabolism (BfR, 2024). The European Commission requested the EFSA to perform a risk assessment on the presence of plant lectins in food 8 ; the assessment is ongoing, expected for finalisation by the CONTAM Panel before the end of 2025. Currently, there is no health‐based guidance value set for lectins. More information is included in Section 3.1.2 on antinutritional factors.
2. DATA AND METHODOLOGIES
2.1. Data
The applicant has submitted a dossier to support the safety evaluation of the present application on pea fibre concentrate (FIPEA) in a variety of food categories (Documentation provided to EFSA n. 1).
In accordance with Art. 38 of the Commission Regulation (EC) No 178/2002 9 and taking into account the protection of confidential information and of personal data in accordance with Articles 39 to 39e of the same Regulation and of the Decision of the EFSA's Executive Director laying down practical arrangements concerning transparency and confidentiality, 10 the non‐confidential version of the dossier is published on Open EFSA. 11
According to Article 32c(2) of Regulation (EC) No 178/2002 12 and to the Decision of EFSA's Executive Director laying down the practical arrangements on pre‐submission phase and public consultations, EFSA carried out a public consultation on the non‐confidential version of the technical dossier from 19 June to 10 July 2024, 13 for which no comments were received 14
Following the requests for additional data sent by EFSA, the applicant provided additional data on 5 December 2023 (Documentation provided to EFSA n. 2), on 24 July 2024 (Documentation provided to EFSA n. 3) and 27 November 2024 (Documentation provided to EFSA n. 4).
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance of the Scientific Committee on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009a) and following the relevant existing Guidance documents from the EFSA Scientific Committee.
The current ‘Guidance for submission for food additive evaluation’ (EFSA ANS Panel, 2012) and the Guidance on the ‘Safety assessment of botanicals and botanical preparations’ (EFSA Scientific Committee, 2009b) and ‘Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles’ (EFSA Scientific Committee, 2021) have been followed by the FAF Panel for evaluating the present application.
3. ASSESSMENT
3.1. Technical data
3.1.1. Identity of the proposed food additive
The proposed food additive, named as pea fibre concentrate (FIPEA) by the applicant, is a powder consisting mainly of dietary fibres (i.e. pectin and hemicellulose) extracted with hot water (> 100°C) from insoluble fibrous material of the seeds of Pisum sativum L., along with low amounts of protein.
P. sativum is a grain legume species that, according to the applicant, is cultivated worldwide, including Europe. It belongs to the Leguminosae family and is also known with the common names: yellow pea, garden pea, field pea, common pea (Documentation provided to EFSA n. 1). The applicant uses cultivars of the yellow pea registered as agricultural species in the EU (Documentation provided to EFSA n. 2).
P. sativum is included in the EFSA's compendium of botanicals 15 , 16 because of the presence of lectins, identified as components of potential toxicological concern. More information is included in Section 3.1.2 on antinutritional factors.
According to literature data provided by the applicant, pea seeds may contain approximately 37%–49% of starch, 21%–33% of protein, 14%–26% of dietary fibre of which 10%–15% insoluble and 2%–9% soluble and 1.2%–2.4% fats, all w/w on a dry basis (Dahl et al., 2012) (Documentation provided to EFSA n. 1).
The applicant provided analytical data for three batches of the insoluble fibrous starting material of pea fibre concentrate (FIPEA) on moisture ■■■■■ and microbiological parameters ■■■■■ (Documentation provided to EFSA n. 1) and for one batch of the insoluble fibrous starting material of pea fibre concentrate (FIPEA) on the main components ■■■■■ along with toxic elements, mycotoxins and microbiological parameters ■■■■■ (Documentation provided to EFSA n. 2).
Additionally, the applicant provided information on the structure of yellow pea seed pectins, based on the study of Noguchi et al. (2020) (Figure 1) (Documentation provided to EFSA n. 1).
FIGURE 1.
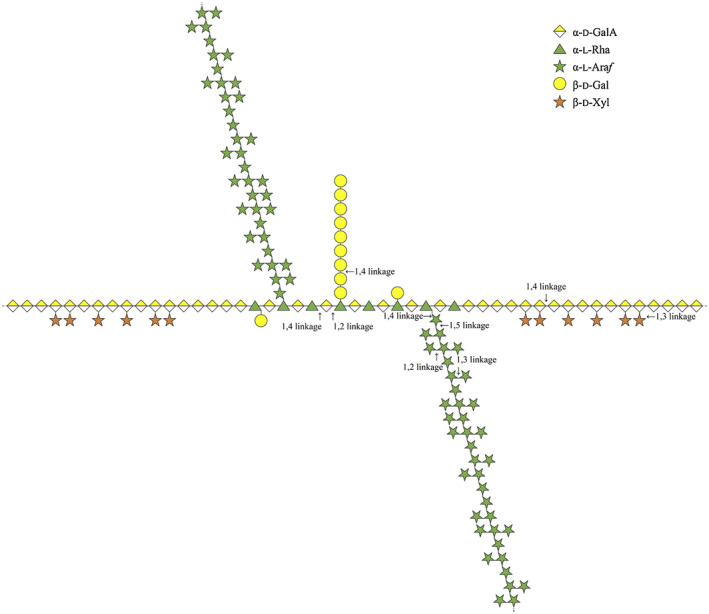
Structural model of yellow pea seed pectins as proposed by Noguchi et al. (2020).
3.1.2. Proposed specifications
The applicant provided specifications for the proposed food additive, i.e. pea fibre concentrate (FIPEA) (Table 1) (Documentation provided to EFSA n. 4).
TABLE 1.
Specifications for pea fibre concentrate (FIPEA) as proposed by the applicant.
| Parameter | Specifications for pea fibre concentrate (FIPEA) as proposed by the applicant |
|---|---|
| Synonyms | Pea fibre concentrate, soluble pea fibre, extracted pea fibre |
| Definition | Pea fibre concentrate (FIPEA) is a refined water‐soluble dietary fibre extracted from side stream of pea starch production. The process involves hot water extraction, followed by further separation, pH adjustment, hydrolysis of starch by commercially available alpha‐amylase enzyme, purification, sterilisation and spray‐drying. |
| Chemical names | Pea polysaccharide, Pea pectin |
| Assay | More than 50% soluble fibre |
| Appearance | FIPEA is a yellowish to light brownish free‐flowing powder |
| pH | 5.5 ± 1.5 (1% aqueous solution) |
| Viscosity | Not more than 200 mPa*s (10% aqueous solution) |
| Solubility | Soluble in hot and cold water without gel formation |
| Loss on drying | Not more than 10.0% |
| Protein | Not more than 10.0% |
| Starch | Not more than 30.0% |
| Ash | Not more than 10.0% |
| Mercury | Not more than 0.1 mg/kg |
| Cadmium | Not more than 0.1 mg/kg |
| Arsenic | Not more than 0.2 mg/kg |
| Lead | Not more than 0.5 mg/kg |
| Escherichia Coli (coliforms) | < 10 cfu/g |
| Yeasts | < 100 cfu/g |
| Moulds | < 100 cfu/g |
| Total plate count | Max 3000 cfu/g |
According to the proposed definition, a step of hydrolysis of starch by commercially available α‐amylase enzyme is performed. The Panel noted that the enzyme used is an α‐amylase from the non‐genetically modified B. amyloliquefaciens strain NZYM‐WR and this should be included in the ‘Definition’ field of the proposed specifications.
Analytical results for five independently produced batches have been provided to demonstrate that each batch complies with the proposed specifications (Documentation provided to EFSA n. 1, 2, 3, 4).
The five independently produced batches of pea fibre concentrate (FIPEA) provided by the applicant were analysed for moisture, protein, ash, starch, dietary fibre, soluble dietary fibre and insoluble dietary fibre, carbohydrates, soluble sugars composition, fat, ash, toxic elements, polycyclic aromatic hydrocarbons (PAHs), microbiological parameters, mycotoxins, pesticide residues, antinutritional factors and physicochemical parameters (i.e. pH, solubility and particle size).
