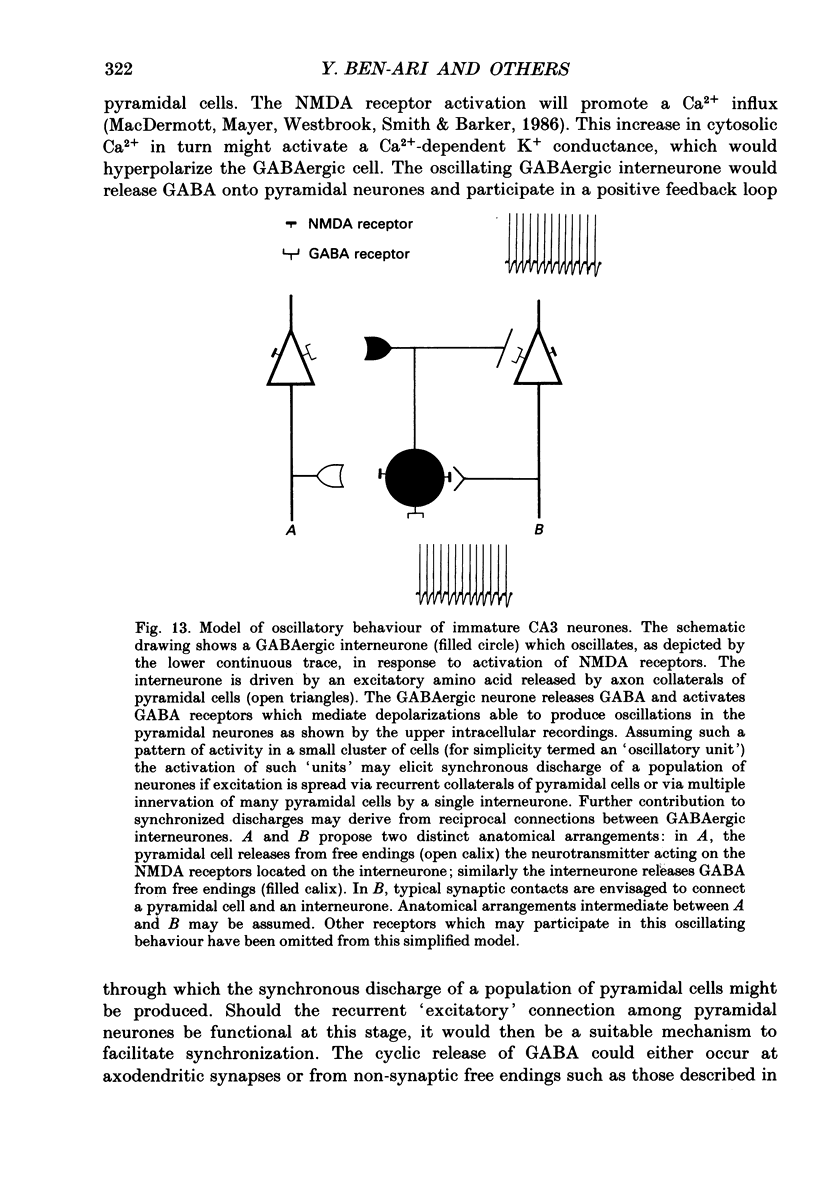
Abstract
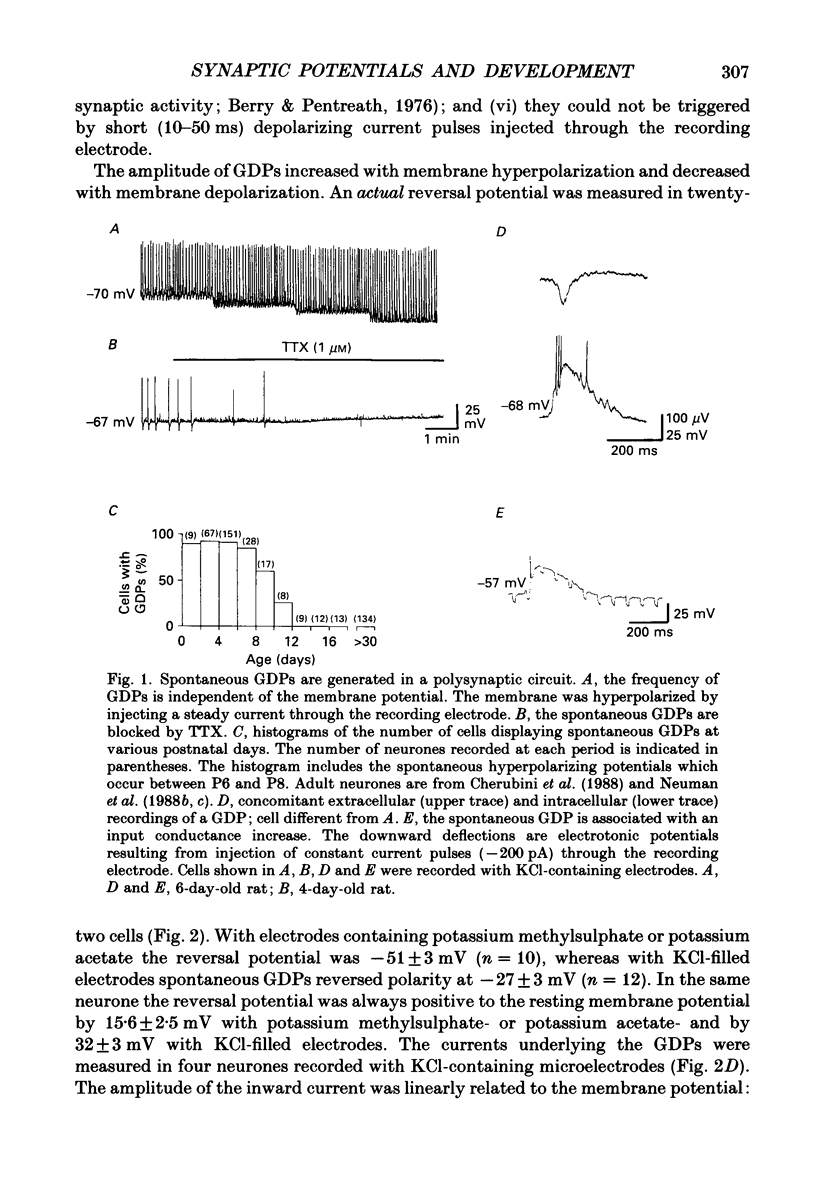
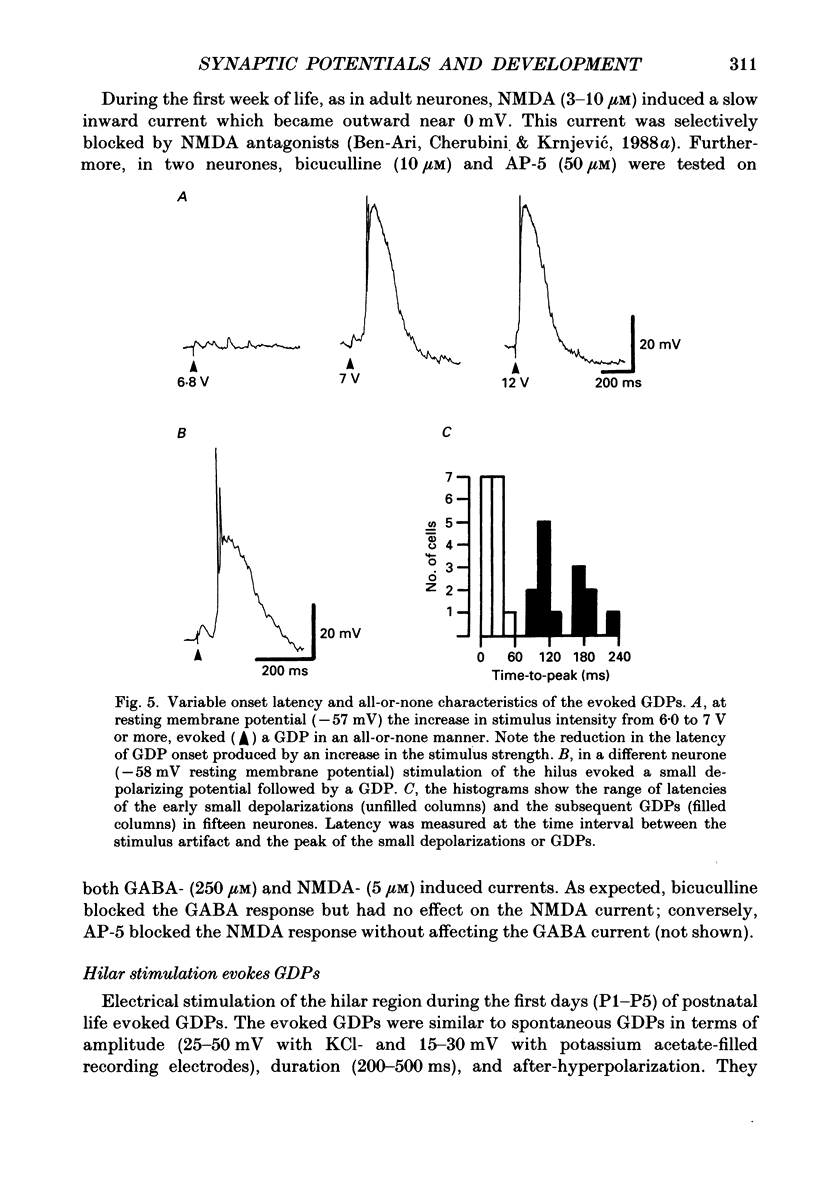
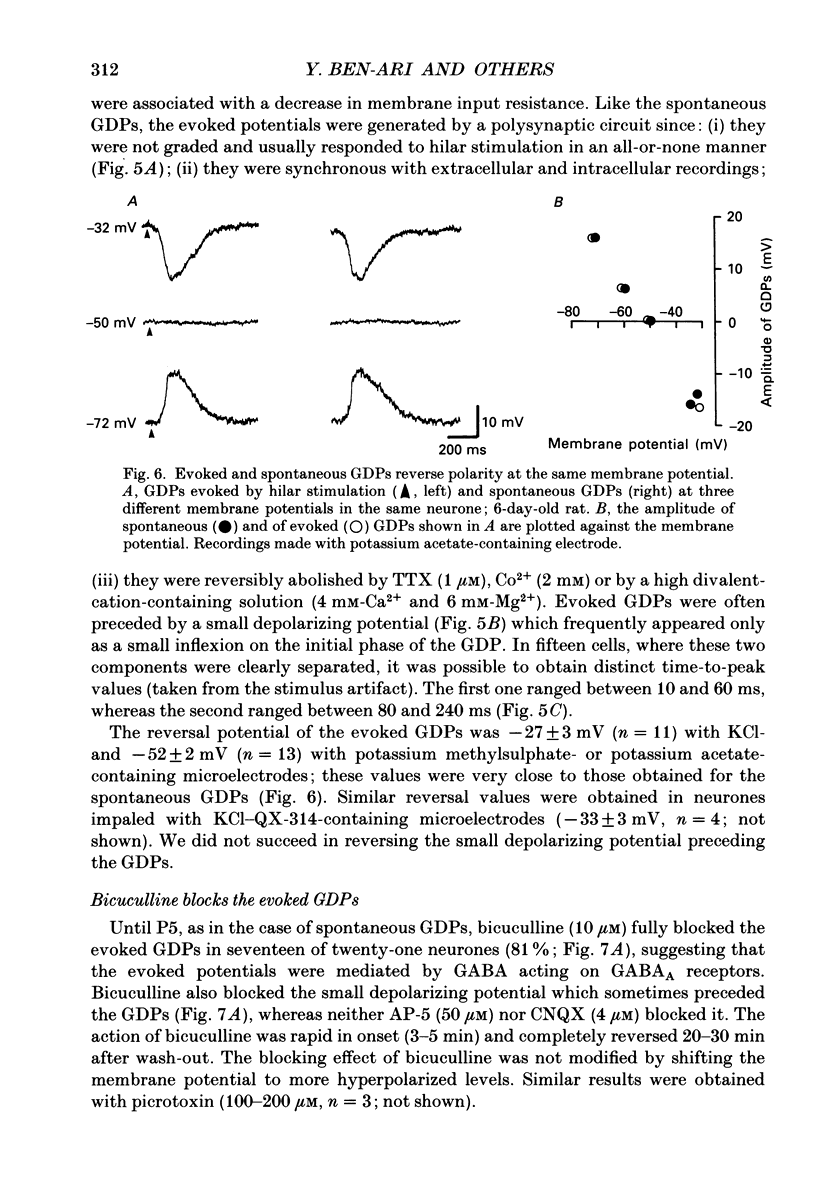
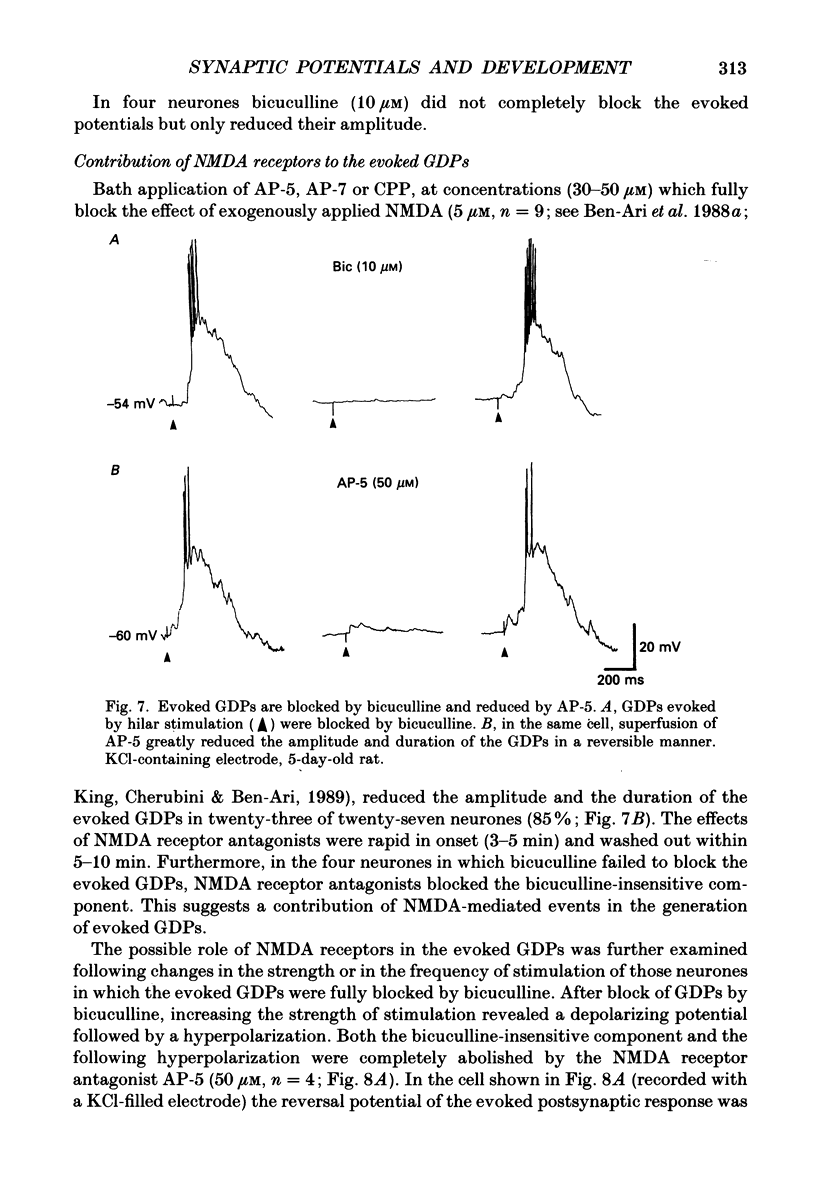
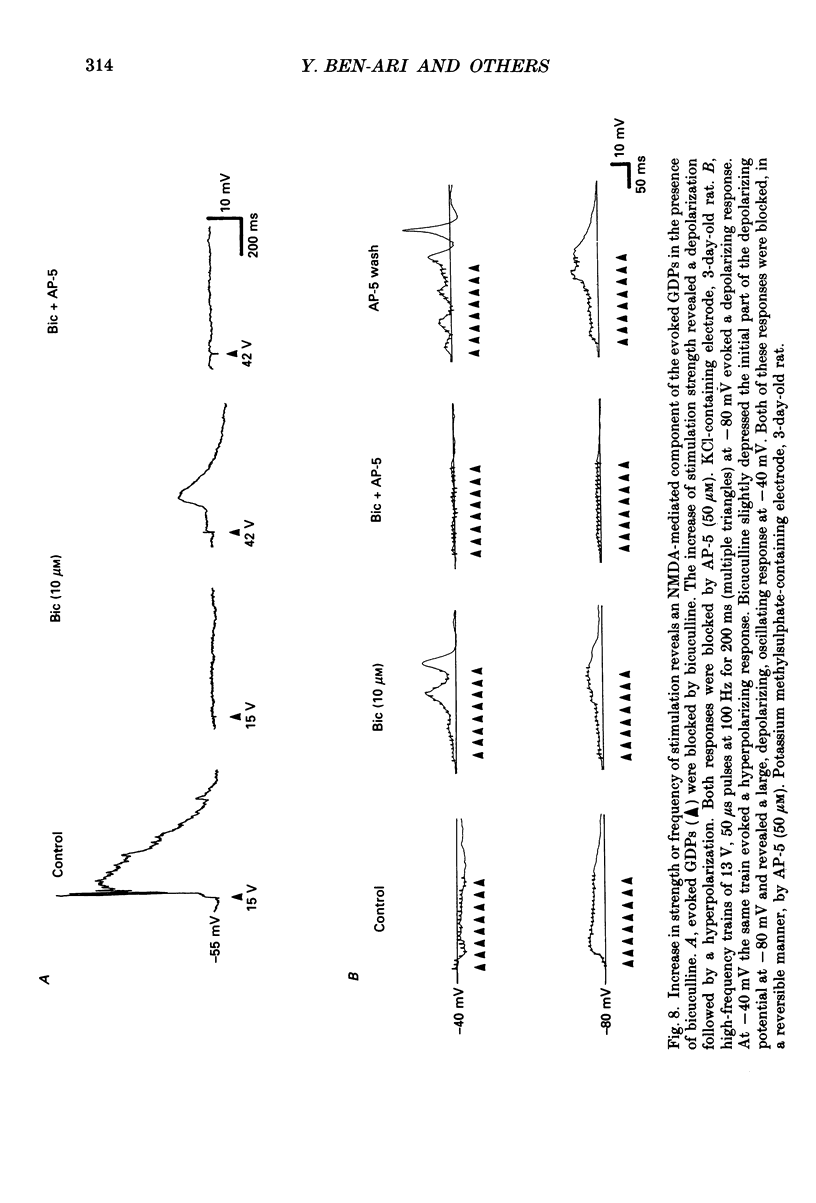
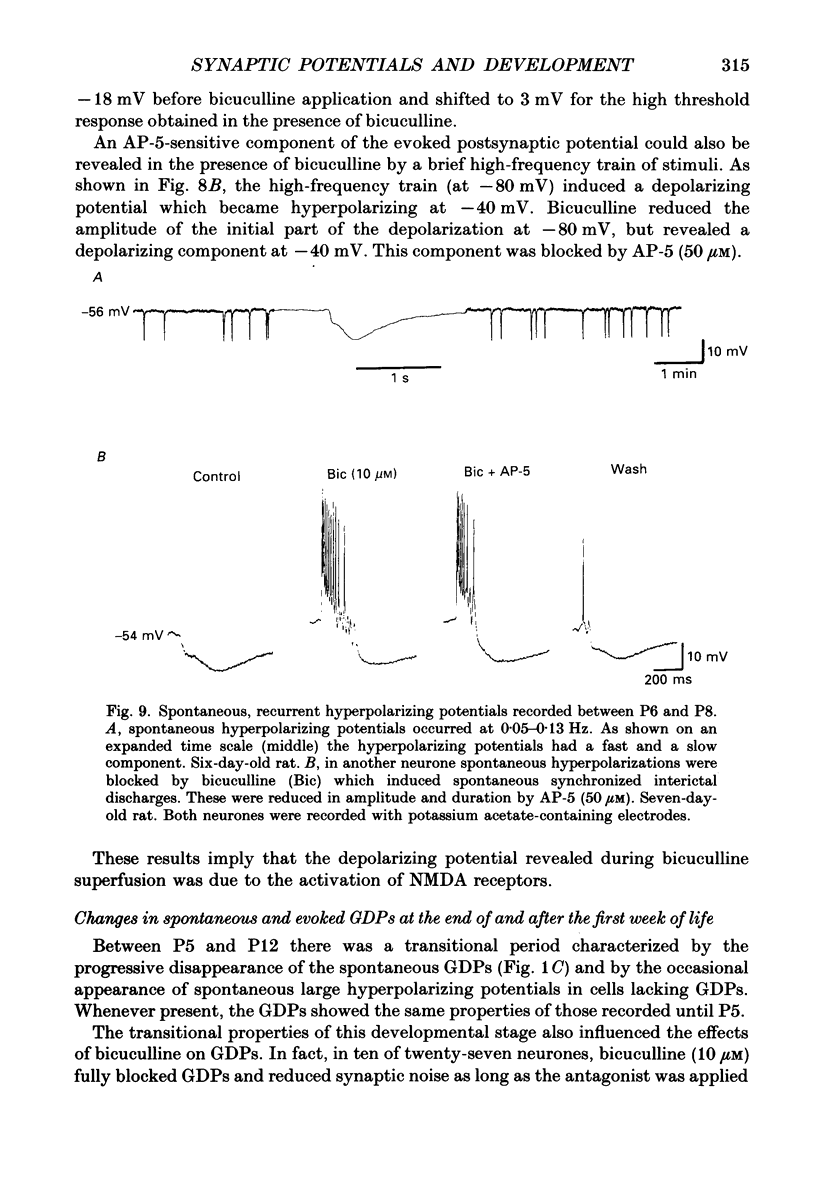
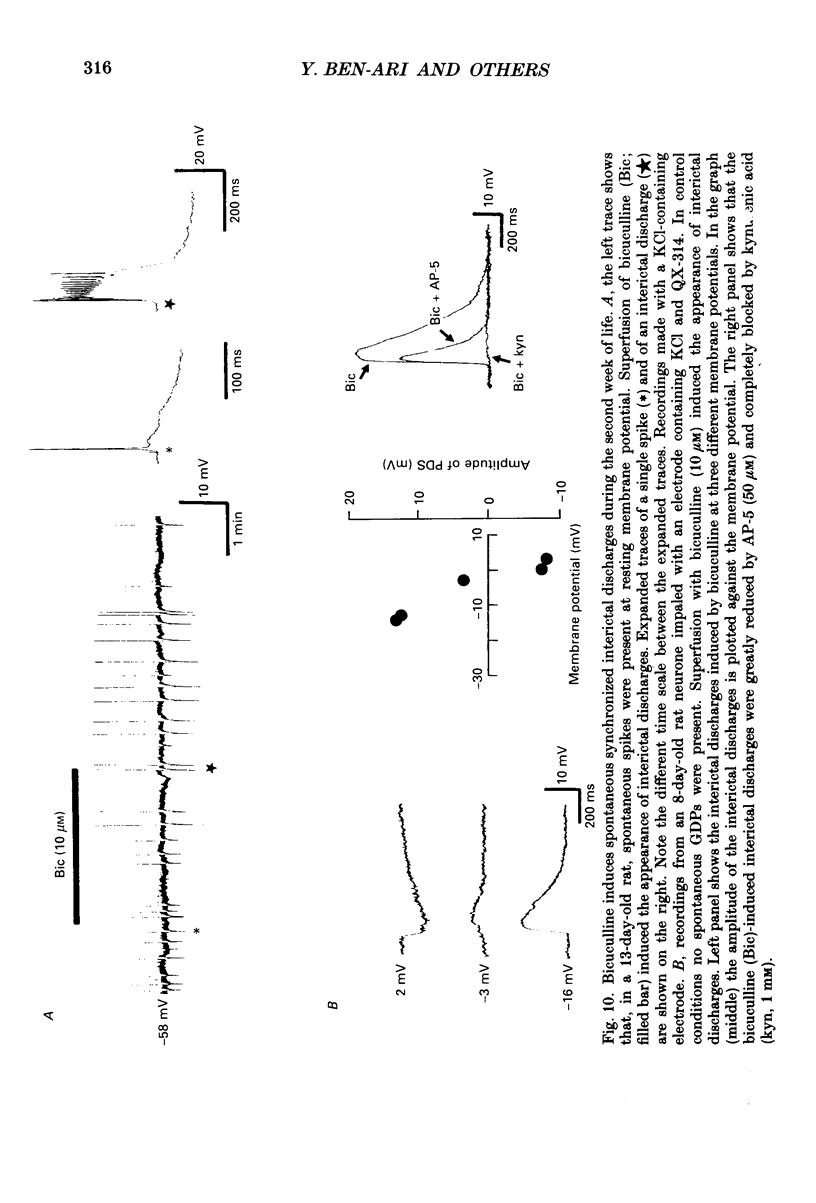
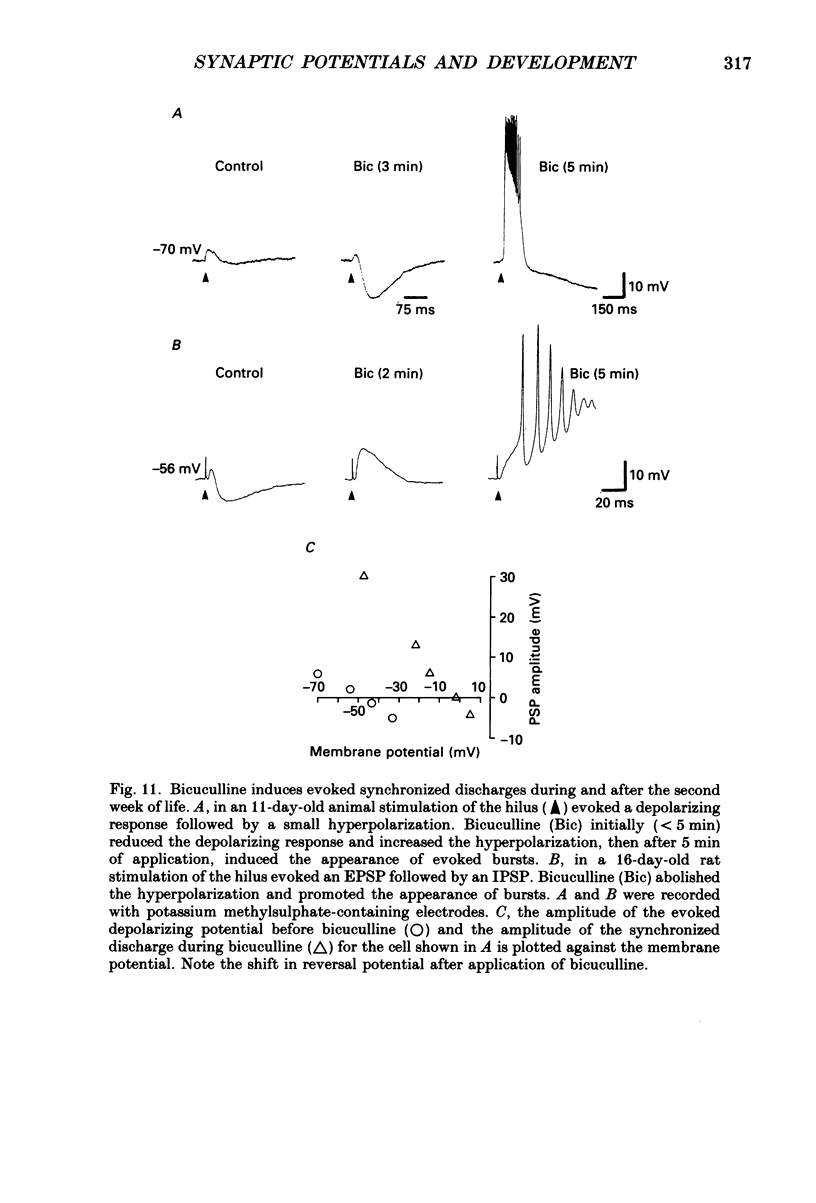
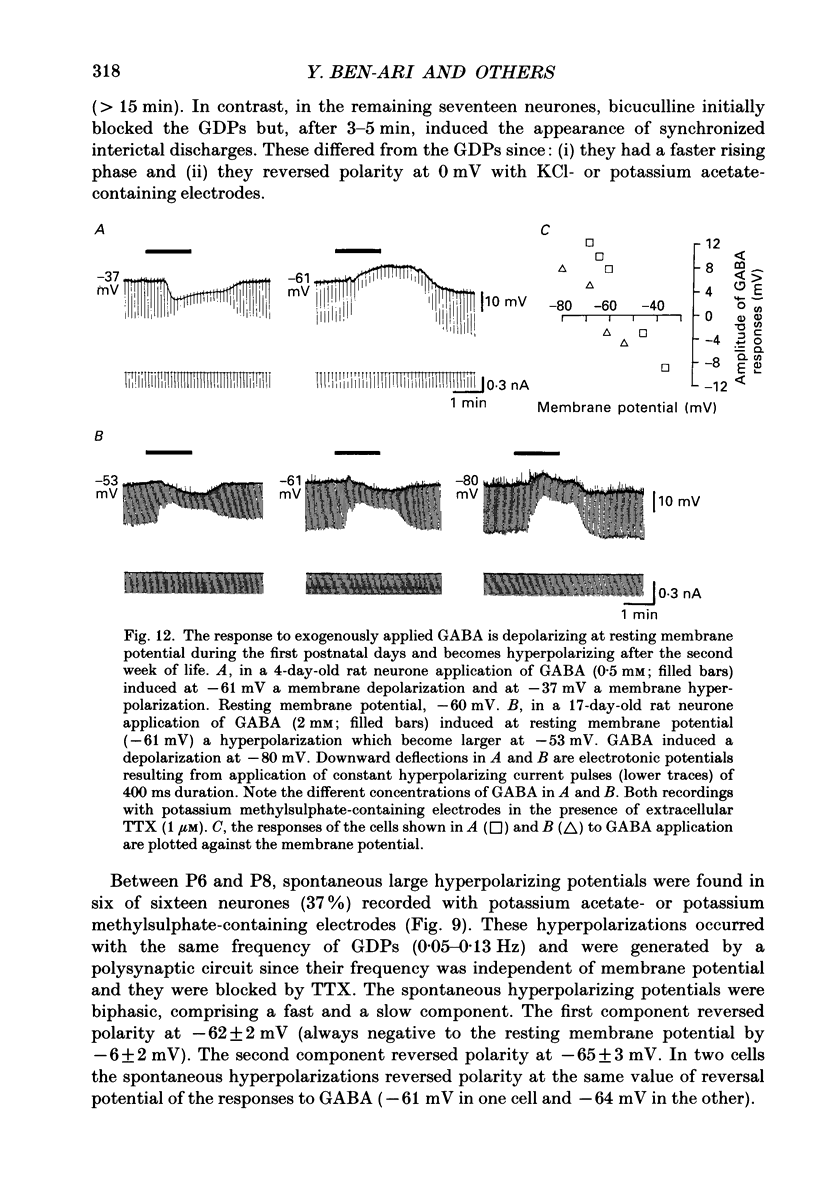
1. Intracellular recordings were made from rat CA3 hippocampal neurones in vitro during the first eighteen days of postnatal life. The cells had resting membrane potentials more negative than -51 mV, action potentials greater than 55 mV and membrane input resistances of 117 +/- 12 M omega. An unusual characteristic of these cells was the presence of spontaneous giant depolarizing potentials (GDPs) which were observed during the first eight postnatal (P) days in over 85% of neurones. They were less frequent between P9 and P12 (48%) and disappeared after P12. 2. The GDPs were synchronously generated by a population of neurones; they reversed polarity at -27 mV when recorded with KCl-containing electrodes and at -51 mV with potassium acetate- or potassium methylsulphate-filled electrodes. 3. The GDPs were blocked by bath application of bicuculline (10 microM) or picrotoxin (100-200 microM). Exogenously applied gamma-aminobutyric acid (GABA; 0.2-1 mM) induced at resting membrane potential a bicuculline-sensitive membrane depolarization which reversed polarity at -25 and -51 mV when recorded with KCl- or potassium methylsulphate-filled electrodes respectively. 4. The GDPs were reduced in frequency or blocked by the N-methyl-D-aspartate (NMDA) receptor antagonists DL-2-amino-7-phosphonoheptanoate (AP-7; 50 microM), D(-)2-amino-5-phosphonovalerate (AP-5, 10-50 microM) and (+-)3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10-50 microM) or NMDA channel blockers phencyclidine (2 microM) and ketamine (20 microM). 5. Stimulation of the hilus during the first week of life evoked a GDP followed by a hyperpolarization. The GDPs were generated by a population of synchronized neurones and reversed polarity at -27 mV with KCl-filled electrodes and at -52 mV with potassium acetate- or potassium methylsulphate-containing electrodes. 6. Bath application of bicuculline (1-10 microM) or picrotoxin (100-200 microM) reversibly blocked the evoked GDPs in the majority of cells. The NMDA receptor antagonists AP-5 (50 microM), AP-7 (50 microM) and CPP (30 microM) usually reduced the amplitude and the duration of the evoked GDPs. In neurones in which evoked GDPs were blocked by bicuculline, a NMDA-mediated component was revealed by increasing the strength or the frequency of stimulation. 7. During the second week of postnatal life, when spontaneous GDPs were extremely rare or absent, superfusion with bicuculline (10 microM) induced, as in adult slices, interictal discharges. These reversed polarity near 0 mV with KCl- or potassium acetate-containing electrodes and were reduced in amplitude and duration by AP-5 (50 microM).(ABSTRACT TRUNCATED AT 400 WORDS)
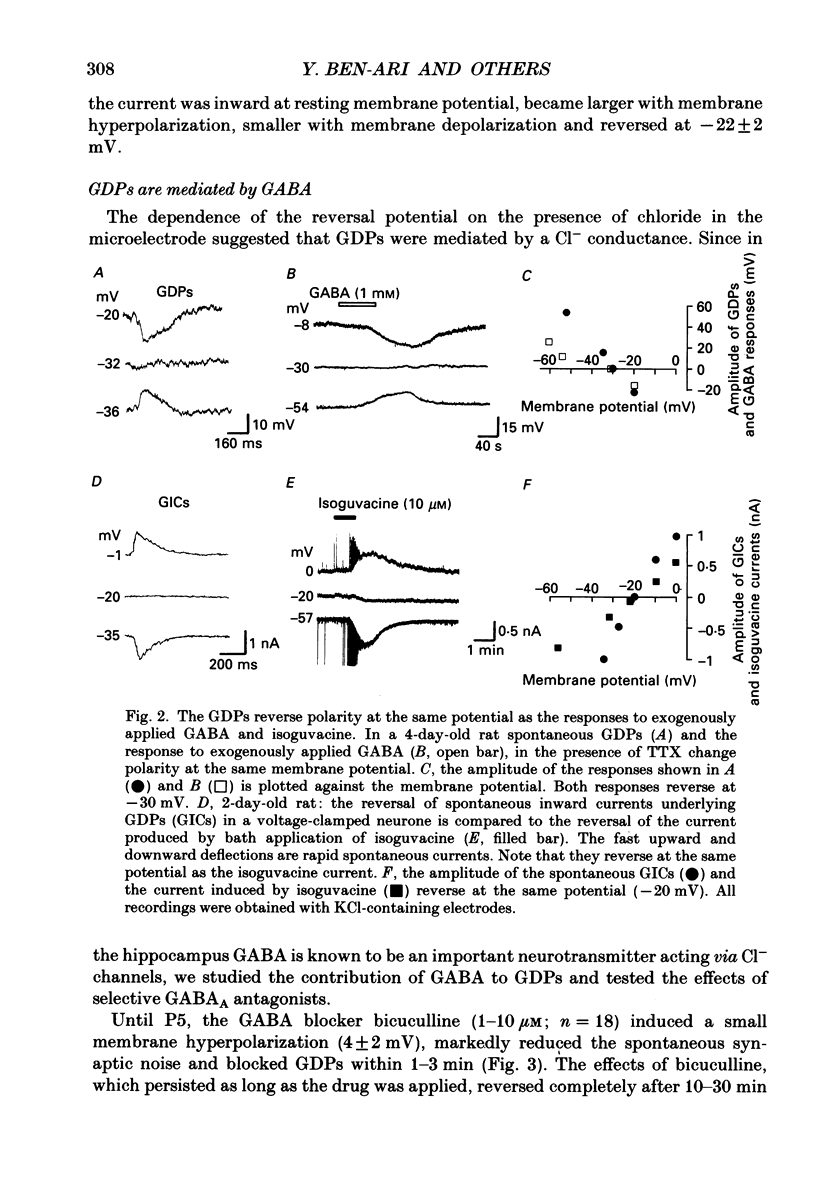
Full text
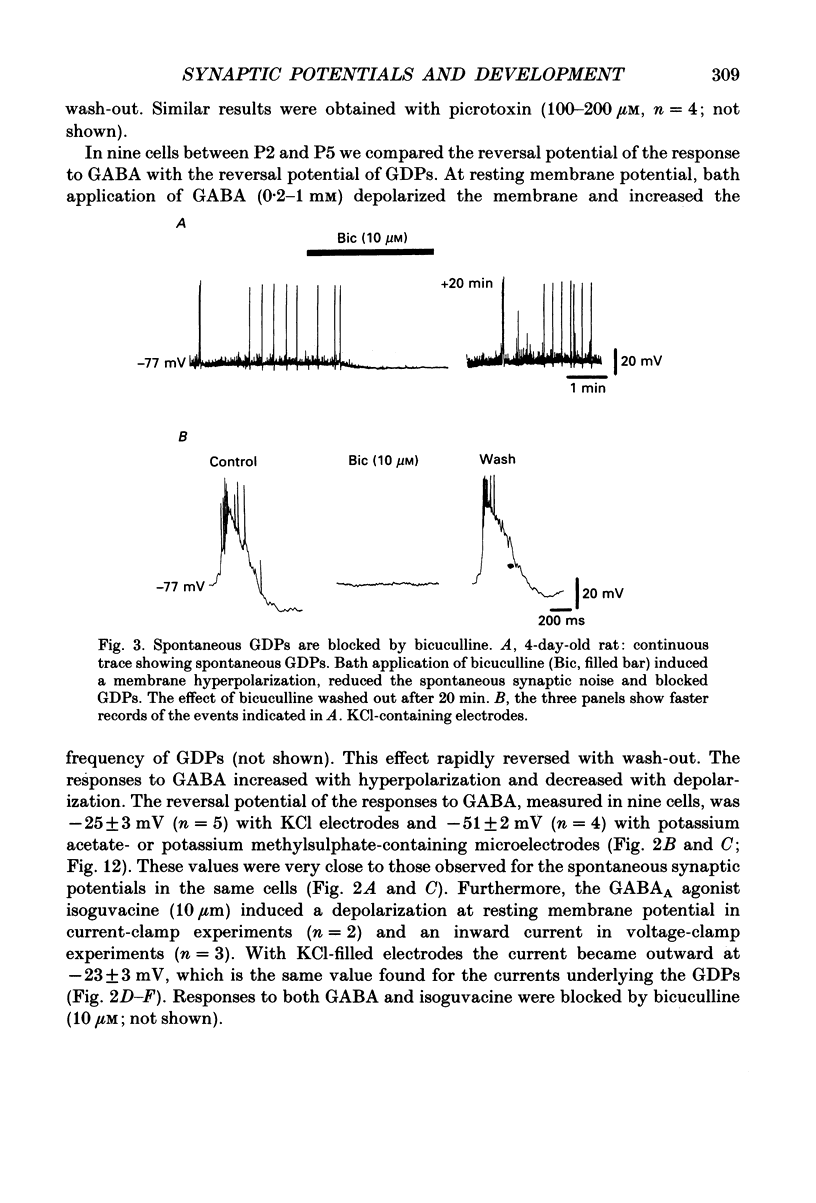
PDF



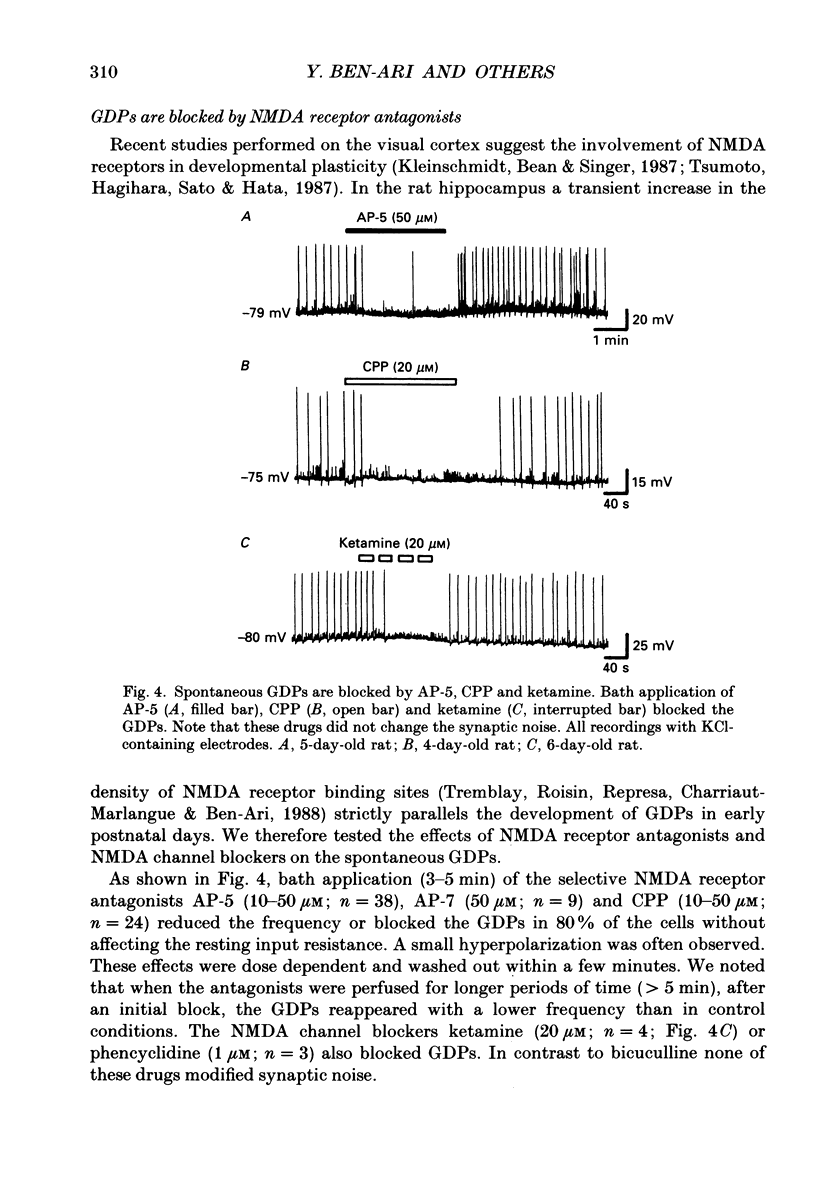






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicardi J., Chevrie J. J. Convulsive status epilepticus in infants and children. A study of 239 cases. Epilepsia. 1970 Jun;11(2):187–197. doi: 10.1111/j.1528-1157.1970.tb03880.x. [DOI] [PubMed] [Google Scholar]