Composition
Regarding the components of pea fibre concentrate (FIPEA), total dietary fibre ranged from 55.2% to 68.4%, soluble dietary fibre from 54.3% to 63.6% and insoluble dietary fibre from 0.4% to 1.3%, carbohydrates from 25.6% to 30.7% of which starch from 25.1% to 28.1%, soluble sugars (sum of maltose, glucose, fructose, sucrose, lactose) from below the LOQ of 0.2%– 2.6%. In addition, moisture ranged from 5.2% to 5.3%, protein from 3.7% to 4.1%, ash from 7.8% to 8.2% and fat from below the LOQ of 0.3%–0.6% in five independently produced batches (Documentation provided to EFSA n. 1).
The analytical data provided by the applicant respect the proposed specification limits for loss of drying, protein, starch, ash and soluble fibre. However, the Panel noted that the measured protein content (3.7%–4.1%) is substantially lower than the proposed specification limit of ‘Not more than 10.0%’.
Considering the nature of the proposed food additive, the applicant determined the soluble sugars composition by high performance anion‐exchange chromatography (HPAEC) with pulsed amperometric detection (PAD) from oligo/polysaccharides after acid hydrolysis. Rhamnose ranged from 2.3% to 2.6%, arabinose from 36.2% to 41.2%, galactose from 5.5% to 6.7%, glucose from 12.8% to 19.0%, mannose was below the limit of detection (LOD) of 0.004% in all batches, xylose ranged from 1.8% to 2.2%, galacturonic acid from 2.5% to 4.9% and glucuronic acid was 0.2% in all batches. In addition, the applicant identified maltose, which ranged from below the LOQ of 0.2%–2.0%, glucose from below the LOQ of 0.2%–0.6% and fructose, lactose and sucrose which were below the LOQ of 0.2% in all batches (Documentation provided to EFSA n. 1).
Toxic elements
As regards to toxic elements, the applicant provided analytical data obtained by inductively coupled plasma mass spectrometry (ICP–MS), according to the DIN EN 15763 method, on five independently produced batches of pea fibre concentrate (FIPEA). Levels of arsenic (As), lead (Pb), cadmium (Cd) and mercury (Hg) were reported to be in all cases below the respective LOQs i.e. below 0.04 mg/kg for As, below 0.015 mg/kg for Pb, below 0.01 mg/kg for Cd and below 0.01 mg/kg for Hg (Documentation provided to EFSA n. 1).
The anticipated impact of the proposed specifications and of the reported analytical data on the potential exposure to these toxic elements is described in Section 3.3.3.
Polycyclic aromatic hydrocarbons (PAHs)
The applicant provided analytical data on five independently produced batches of pea fibre concentrate (FIPEA) on the PAH profile, determined with gas chromatography/mass spectrometry (GC/MS). In all cases, the results were below the respective LOQs (e.g. sum of PAH4 (benzo(a)anthracene, benzo(a)pyrene, benzo(b)fluoranthene, chrysene) below the LOQ of 0.2 μg/kg, sum of PAH16 below the LOQ of 0.5 μg/kg in all batches) (Documentation provided to EFSA n. 1).
The Panel considered that there is no concern with respect to a potential contamination by PAHs in the proposed food additive and no need to introduce limit values for PAHs in the proposed food additive specifications.
Microbiological parameters
Five independently produced batches of pea fibre concentrate (FIPEA) were analysed for the presence of possible microbiological contaminants (Documentation provided to EFSA n. 1).
Individual types of microorganisms, including L. monocytogenes, Salmonella spp. and Cronobacter spp., were consistently not detectable in all tested batches. Coliforms, E. Coli, Coagulase‐positive‐staphylococci, Bacillus cereus, spores of sulfite‐reducing bacteria growing under anaerobic conditions (incl. Clostridia), Clostridium perfringens, anaerobic mesophilic sporulating bacteria and Enterobacteriaceae resulted to be below 10 colony forming unit (cfu)/g in all analysed batches, while yeasts and moulds resulted to be below 100 cfu/g in all analysed batches. The total viable count ranged from below 10 to below 100 cfu/g.
The Panel considered that the data provided support the proposed specifications for microbiological parameters.
Mycotoxins
Because of the botanical origin of the proposed food additive, mycotoxins might be possible contaminants. The applicant provided analytical data on mycotoxins obtained by analysis using liquid chromatography with tandem mass spectrometry (LC–MS/MS) on five independently produced batches (Documentation provided to EFSA n. 1). Aflatoxins B1, B2, G1 and G2 were below the LOQ of 0.2 μg/kg in all batches, and ochratoxin A was below the LOQ of 0.5 μg/kg in all batches.
The Panel considered that there is no concern with respect to a potential contamination by mycotoxins in the proposed food additive and no need to introduce limit values for mycotoxins in the proposed food additive specifications.
Pesticide residues
Regarding pesticide residues, the applicant provided data obtained by analysis using gas chromatography with tandem mass spectrometry (GC–MS/MS) and LC–MS/MS of five independently produced batches. No pesticides residues were quantified (Documentation provided to EFSA n. 1).
Despite the botanical origin of the food additive, the Panel saw no need to recommend limit values for pesticides in the specifications of pea fibre concentrate (FIPEA).
Antinutritional factors
Upon EFSA's request, the applicant provided analytical data on the antinutritional factors phytic acid (%) analysed by inductively coupled plasma optical emission spectroscopy (ICP‐OES), trypsin inhibitor activity (TIU/g) analysed by spectrophotometry (UV/VIS) and the lectin activity (HAU/g) tested by the haemagglutination assay in five independently produced batches of the proposed food additive. Phytic acid and trypsin inhibitor activity were below the LOQ of 0.14% and 450 TIU/g in all batches, respectively, while the activity of lectins ranged from 3830 to 3840 (HAU/g). The impact of processing on the concentration and activity of the antinutritional factors was not discussed by the applicant (Documentation provided to EFSA n. 2).
In response to a further request by EFSA, the applicant provided analytical data on five batches of raw, yellow peas and five batches of the insoluble fibrous starting material of pea fibre concentrate (FIPEA) on the activity of lectins using the same test method (haemagglutination assay). For the raw, yellow peas, the activity of lectins was below the LOQ of 120 HAU/g while for the insoluble fibrous starting material of pea fibre concentrate (FIPEA) was below the LOD of 420 HAU/g for all batches (different LOD/LOQs because a different extraction ratio was needed depending on the sample type) (Documentation provided to EFSA n. 3).
Plant lectins are proteins found in certain plants, which bind to carbohydrate structures of cells in the human intestine or blood and thereby induce cell division, cause agglutination (‘clumping’) of red blood cells (haemagglutination), influence the immune system, change the permeability of the intestinal barrier and generally disrupt cell metabolism (BfR, 2024). The European Commission requested the European Food Safety Authority to perform a risk assessment on the presence of plant lectins in food (EFSA‐Q‐2024‐00195); the assessment is ongoing. Currently, there is no health‐based guidance value (HBGV) set for lectins.
The Panel noted that the haemagglutination assay used to monitor the lectin activity is not a direct measurement of their concentration; in addition, it has several drawbacks and limitations, such as low specificity and reproducibility, which may easily result in false positive or negative results. The Panel, also, noted that the proposed food additive contains carbohydrates in levels of 25.6%–30.7%, sugars from the LOQ of 0.2%–2.6%, and dietary fibre in levels of 55.2%–68.4%. Such components may exhibit non‐specific binding to erythrocytes and lead to false positive results. The variation in the preparation of the erythrocytes (e.g. trypsin treatment, which may lead to greater agglutination activity outcomes by lectins), the type of erythrocytes used (from animals or humans) and the conditions under which the testing is performed may lead to significantly different results.