- Alger B. E. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984 Nov;52(5):892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. G., Dent J. A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981 Jan 1;195(1):51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Krnjevic K. Changes in voltage dependence of NMDA currents during development. Neurosci Lett. 1988 Nov 22;94(1-2):88–92. doi: 10.1016/0304-3940(88)90275-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Gho M. Long-lasting modification of the synaptic properties of rat CA3 hippocampal neurones induced by kainic acid. J Physiol. 1988 Oct;404:365–384. doi: 10.1113/jphysiol.1988.sp017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Krnjević K., Reiffenstein R. J., Reinhardt W. Inhibitory conductance changes and action of gamma-aminobutyrate in rat hippocampus. Neuroscience. 1981;6(12):2445–2463. doi: 10.1016/0306-4522(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Blue M. E., Parnavelas J. G. The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol. 1983 Aug;12(4):697–712. doi: 10.1007/BF01181531. [DOI] [PubMed] [Google Scholar]
- Bähr S., Wolff J. R. Postnatal development of axosomatic synapses in the rat visual cortex: morphogenesis and quantitative evaluation. J Comp Neurol. 1985 Mar 15;233(3):405–420. doi: 10.1002/cne.902330309. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Neuman R., Rovira C., Ben Ari Y. Epileptogenic properties of the mast cell degranulating peptide in CA3 hippocampal neurones. Brain Res. 1988 Mar 29;445(1):91–100. doi: 10.1016/0006-8993(88)91077-3. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985 Jan 7;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti R., Gaïarsa J. L., Ben-Ari Y. D-aminophosphonovaleric acid-sensitive spontaneous giant EPSPs in immature rat hippocampal neurones. Eur J Pharmacol. 1988 Sep 13;154(2):221–222. doi: 10.1016/0014-2999(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Flatman J. A., Ganong A. H., Perkins M. N. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J Physiol. 1986 Sep;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer J., Honoré T. New quinoxalinediones show potent antagonism of quisqualate responses in cultured mouse cortical neurons. Neurosci Lett. 1988 Apr 22;87(1-2):104–108. doi: 10.1016/0304-3940(88)90153-x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V. Age-related differences in the in vitro rat hippocampus. Development of inhibition and the effects of hypoxia. Dev Neurosci. 1981;4(3):165–175. doi: 10.1159/000112753. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer F. B. The organization and development of the hippocampal mossy fiber system. Brain Res. 1986 Dec;396(4):335–357. doi: 10.1016/0165-0173(86)90004-4. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R., Lockerbie R. O., Pearce B. R. Uptake and release of [3H]GABA by growth cones isolated from neonatal rat brain. Neurosci Lett. 1984 Nov 23;52(1-2):205–210. doi: 10.1016/0304-3940(84)90375-6. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Johnston D. Endogenous nature of spontaneous bursting in hippocampal pyramidal neurons. Cell Mol Neurobiol. 1981 Dec;1(4):325–334. doi: 10.1007/BF00716267. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Teyler T. J. Evidence for late development of inhibition in area CA1 of the rat hippocampus. Brain Res. 1983 Jun 6;268(2):339–343. doi: 10.1016/0006-8993(83)90500-0. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Ruwe W. D., Veale W. L. Release of gamma-aminobutyric acid from the visual cortex of young kittens. Brain Res. 1986 Jan;389(1-2):299–304. doi: 10.1016/0165-3806(86)90200-2. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kehl S. J., McLennan H. Evidence for a bicuculline-insensitive long-lasting inhibition in the CA3 region of the rat hippocampal slice. Brain Res. 1983 Nov 21;279(1-2):278–281. doi: 10.1016/0006-8993(83)90192-0. [DOI] [PubMed] [Google Scholar]