According to Adamcová et al. (2021) raw sugar peas seed (P. sativum) show a lectin activity of 414 HAU/g and sugar snaps (including the legume pod) a lectin activity of 208 HAU/g (non‐trypsin treated), which after 5 mins of boiling of the raw material is not further detected. The Panel noted that in this study, the hemagglutination assay was performed under different experimental conditions, e.g. using rabbit erythrocytes, while in the provided results of the applicant, the hemagglutination assay was calculated using human red blood cells, and thus the results cannot be compared directly although the instability towards heat and rapid inactivation seems to be clear from Adamcová et al. (2021). In the same review paper, it is also mentioned that pea leaves, sugar peas and sugar snaps are consumed fresh without soaking and heat treatment, and the lectins are therefore ‘consumed in an active form but classified as non‐toxic or slightly toxic’.
According to the information provided by the applicant, lectin activity is typically reduced by heating (e.g. boiling legumes for at least 30 min) (Documentation provided to EFSA n. 3; Adamcová et al., 2021). According to Shi et al. (2018), the hemagglutinating activity of raw whole yellow peas decreased more than 30‐fold from 5.64 HAU/g to 0.17 HAU/g after cooking at 95°C for 1 h. These findings are consistent with other studies, which also report a reduction in lectin activity after heat treatment (Hernández‐Infante et al., 1998; Leontowicz et al., 1998).
With regard to the manufacturing process of pea fibre concentrate (FIPEA), the dry insoluble pea raw material is initially heated in hot water (> 100°C) for an extensive period of time (more than 40 min) and goes though further heating steps (see Section 3.1.3). Considering the impact of heat treatment on lectin activity and the duration and temperature of the heating steps during the processing of pea fibre concentrate (FIPEA), the Panel would expect a very significant decrease in the lectin activity. No cases have been reported in the EU or outside, of food poisoning from lectins in peas. Most reports were related to the consumption of (red kidney) beans not sufficiently heat‐treated. According to BfR (2024), when legumes are consumed, most lectins are not expected to have any adverse effects on human health. However, ingesting certain lectins, which are mainly found in raw and inadequately prepared legumes, can lead to adverse health effects, including symptoms such as nausea, vomiting and diarrhoea (BfR, 2024). In 2020, an alert notification was published thought the Rapid Alert System for Food and Feed (RASFF) system concerning food poisoning, due to high amount of lectins in ‘precooked’ frozen red kidney beans 17 (6989 HAU /g). Different lectins are able to recognise different saccharides, therefore lectins from different legumes cannot be compared for their adverse activity. In addition, because of the low specificity and reproducibility of the HAU assay, the Panel considered that the information from the RASFF cannot be compared with the activity of lectins in the proposed food additive as measured by the applicant.
Taking account of all the information above, the Panel considered that lectins in the proposed food additive do not pose a safety concern.
Physicochemical characterisation
pH
In the five analysed batches, the pH (1% w/w in water) ranged from 5.7 to 6.3, respecting the proposed specifications for this parameter (Documentation provided to EFSA n. 1).
Particle size
Information on the particle size distribution based on dynamic light scattering (DLS) measurements for five batches of the proposed food additive was provided by the applicant. The median particle size was reported ranging from 41 to 52 μm (Documentation provided to EFSA n. 1).
The Panel noted that DLS is not considered a suitable method to investigate the presence of nanosized particles as it does not provide information on the size of the constituent particles as required by the Guidance on Particle‐TR (EFSA Scientific Committee, 2021) and DLS is prone to bias for polydisperse materials (Mech, Rauscher, Babick, et al., 2020; Mech, Rauscher, Rasmussen, et al., 2020; Rauscher et al., 2019).
Based on the data provided, the Panel cannot confirm or exclude the presence of a fraction of small particles including nanoparticles in the proposed food additive.
Solubility
The applicant provided information on the solubility in water of the proposed food additive at 20°C. The method used was neither an accredited one nor described in full. The solubility in water was tested as a part of the stability study. The solubility for five batches at the beginning of the storage time was ranging from 6.1 to 6.9 g/100 mL at 20°C (Documentation provided to EFSA n. 1).
The Panel noted that the solubility test provided variable results and was not performed following the EFSA Guidance on Particle‐TR (EFSA Scientific Committee, 2021), however the Panel considered the data suitable for the assessment of the solubility of the proposed food additive.
3.1.3. Manufacturing process
The proposed food additive pea fibre concentrate (FIPEA) is extracted with hot water (> 100°C) from the insoluble fibrous material of yellow peas (P. sativum).
The pea‐derived starting material is a side stream by‐product that remains after separation of starch during pea processing in the food industry. Yellow peas are dehulled, sifted, milled and mixed with water to extract starch. The remaining insoluble pea material is dried, blended, sifted and after metal detection, stored as the starting material of pea fibre concentrate (FIPEA) (Documentation provided to EFSA n. 1, 2).
As a next step, the insoluble fibrous material is mixed with hot water at a temperature above 100°C for more than 40 min to extract the soluble fibres. The extracted soluble fibres in the liquid phase are separated from the rest of the mixture ■■■■■. The separated liquid fraction is then pH adjusted ■■■■■, enzymatically treated with an α‐amylase to hydrolyse the remaining starch ■■■■■, purified by ultra‐filtration, sterilised by ultra‐high temperature (UHT) treatment and spray‐dried ■■■■■. The resulting pea fibre concentrate (FIPEA) powder is sifted, metal detected, packaged and stored (Documentation provided to EFSA n. 1, 2).
The Panel noted that the enzyme used in the manufacturing process is an α‐amylase (4‐α‐d‐glucan glucanohydrolase; EC 3.2.1.1) from the non‐genetically modified B. amyloliquefaciens strain NZYM‐WR, assessed as safe under the intended conditions of use by the EFSA CEP Panel (2023). Based on the data provided, the qualification for the qualified presumption of safety (QPS) status of the production strain and the absence of concern arising from the food enzyme manufacturing process, the Panel concluded that the food enzyme α‐amylase produced with the non‐genetically modified B. amyloliquefaciens strain NZYM‐WR does not give rise to safety concerns under the intended conditions of use.
The production yield of FIPEA from yellow peas is ■■■■■■■■■■ ■■■■■ ■■■■■ is derived from 1 kg yellow peas). The production yield of FIPEA from the insoluble fibrous starting material is ■■■■■ (Documentation provided to EFSA n. 3, 4).
According to the applicant, pea fibre concentrate (FIPEA) does not contain, consist of or is produced from genetically modified organisms (GMO) and therefore, does not fall in the scope of Regulation (EC) 1829/2003 18 (Documentation provided to EFSA n. 1).
3.1.4. Methods of analysis in food
According to the applicant, pea fibre concentrate (FIPEA) is characterised by a high content of dietary fibre (55%–68%) and more specifically of soluble dietary fibre (54%–64%).
The applicant proposed a modified version (ASU L00.00–18 Mod. 1997‐01 Corr. 2017‐10 Mod.: determination of protein with Dumas) of the enzymatic‐gravimetric method of AOAC 985.29 for the determination of total dietary fibre in foods, which allows to determine and differentiate between total dietary fibre, soluble dietary fibre and insoluble dietary fibre. In the modified version, an additional filtration step is performed to separate the residue (insoluble dietary fibre) and filtrate (soluble dietary fibre) (Documentation provided to EFSA n. 1, 2).
3.1.5. Stability, reaction and fate in food of the proposed food additive
The applicant investigated the stability of five independent batches of pea fibre concentrate (FIPEA), stored under normal conditions (25°C, 60% relative humidity (RH), for 24 months) and accelerated storage conditions (40°C, 75% RH, for 6 months). Throughout the storage period, samples were tested for moisture, total dietary fibre, soluble and insoluble dietary fibre and galactose contents and microbial quality (total plate count, yeasts, moulds, E. coli, Salmonella spp.) (Documentation provided to EFSA n. 1).
The analytical results are in accordance with the proposed specification limits, with the exception of soluble dietary fibre results being lower than the specifications value of 50%, in a small number of the measurements made for the different batches and timepoints, with no evident trend. Based on the results, the applicant proposed a shelf‐life of 2 years for pea fibre concentrate (FIPEA), if stored under dry conditions at room temperature (Documentation provided to EFSA n. 1).
The Panel noted that the results indicate that pea fibre concentrate (FIPEA) is stable under the tested storage conditions.
3.2. Proposed uses and use levels
Through the current application, authorisation of pea fibre concentrate (FIPEA) as a food additive is sought with regards to the food categories listed in Table 2.
TABLE 2.
Proposed uses and maximum/typical use levels of pea fibre concentrate (FIPEA) (mg FIPEA/kg food).
| Food category number | Food category name | Restrictions/exceptions | Proposed maximum use levels (mg FIPEA/kg food) | Proposed typical use levels (mg FIPEA/kg food) |
|---|---|---|---|---|
| 01.4 | Flavoured fermented milk products including heat‐treated products | 10,000 | 1000 | |
| 01.7.5 | Processed cheese | 10,000 | 1000 | |
| 01.8 | Dairy analogues, including beverage whiteners | 10,000 | 1000 | |
| 06.5 | Noodles | 5000 | 100 | |
| 07.2 | Fine bakery wares | 10,000 | 1000 | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) 19 | 10,000 | 1000 | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | 10,000 | 1000 | |
| 14.1.4 | Flavoured drinks | 10,000 | 1000 |
The Panel noted that the applicant has submitted proposed maximum use levels of 5000 mg FIPEA/kg food and 10,000 mg FIPEA/kg food, depending on the food category. Categories are identified according to Part D of Annex II of Regulation (EC) No 1333/2008.
Concentration levels used to estimate the dietary exposure to pea fibre concentrate (FIPEA) are listed in Annex A, Table A.1.
3.3. Exposure data
3.3.1. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
To assess whether the proposed uses and use levels (Table 2) pose a possible health concern, the potential chronic dietary exposure to pea fibre concentrate (FIPEA) was calculated by the Panel using the proposed use levels with the Food Additive Intake Model (FAIM; version 2.1) (Documentation provided to EFSA n. 1, 3). FAIM contains food consumption data from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database). Since 2010, this database has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011)). The version of the Comprehensive database taken into account in this assessment was published in December 2022 and its linkage with the food classification system 20 was updated in November 2023.
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons may not be appropriate. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects' underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from infants, toddlers, children, adolescents, adults and the elderly were used in the exposure assessment with FAIM. For the present assessment, food consumption data were available from 43 different dietary surveys carried out in 22 European countries (Table 3).
TABLE 3.
Population groups considered for the exposure estimates of pea fibre concentrate (FIPEA).
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Portugal, Slovenia, Spain |
| Toddlers a | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, the Netherlands, Portugal, Slovenia, Spain |
| Children a | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, the Netherlands, Portugal, Spain, Sweden |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| The elderly b | From 65 years of age and older | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
The term ‘toddlers’ in the Comprehensive Database (EFSA, 2011) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013. 21
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in Comprehensive Database (EFSA, 2011).
Consumption records were codified according to the FoodEx2 classification system (EFSA, 2015). Nomenclature from the FoodEx2 classification system was linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform the exposure assessments. In practice, the FoodEx2 food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of pea fibre concentrate (FIPEA)
The food categories in which the use of pea fibre concentrate (FIPEA) is proposed (see Table 2) were considered using FAIM. 22
Food categories 13.2 ‘Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5)’ and 13.3 ‘Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet)’ are for foods for special medical purposes consumed by children, adolescents, adults and the elderly population groups. These foods are very diverse and their consumption is not always well reported in dietary surveys. Therefore, eating occasions belonging to these food categories have been mostly reclassified under food categories in accordance to their main component (e.g. meal replacement drinks as flavoured drinks).
3.3.2. Exposure to pea fibre concentrate (FIPEA) from its proposed use as food additive
The Panel noted that the applicant provided estimates of exposure to pea fibre concentrate (FIPEA) based on FAIM and using the maximum and typical use levels (Table 2; Documentation provided to EFSA n. 1, 3). However, the Panel noted that the applicant did not correctly allocate the proposed use levels to the parent food categories. Therefore, the Panel performed an updated estimation using FAIM with the proposed maximum and proposed typical use levels. The summary of the results per population group is presented in Table 4. Detailed results per population group and survey are presented in Annex A, Table A.2.
At the proposed maximum use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 1.6 mg/kg bw per day in infants to 267 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 11 mg/kg bw per day in infants to 719 mg/kg bw per day in toddlers.
At the proposed typical use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 0.2 mg/kg bw per day in infants to 27 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 1.1 mg/kg bw per day in infants to 72 mg/kg bw per day in toddlers.
Main food categories contributing to exposure to pea fibre concentrate (FIPEA)
In the proposed maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates of pea fibre concentrate (FIPEA) for all population groups were flavoured drinks (FC 14.1.4) and fine bakery wares (FC 7.2). For infants, toddlers and children, the food category flavoured fermented milk products, including heat‐treated products (FC 01.4), was also a main contributor to the mean exposure.
Annex A, Table A.3 lists all the contributing food categories by population group.
Uncertainty analysis
Potential sources of uncertainty in the exposure assessment of FIPE have been presented above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
TABLE 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate.
| Sources of uncertainties | Direction a |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Methodology used to estimate high percentiles (95th) long‐term (chronic) exposure based on data from food consumption surveys covering only a few days | + |
| Correspondence of proposed use levels to the food items in the EFSA Comprehensive Database: uncertainties to which types of food the levels refer | +/− |
| Uncertainty in possible national differences in use levels of food categories | +/− |
| Food categories selected for the exposure assessment: foods belonging to food categories 13.2 and 13.3 considered through reclassification under other food categories in accordance with their main component | − |
| Concentration data: | |
| Proposed maximum/typical use levels considered applicable to all foods within the entire food category, whereas it is not likely that pea fibre concentrate will be added as a food additive to all foods belonging to a proposed food category | + |
+, uncertainty with potential to cause overestimation of exposure; −, uncertainty with potential to cause underestimation of exposure.
Pea fibre concentrate (FIPEA) is requested to be authorised in 8 food categories. For all food categories considered, it was assumed that 100% of the foods belonging to these food categories will contain pea fibre concentrate (FIPEA) at the proposed maximum or typical use levels. Therefore, overall, the Panel considered that the uncertainties identified resulted in an overestimation of the exposure to pea fibre concentrate (FIPEA) at the maximum/typical proposed use levels in European countries considered in the EFSA Comprehensive database.
3.3.3. Anticipated exposure to toxic elements from proposed specifications
The applicant provided maximum limits for As (0.2 mg/kg), Pb (0.5 mg/kg), Cd (0.1 mg/kg) and Hg (0.1 mg/kg) in the proposed food additive for the purpose of defining appropriate specifications (Table 1). The Panel noted that the occurrence data on toxic elements for five independently produced batches of the proposed food additive submitted by the applicant are substantially lower than the proposed specification limits, being below the respective LOQs, i.e. < 0.04 mg/kg for As, < 0.015 mg/kg for Pb, < 0.01 mg/kg for Cd and < 0.01 mg/kg for Hg in all batches (Documentation provided to EFSA n. 1).
The potential exposure to impurities from the use of the proposed food additive was calculated by assuming that they are present in the food additive up to a certain limit value and then by calculation pro‐rata to the estimates of exposure to the food additive itself.
The highest exposure levels for the mean and 95th percentile among the different population groups were considered, i.e. 267 and 719 mg/kg bw per day respectively, for toddlers (Table 4).
The potential levels of the toxic elements in the proposed food additive combined with the estimated exposure levels presented in Table 4, result in exposure estimates that can be compared with the following reference points (RP) or HBGV (Table 6) for the toxic elements. It is considered that any Hg or As in the proposed food additive corresponds to the element in the inorganic form rather than an organic form. Consequently, the HBGV for inorganic mercury and the RP for inorganic arsenic were used for comparison (Table 6).
TABLE 6.