- King A. E., Cherubini E., Ben-Ari Y. N-methyl-D-aspartate induces recurrent synchronized burst activity in immature hippocampal CA3 neurones in vitro. Brain Res Dev Brain Res. 1989 Mar 1;46(1):1–8. doi: 10.1016/0165-3806(89)90138-7. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Suppes T., Prince D. A. Cellular and synaptic physiology and epileptogenesis of developing rat neocortical neurons in vitro. Brain Res. 1987 Aug;431(2):161–171. doi: 10.1016/0165-3806(87)90206-9. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in developing axonal processes and presynaptic terminals of rodent cerebellum. Brain Res. 1975 Mar 7;85(3):355–371. doi: 10.1016/0006-8993(75)90813-6. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Taube J. S., Schwartzkroin P. A. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J Neurosci. 1984 Mar;4(3):860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman R. S., Ben-Ari Y., Gho M., Cherubini E. Blockade of excitatory synaptic transmission by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the hippocampus in vitro. Neurosci Lett. 1988 Sep 23;92(1):64–68. doi: 10.1016/0304-3940(88)90743-4. [DOI] [PubMed] [Google Scholar]
- Neuman R. S., Cherubini E., Ben-Ari Y. Endogenous and network bursts induced by N-methyl-D-aspartate and magnesium free medium in the CA3 region of the hippocampal slice. Neuroscience. 1989;28(2):393–399. doi: 10.1016/0306-4522(89)90186-3. [DOI] [PubMed] [Google Scholar]
- Neuman R., Cherubini E., Ben-Ari Y. Epileptiform bursts elicited in CA3 hippocampal neurons by a variety of convulsants are not blocked by N-methyl-D-aspartate antagonists. Brain Res. 1988 Sep 6;459(2):265–274. doi: 10.1016/0006-8993(88)90642-7. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Hagihara K., Sato H., Hata Y. NMDA receptors in the visual cortex of young kittens are more effective than those of adult cats. Nature. 1987 Jun 11;327(6122):513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. J Neurophysiol. 1983 Feb;49(2):442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Sawada S., Takada S. Suppressing action of 2-amino-4-phosphonobutyric acid on mossy fiber-induced excitation in the guinea pig hippocampus. Exp Brain Res. 1983;51(1):128–134. doi: 10.1007/BF00236810. [DOI] [PubMed] [Google Scholar]