Reference points/health‐based guidance value for toxic elements potentially present in the proposed food additive.
| Element/HBGV/RP | Basis |
|---|---|
| Lead (Pb)/0.5 mg/kg bw per day (BMDL01) | The reference point is based on a study demonstrating perturbation of intellectual development in children with the critical response size of 1 point reduction in IQ. The EFSA CONTAM Panel mentioned that a 1 point reduction in IQ is related to a 4.5% increase in the risk of failure to graduate from high school and that a 1 point reduction in IQ in children can be associated with a decrease of later productivity of about 2%. A risk cannot be excluded if the exposure exceeds the BMDL01 (MOE lower than 1). EFSA CONTAM Panel (2010) |
| Inorganic mercury (iHg)/4 mg/kg bw per week (TWI) | The HBGV was set using kidney weight changes in male rats as the pivotal effect. Based on the BMDL10 of 0.06 mg/kg bw per day, expressed as mercury, and an uncertainty factor of 100 to account for inter and intra species differences, with conversion to a weekly basis and rounding to one significant figure, a TWI for inorganic mercury of 4 μg/kg bw per week, expressed as mercury was established. EFSA CONTAM Panel (2012) |
| Cadmium (Cd)/2.5 mg/kg bw per week (TWI) | The derivation of the reference point is based on a meta‐analysis to evaluate the dose–response relationship between selected urinary cadmium and urinary beta‐2‐microglobulin as the biomarker of tubular damage recognised as the most useful biomarker in relation to tubular effects. A group‐based BMDL5 of 4 μg Cd/g creatinine for humans was derived. A chemical specific adjustment factor of 3.9 was applied to account for human variability in urinary cadmium within each dose‐subgroup in the analysis resulting in a reference point of 1.0 μg Cd per g creatinine. In order to remain below 1 μg Cd/g creatinine in urine in 95% of the population by age 50, the average daily dietary cadmium intake should not exceed 0.36 μg Cd/kg bw, corresponding to a weekly dietary intake of 2.5 μg Cd/kg bw. EFSA CONTAM Panel (2009) |
| Inorganic arsenic (iAs)/0.06 μg/kg bw per day (BMDL05) |
The reference point is based on a benchmark dose lower confidence limit (BMDL05) of 0.06 μg/kg bw per day identified for skin cancer. The reference point is considered to cover lung cancer, bladder cancer, skin lesions, ischemic heart disease, chronic kidney disease, respiratory disease, spontaneous abortion, stillbirth, infant mortality and neurodevelopmental effects. A MOE of 1 would correspond to the exposure level that is associated with a 5% increase relative to the background incidence for skin cancer, based on the available data. A MOE of 1 raises a health concern. Because there are no precedents in EFSA for identification of a MOE of low concern, when using a BMDL derived from human cancer data the CONTAM Panel decided not to determine a value for a MOE of low concern. EFSA CONTAM Panel (2024) |
Abbreviations: BMDL, benchmark dose (lower confidence limit); HBGV, Health‐based guidance value; IQ, intelligence quotient; MOE, margin of exposure; RP, Reference point; TWI, tolerable weekly intake.
The risk assessment of the impurities helps to determine whether there could be a possible health concern if these impurities would be present at a certain level in the proposed food additive. The assessment is performed by calculating e.g. the MOE (margin of exposure) by dividing the RP (i.e. BMDL, Table 6) by the exposure estimate (Table 4).
The Panel assessed the risk that would result if these toxic elements were present in pea fibre concentrate (FIPEA) according to two concentration scenarios: (i) considering their presence at the proposed specification limits and (ii) considering the analytical data provided, which correspond to the LOQs of the toxic elements.
The outcome of the risk assessment of the Panel is presented in Table 7.
Considering the results of the exposure to the toxic elements Pb, Cd and iHg, the Panel noted that their presence in PIFEA at both scenarios would not give rise to concern. In the case of iAs, the Panel noted that its presence at the proposed specification limit value would lead to an MOE of 0.4 and 1.1, while in the analytical data scenario, it would lead to an MOE of 2.1 and 5.6 for the 95th percentile and mean, respectively.
The Panel recommends to lower the specification limits proposed by the applicant for all four toxic elements (Pb, Cd, Hg, As), taking into account (i) the results of the calculations performed by the Panel (the MOE for Pb and As are close to or lower than the MOE of 1 at which risk cannot be excluded, Table 7), (ii) the fact that the proposed food additive is not the only potential dietary source of toxic elements and that (iii) the maximum limits should be established based on actual levels in the commercial food additive.
The Panel considered that the choice of maximum limits for toxic elements in the specifications is in the remit of risk manager(s). The values used here were merely taken to support the risk assessment of these toxic elements as presented above.
3.4. Biological and toxicological data
3.4.1. Absorption, distribution, metabolism and excretion
FIPEA contains polysaccharides similar to water‐soluble soybean polysaccharides; in addition, it has similar high dietary fibre content. In the re‐evaluation of refined water‐soluble soybean polysaccharides, soybean hemicellulose (E 426) (EFSA ANS Panel, 2017a, 2017b), the ANS Panel considered that ‘certain high molecular weight dietary polysaccharides could be partially broken down by microbiota in the human large intestine. In addition to intermediate metabolites such as lactic, acrylic or fumaric acid, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, which are absorbed from the colon’. The data indicated that hemicelluloses would be most probably not absorbed intact but fermented by the intestinal microbiota in animals and humans. The ANS Panel considered that the potential formation of fermentation products from soybean hemicellulose does not raise a safety concern.
The Panel considered that pea fibre concentrate (FIPEA) has a similar toxicokinetic profile to water‐soluble soybean polysaccharides: it is most likely not absorbed in intact form but is extensively fermented to SCFA by the intestinal microbiota in humans.
The Panel noted that a‐galactosides, possible minor components of the proposed food additive, may be potential fermentable compounds in FIPEA; a‐galactosides are fermented by colonic α‐galactosidase bacteria producing gases (e.g. H2 and CO2 and traces of CH4) (Camara et al., 2017).
3.4.2. Acute toxicity
No data on acute toxicity were submitted by the applicant.
3.4.3. Short‐term and subchronic toxicity
The applicant did not provide specific toxicity studies on pea fibre concentrate (FIPEA), instead supported its assessment reporting information on water‐soluble soybean fibres from the ANS Panel opinion of 2017. In this opinion, no adverse effects were reported for water‐soluble soybean fibres in a dietary 90‐day study in rats at the highest dose tested of 2430 mg/kg bw per day for males and 2910 mg/kg bw per day for females (Takahashi et al., 2003).
The Panel considered that pea fibre concentrate (FIPEA) has a similar toxicity profile based on similar structure to water‐soluble soybean fibres.
3.4.4. Genotoxicity
The applicant did not provide genotoxicity studies on pea fibre concentrate (FIPEA), instead supported its assessment reporting information on water‐soluble soybean fibres from the ANS Panel opinion in 2017. Despite that in this 2017 opinion, only an Ames test was described to investigate the genotoxicity of water‐soluble soybean fibres, the ANS Panel concluded that, similarly to other dietary polysaccharides, soybean hemicellulose was not of genotoxic concern.
The Panel noted that the available data set is not aligned with current requirements for genotoxicity hazard identification. However, based on (i) the nature of pea fibre concentrate (FIPEA), mainly consisting of dietary fibres, and its source, (ii) the similar properties between the components of the pea fibre concentrate (FIPEA) and the water‐soluble soya fibre previously evaluated by the ANS Panel in 2017, (iii) the absence of structural alerts for genotoxicity for the components of the proposed food additive based on expert judgement and (iv) the absence of by‐products of concern from the manufacturing process, the Panel overall considered that there are no indications for concerns on genotoxicity. Therefore, in this specific case, the Panel did not request experimental data for genotoxicity on the proposed food additive.
3.4.5. Chronic toxicity and carcinogenicity
No data on chronic toxicity and carcinogenicity were submitted by the applicant.
3.4.6. Reproductive and developmental toxicity
No data on reproductive and developmental toxicity were submitted by the applicant.
3.4.7. Hypersensitivity, allergenicity and food intolerance
No information was submitted on hypersensitivity, allergenicity and food intolerance. The Panel noted that the manufacturing process would not lead to the generation of new proteins compared to the proteins present in the starting material (yellow peas).
4. DISCUSSION
The European Commission requests EFSA to provide a scientific opinion on the safety of the proposed use of pea fibre concentrate (FIPEA) as a food additive, in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
The proposed food additive is a powder consisting mainly of dietary fibres (i.e. pectin and hemicellulose), along with low amounts of protein. The source material is yellow pea (P. sativum).
The manufacturing process begins with hot water extraction (> 100°C) from of the insoluble fibrous material of yellow peas (side stream by‐product that remains after separation of starch during pea processing in the food industry), followed by further separation, pH adjustment, hydrolysis of starch by commercially available α‐amylase enzyme, purification, sterilisation and spray‐drying. The Panel noted that the enzyme used in the manufacturing process is an α‐amylase (4‐α‐d‐glucan glucanohydrolase; EC 3.2.1.1) from the non‐genetically modified B. amyloliquefaciens strain NZYM‐WR (EFSA CEP Panel, 2023) and this should be included in the definition of the proposed specifications.
The applicant provided analytical data on five batches of the proposed food additive, showing that pea fibre concentrate (FIPEA) is produced according to the proposed specifications. The Panel considered the specifications provided by the applicant sufficient to properly characterise the proposed food additive.
Dietary fibre in pea fibre concentrate (FIPEA) ranged from 55.2% to 68.4% in the five analysed batches. Considering the highest value of 68.4% of dietary fibre in pea fibre concentrate (FIPEA) and the maximum dietary exposure estimates of FIPEA (Table 4), the corresponding estimated dietary intake of fibres from pea fibre concentrate (FIPEA) in adults (bw 70 kg) would be 2.8 g/day at the mean and 8.3 g/day at the 95th percentile. In toddlers (bw 12 kg), the estimated dietary intake of fibres from pea fibre concentrate (FIPEA) would be 2.2 g/day at the mean and 5.9 g/day at the 95th percentile. The NDA Panel considered dietary fibre intakes of 25 g/day and 10 g/day to be adequate for normal bowel function in adults and toddlers, respectively (EFSA NDA Panel, 2010). Considering the overestimation of the exposure to pea fibre concentrate (FIPEA) at the maximum/typical proposed use levels, the Panel considered that the additional contribution of pea fibre concentrate (FIPEA) to the total fibre intake in adults and toddlers would be acceptable taking into account the levels that are considered to be adequate for normal bowel function by the NDA Panel, also noting the health benefit of a diet rich in fibre (EFSA NDA Panel, 2010).
Upon EFSA's request, the applicant provided analytical data regarding antinutritional factors, including lectin activity in five batches of pea fibre concentrate (FIPEA), ranging from 3830 to 3840 HAU/g. The European Commission requested EFSA to perform a risk assessment on the presence of plant lectins in food (EFSA‐Q‐2024‐00195); the assessment is ongoing. Currently, there is no HBGV set for lectins. The Panel noted that the haemagglutination assay used to monitor the lectin activity is not a direct measurement of their concentration; in addition, it has several drawbacks and limitations, such as low specificity and reproducibility, leading to inconsistent or misleading results. No cases of food poisoning from lectins in EU and outside are reported for peas (BfR, 2024), rather than for (red kidney) beans. 23 The Panel noted that lectins among legumes differ. According to BfR (2024), when legumes are consumed, most lectins are not expected to have any adverse effects on human health. However, ingesting certain lectins, which are mainly found in raw and insufficiently prepared legumes, can lead to adverse health effects, including symptoms such as nausea, vomiting and diarrhoea (BfR, 2024). Studies demonstrate that lectin activity is significantly reduced by heat treatment (Adamcová et al., 2021; Hernández‐Infante et al., 1998; Leontowicz et al., 1998; Shi et al., 2018). The Panel noted that the manufacturing process of FIPEA entails extensive heat treatments (e.g. > 100°C for more than 40 min), conditions which lead to inactivation of lectins. Based on the above, the Panel considered that lectins in the proposed food additive do not pose a safety concern.
Analytical data on the levels of As, Pb, Cd and Hg were provided by the applicant for five samples of the proposed food additive. The Panel assessed the risk that would result if those toxic elements were present in pea fibre concentrate (FIPEA) at two concentration scenarios (i) considering their presence at the proposed specification limits and (ii) considering the analytical data provided, which correspond to the LOQs of the toxic elements. The Panel recommended to lower the specification limits proposed by the applicant for all four toxic elements (Pb, Cd, Hg, As), taking into account (i) the results of the calculations performed by the Panel (the MOE for Pb and As are close to or lower than the MOE of 1 at which risk cannot be excluded, Table 7), (ii) the fact that the proposed food additive is not the only potential dietary source of toxic elements and that (iii) the maximum limits should be established based on actual levels in the commercial food additive.
The applicant provided particle size distribution for five batches of the proposed food additive using DLS, a method not suitable for detecting nanosized particles due to its limitations in providing detailed particle size information and its bias for polydisperse materials reporting median particle sizes ranging from 41 to 52 μm. Based on these data provided, the Panel cannot confirm or exclude the presence of small particles, including nanoparticles, in the proposed food additive. The applicant also provided solubility data, tested using an unspecified, unaccredited method, showing solubility of pea fibre concentrate (FIPEA) in water ranging from 6.1 to 6.9 g/100 mL at 20°C. The Panel noted that although the solubility test provided variable results and it was not performed following the EFSA Guidance on Particle‐TR (EFSA Scientific Committee, 2021), the results indicate solubility is higher than the threshold value of 33.3 g/L as a decision criterion for demonstrating that the material does not require specific assessment at the nanoscale.
The Panel noted that the proposed food additive pea fibre concentrate (FIPEA) is extracted with hot water (> 100°C) from the insoluble fibrous material of dehulled yellow peas (P. sativum), which are commonly consumed in the human diet after boiling. The functional component is soluble dietary fibre (SDF), which is commonly known to be hydrophilic, non‐crystalline and easily wetted by aqueous gastrointestinal fluid, forming viscous colloidal dispersions or gels when hydrated. Due to these properties, SDF is widely used in the food industry to modify texture and rheology and to influence the colligative properties of food systems (Li et al., 2017). Considering the solubility of the proposed food additive (6.1–6.9 g/100 mL) and the properties of SDF, the Panel considered that the consumers will not be exposed to small particles, including nanoparticles of pea fibre concentrate (FIPEA) under the proposed conditions of use. Therefore, the Panel considered that the proposed food additive can be assessed following conventional risk assessment, i.e. Guidance for submission for food additive evaluations should be followed (EFSA ANS Panel, 2012).
The applicant conducted stability tests under normal (25°C, 60% RH, for 24 months) and accelerated conditions (40°C, 75% RH, for 6 months) on five batches of the proposed food additive. The results indicate that pea fibre concentrate (FIPEA) is stable under the tested storage conditions.
At the proposed maximum use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 1.6 mg/kg bw per day in infants to 267 mg/kg bw per day in toddlers, and the 95th percentile of exposure ranged from 11 mg/kg bw per day in infants to 719 mg/kg bw per day in toddlers. At the proposed typical use levels, the mean exposure to pea fibre concentrate (FIPEA) from its use as a food additive ranged from 0.2 mg/kg bw per day in infants to 27 mg/kg bw per day in toddlers, and the 95th percentile of exposure from 1.1 mg/kg bw per day in infants to 72 mg/kg bw per day in toddlers.
The applicant did not provide specific toxicity studies on pea fibre concentrate (FIPEA), instead supported its assessment by reporting information on water‐soluble soybean polysaccharides from the ANS Panel 2017. The Panel considered that, similarly to water‐soluble soybean polysaccharides and other dietary polysaccharides, pea fibre concentrate (FIPEA) is most likely not absorbed intact but undergoes extensive fermentation by the intestinal microbiota in humans. There is no concern with respect to genotoxicity. Dry peas, the raw material used for the manufacture of pea fibre concentrate (FIPEA), are a staple food, with a very long history of safe use in the EU. The proposed food additive is extracted with hot water (> 100°C) from the insoluble fibrous material of dehulled yellow peas (P. sativum), therefore the structure of the fibres is not chemically modified, and no new by‐products or components of toxicological concern are expected from the manufacturing process. Therefore, the Panel considered that there was no need for a numerical ADI.
5. CONCLUSIONS
The Panel concluded that that there was no need for a numerical ADI and that the use of pea fibre concentrate (FIPEA) as a new food additive does not raise a safety concern at the proposed use and use levels.
6. DOCUMENTATION PROVIDED TO EFSA
FUJI OIL HOLDINGS INC. 2023. Technical dossier for the request on the safety in use of pea fibre concentrate as a food additive. Submitted on 14 March 2023. 24
FUJI OIL HOLDINGS INC. 2023. Clarification on the data submitted for request on the safety in use of pea fibre concentrate as a food additive. Submitted on 05 December 2023.
FUJI OIL HOLDINGS INC. 2024. Clarification on the data submitted for the request on the safety in use of pea fibre concentrate as a food additive. Submitted on 24 July 2024.
FUJI OIL HOLDINGS INC. 2024. Clarification on the data submitted for the request on the safety in use of pea fibre concentrate as a food additive. Submitted on 27 November 2024.
ABBREVIATIONS
- ADI
acceptable daily intake
- ANS panel
Panel on Food Additives and Nutrient Sources added to Food
- BfR
Bundesinstitut für Risikobewertung
- BMDL
Benchmark dose lower confidence limit
- BW
body weight
- CEP Panel
Panel on Food Contact Materials
Enzymes and Processing Aids
- CFU
colony forming unit
- CONTAM Panel
Panel on Contaminants in the Food Chain
- DLS
dynamic lighter scattering
- FAF panel
Panel on Food Additives and Flavourings
- FAIM
Food Additive Intake Model
- FC
Food Category
- FCS
Food categorisation system
- FIPEA
pea fibre concentrate
- FoodEx2
Food classification standardisation
- GC/MS
gas chromatography/mass spectrometry
- GC–MS/MS
gas chromatography with tandem mass spectrometry
- GMO
genetically modified organisms
- HAU
hemagglutination unit
- HBGV
health‐based guidance values
- HPAEC
high performance anion‐exchange chromatography
- ICP‐MS
inductively coupled plasma mass spectrometry
- ICP‐OES
inductively coupled plasma optical emission spectroscopy
- IQ
intelligence quotient
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MOE
margin of exposure
- MPL
maximum permitted level
- NDA panel
Panel on Nutrition
Novel food and Food Allergens
- PAD
pulsed amperometric detection
- PAHs
polycyclic aromatic hydrocarbons
- QPS
qualified presumption of safety
- RASFF
Rapid Alert System for Food and Feed
- RH
relative humidity
- RP
Reference point
- SCF
Scientific Committee on Food
- SCFA
short‐chain fatty acids
- SDF
soluble dietary fibre
- TIU
trypsin inhibitor unit
- TWI
tolerable weekly intake
- UHT
ultra‐high temperature
- UV/VIS
ultraviolet–Visible
- WG
Working group
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2022‐00795
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Monica Andreassen, Gabriele Aquilina, Maria Lourdes Bastos, Polly Boon, Laurence Castle, Biagio Fallico, Reginald FitzGerald, Maria Jose Frutos Fernandez, Bettina Grasl‐Kraupp, Ursula Gundert‐Remy, Rainer Gürtler, Eric Houdeau, Marcin Kurek, Henriqueta Louro, Patricia Morales, and Sabina Passamonti.
NOTE
Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by EFSA. The full output has been shared with the European Commission, EU Member States (if applicable) and the applicant. The blackening may be subject to review once the decision on the confidentiality requests is adopted by EFSA and in case it rejects some of the confidentiality requests.
Supporting information
Exposure data and estimates
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: Eirini Kouloura and Sam Vermeiren.
ANNEX A.
Table A.1: Concentration levels of pea fibre concentrate (FIPEA) used in the exposure assessment scenarios (mg/kg).
Table A.2: Summary of total estimated exposure to pea fibre concentrate (FIPEA) from use as a food additive for the proposed maximum level exposure scenario and the proposed typical level exposure assessment scenario per population group and survey: Mean and 95th percentile (mg/kg bw per day).
Table A.3: Main food categories contributing to exposure to pea fibre concentrate (FIPEA) using the maximum level exposure assessment scenario and the refined exposure assessment scenario (> 5% to the total mean exposure).
Annex A can be found in the online version of this output (‘Supporting information’ section).
EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Castle, L. , Andreassen, M. , Aquilina, G. , Bastos, M. L. , Boon, P. , Fallico, B. , FitzGerald, R. , Frutos Fernandez, M. J. , Grasl‐Kraupp, B. , Gundert‐Remy, U. , Gürtler, R. , Houdeau, E. , Kurek, M. , Louro, H. , Morales, P. , Passamonti, S. , Barat Baviera, J. M. , … Ruggeri, L. (2025). Safety evaluation of pea fibre concentrate (FIPEA) as food additive. EFSA Journal, 23 (3), e9255. 10.2903/j.efsa.2025.9255
Adopted: 28 January 2025
The declarations of interest of all scientific experts active in EFSA's work are available at https://open.efsa.europa.eu/experts
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. OJ L 354, 31.12.2008, p. 1–6.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a program for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Available online: https://zenodo.org/record/1212388/files/BOTANIC.xls?download=1.
Available online: https://www.efsa.europa.eu/en/microstrategy/botanical‐summary‐report.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Decision available online: https://www.efsa.europa.eu/en/corporate‐pubs/transparency‐regulation‐practical‐arrangements.
The non‐confidential version of the dossier, following EFSA's assessment of the applicant's confidentiality requests, is published on Open.EFSA and is available at the following link: https://open.efsa.europa.eu/dossier/FAD‐2022‐8071.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Accessible at: https://connect.efsa.europa.eu/RM/s/consultations/publicconsultation2/a0lTk00000029nB/pc0756.
Accessible at: https://open.efsa.europa.eu/consultations/a0cTk0000002JzRIAU.
Available online: https://www.efsa.europa.eu/en/data‐report/compendium‐botanicals.
Available online: https://www.efsa.europa.eu/en/microstrategy/botanical‐summary‐report.
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. OJ L 268, 18.10.2003, p. 1–23.
Directive 1999/21/EC was repealed by Regulation (EU) 609/2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control.
Food categories in Part D of Annex II to Regulation (EC) No 1333/2008 on Food Additives.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
The non‐confidential version of the dossier, following EFSA's assessment of the applicant's confidentiality requests, is published on Open.EFSA and is available at the following link: https://open.efsa.europa.eu/dossier/FAD‐2022‐8071.
REFERENCES
- Adamcová, A. , Laursen, K. H. , & Ballin, N. Z. (2021). Lectin activity in commonly consumed plant‐based foods: Calling for method harmonization and risk assessment. Food, 10(11), 2796. 10.3390/foods10112796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung) . (2024). Lectins in plant‐based foods: Is there a health risk? BfR Opinion No. 003/2024 of January 23, 2024, BfR statements. The German Federal Institute for Risk Assessment. 10.17590/20240121-185904-0, https://www.bfr.bund.de/cm/343/lektine‐in‐pflanzenbasierten‐lebensmitteln‐gibt‐es‐ein‐gesundheitliches‐risiko.pdf [DOI]
- Camara, M. , Fernandez‐Ruiz, V. , Morales, P. , & Cortes Sanchez‐Mata, M. (2017). Fiber compounds and human health. Current Pharmaceutical Design, 23(19), 2835–2849. 10.2174/1381612823666170216123219 [DOI] [PubMed] [Google Scholar]
- Dahl, W. J. , Foster, L. M. , & Tyler, R. T. (2012). Review of the health benefits of peas (Pisum sativum L.). British Journal of Nutrition, 108(S1), S3–S10. 10.1017/S0007114512000852 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2007). Scientific opinion of the scientific committee related to uncertainties in dietary exposure assessment. EFSA Journal, 5(1), 438. 10.2903/j.efsa.2007.43 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011). Use of the EFSA comprehensive European food consumption database in exposure assessment. EFSA Journal, 9(3), 2097. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015). The food classification and description system FoodEx 2 (revision 2). EFSA Journal, 12(5), 804E. 10.2903/sp.efsa.2015.EN-804 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources) . (2012). Guidance for submission for food additive evaluations. EFSA Journal, 10(7), 2760. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen, A. , Aguilar, F. , Crebelli, R. , Di Domenico, A. , Dusemund, B. , Frutos, M. J. , Galtier, P. , Gott, D. , Gundert‐Remy, U. , Lambré, C. , Leblanc, J.‐C. , Lindtner, O. , Moldeus, P. , Mosesso, P. , Oskarsson, A. , Parent‐Massin, D. , Stankovic, I. , Waalkens‐Berendsen, I. , … Woutersen, R. A. (2017a). Scientific Opinion on the re‐evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA Journal, 15(7), 4866. 10.2903/j.efsa.2017.4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen, A. , Aguilar, F. , Crebelli, R. , Di Domenico, A. , Dusemund, B. , Frutos, M. J. , Galtier, P. , Gott, D. , Gundert‐Remy, U. , Lambré, C. , Leblanc, J.‐C. , Lindtner, O. , Moldeus, P. , Mosesso, P. , Oskarsson, A. , Parent‐Massin, D. , Stankovic, I. , Waalkens‐Berendsen, I. , … Woutersen, R. A. (2017b). Scientific opinion on the re‐evaluation of soybean hemicellulose (E 426) as a food additive. EFSA Journal, 15(3), 4721. 10.2903/j.efsa.2017.4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , Lambré, C. , Barat Baviera, J. M. , BolognesI, C. , Cocconcelli, P. S. , Crebelli, R. , Gott, D. M. , Grob, K. , Lampi, E. , Mengelers, M. , Mortensen, A. , Rivière, G. , Steffensen, I.‐L. , Tlustos, C. , Van Loveren, H. , Vernis, L. , Zorn, H. , Roos, Y. , Andryszkiewicz, M. , … Chesson, A. (2023). Safety evaluation of the food enzyme α‐amylase from the non‐genetically modified Bacillus amyloliquefaciens strain NZYM‐WR. EFSA Journal, 21(12), 1–13. 10.2903/j.efsa.2023.8394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2009). Cadmium in food‐scientific opinion of the panel on contaminants in the food chain. EFSA Journal, 7(3), 980. 10.2903/j.efsa.2009.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2010). Scientific opinion on lead in food. EFSA Journal, 8(4), 1570. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2012). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal, 10(12). 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2024). Update of the risk assessment of inorganic arsenic in food. EFSA Journal, 22(1), e8488. 10.2903/j.efsa.2024.8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes, M. , Aquilina, G. , Castle, L. , Engel, K.‐H. , Fowler, P. , Frutos Fernandez, M. J. , Fürst, P. , Gürtler, R. , Husøy, T. , Manco, M. , Mennes, W. , Moldeus, P. , Passamonti, S. , Shah, R. , Waalkens‐Berendsen, I. , Wölfle, D. , Wright, M. , Dusemund, B. , … Gundert‐Remy, U. (2021). Opinion on the re‐evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives in foods for infants below 16 weeks of age and follow‐up of their re‐evaluation as food additives for uses in foods for all population groups. EFSA Journal, 19(1), 6387. 10.2903/j.efsa.2021.6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (Panel on Dietetic Products, Nutrition, and Allergies) . (2010). Scientific Opinion on dietary reference values for carbohydrates and dietary fibre. EFSA Journal, 8(3), 1462. 10.2903/j.efsa.2010.1462 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2009a). Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal, 7(5), 1051. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2009b). Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal, 7(9), 1249. 10.2093/j.efsa.2009.1249 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2021). Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal, 19(8), 6769. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Infante, M. , Sousa, V. , Montalvo, I. , & Tena, E. (1998). Impact of microwave heating on hemagglutinins, trypsin inhibitors and protein quality of selected legume seeds. Plant Foods for Human Nutrition, 52, 199–208. 10.1023/A:1008033610737 [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO, WHO Expert Committee on Food Additives) . (1981). Evaluation of certain food additives. Twenty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series, No 669.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (1974). Toxicological evaluation of some foodadditives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. 340. Pectin. WHO Food Additives Series, No. 5. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (2015). Safety evaluation of certain food additives. Pectins (addendum). Seventy‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series, No. 70, 139 pp. https://iris.who.int/bitstream/handle/10665/171781/9789240693982_eng.pdf?sequence=3#page=147
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (2016). Eighty second report of the joint FAO/WHO expert committee on food additives. WHO Technical Report Series, No. 1000, 176 pp. http://apps.who.int/iris/bitstream/10665/250277/1/9789241210003‐eng.pdf#page=46
- Leontowicz, H. , Kostyra, H. , Leontowicz, M. , & Kulasek, W. (1998). The inactivation of legume seed haemagglutinin and trypsin inhibitors by boiling. In Jansman A. J. M., Hill G. D., Huisman J., & van der Poel A. F. B. (Eds.), Recent advances of research in antinutritional factors in legume seeds and rapeseed (pp. 429–432). Wageningen Pers. [Google Scholar]
- Li, Q. , Liu, R. , Wu, T. , & Zhang, M. (2017). Aggregation and rheological behavior of soluble dietary fibers from wheat bran. Food Research International, 102, 291–302. 10.1016/j.foodres.2017.09.064 [DOI] [PubMed] [Google Scholar]
- Mech, A. , Rauscher, H. , Babick, F. , Hodoroaba, V.‐D. , Wohlleben, W. , Marvin, H. , Weigel, S. , Brüngel, R. , & Friedrich, C. M. (2020). The NanoDefine methods manual, EUR 29876 EN. Publications Office of the European Union, Luxembourg, 2020, ISBN 978–92–76‐12335‐4, JRC117501.
- Mech, A. , Rauscher, H. , Rasmussen, K. , Babick, F. , Hodoroaba, V.‐D. , Ghanem, A. , Wohlleben, W. , Marvin, H. J. P. , Brüngel, R. , & Friedrich, C. (2020). Nano or not Nano a structured approach for identifying nanomaterials according to the European Commission's definition. Small, 16(36), 2002228. 10.1002/smll.202002228 [DOI] [PubMed] [Google Scholar]
- Noguchi, M. , Hasegawa, Y. , Suzuki, S. , Nakazawa, M. , Ueda, M. , & Sakamoto, T. (2020). Determination of chemical structure of pea pectin by using pectinolytic enzymes. Carbohydrate Polymers, 231, 115738. 10.1016/j.carbpol.2019.115738 [DOI] [PubMed] [Google Scholar]
- Rauscher, H. , Mech, A. , Gibson, N. , Gilliland, D. , Held, A. , Kestens, V. , Koeber, R. , Linsinger, T. , & Stefaniak, E. (2019). Identification of nanomaterials through measurements Publications Office of the European Union. https://data.europa.eu/doi/10.2760/053982
- SCF (Scientific Committee for Food) . (1978). Report of the Scientific Committee for Food, 7 Series, 44 pp.
- Shi, L. , Arntfield, S. D. , & Nickerson, M. (2018). Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Research International, 107, 660–668. 10.1016/j.foodres.2018.02.056 [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , Nakamura, A. , Kato, M. , Maeda, H. , Mandella, R. C. , Broadmeadow, A. , & Ruckman, S. A. (2003). Soluble soybean fiber: A 3‐month dietary toxicity study in rats. Food and Chemical Toxicology, 41(8), 1111–1121. 10.1016/S0278-6915(03)00065-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exposure data and estimates


