Abstract
Background
Endodontic sealers are expected to provide a favorable adhesion for a successful seal. This study aimed to evaluate the effect of ethylenediaminetetraacetic acid (EDTA) and Hydroxyethylidene Diphosphonic acid (HEDP) on bond strength of AH Plus and EndoSequence BC HiFlow sealers to root canal walls.
Methods
This study utilized 144 mandibular human premolars. Root canals were shaped using ProTaper Next system. Teeth were divided into 4 groups of 36 according to final irrigation: Group 1: NaOCl, EDTA; Group 2: NaOCl, HEDP (mixed with distilled water); Group 3: HEDP (mixed with NaOCl); Group 4: NaOCl only. Teeth were then divided into 2 subgroups (n = 18) and filled by AH Plus or BC Sealer HiFlow using warm vertical compaction. Teeth were kept at 37 °C with 100% humidity for 7 days. Using the IsoMet device, horizontal sections with a thickness of approximately 1 mm were taken from each sample from the apical, middle and coronal third of the root. Bond strength was tested using an Instron universal testing device at a constant speed of 1 mm/min until failure. Statistical analysis was performed using Shapiro-Wilk normality test, one-way analysis of variance and Three-Way ANOVA for intergroup comparisons.
Results
Statistically significant differences were observed between all groups (p = 0,0001). Push-out averages of NaOCl, EDTA group were significantly higher than NaOCl, HEDP; HEDP (NaOCl) and NaOCl groups (p = 0,0001). Push-out averages of NaOCl group were significantly lower than NaOCl, HEDP and HEDP (NaOCl) groups (p = 0,0001). No significant difference was observed between AH Plus and BC Sealer HiFlow (p = 0,883). Push out averages of the apical region were significantly higher than others and those of middle region were significantly higher than coronal (p = 0,0001). Cohesive failure was prevalent in NaOCl, EDTA, NaOCl, HEDP and HEDP (NaOCl) groups and mixed failure was prevalent in NaOCl group.
Conclusions
Irrigation using chelating agents such as EDTA and HEDP mixed with NaOCl gave higher adhesion compared to teeth where only NaOCl was used. EDTA and HEDP mixed with NaOCl can be advocated both for resin based and calcium silicate based sealers.
Keywords: Bond strength, NaOCl, EDTA, HEDP, EndoSequence BC Sealer HiFlow, AH Plus
Introduction
During root canal preparation, a debris layer, resembling sludge composed of mineralized and organic tissues, is formed on dentin walls due to the use of hand instruments or rotary instruments. This layer is referred to as the smear layer [1]. There are varying opinions in the literature regarding whether to keep or remove the smear layer during canal treatment. Some researchers argue that the smear layer acts as a defensive barrier, preventing penetration of bacteria and toxins into dentin tubules [2–4]. On the other hand, some suggest that the smear layer creates a breeding ground for microorganisms, facilitates their passage into dentin tubules, and reduces the effectiveness of irrigation solutions. The prevailing contemporary view leans towards the removal of the smear layer [5].
Irrigation of the root canal system with antibacterial and tissue-dissolving solutions, is essential in endodontics [6]. Sodium hypochlorite is a broad-spectrum antibacterial solution with the ability to dissolve vital and necrotic tissues [7]. Although effective in surface debris removal, it cannot eliminate the smear layer [8]. Therefore, chelating agents are recommended for use in conjunction with sodium hypochlorite to remove the smear layer [9]. EDTA, a colorless, water-soluble chelating agent, is recommended for demineralizing the smear layer [10]. It forms a chelate by binding with calcium ions in dentin, assisting in the removal of inorganic tissue. EDTA is typically used at a neutral pH and a concentration of 17% [11]. It is often recommended to be used in conjunction with sodium hypochlorite to remove organic residues [12, 13]. Etidronic acid, also known as Etidronate, is an alternative chelating agent in endodontics. It is considered suitable due to its weak and non-toxic nature [14]. The combination of sodium hypochlorite and HEDP in continuous chelation during root canal irrigation has clinical advantages, since it prevents and reduces smear layer formation and reducing stresses during instrumentation [15–17].
Favorable adhesion of filling materials is an essential factor for the attainment of success in endodontic treatment. Solid root canal filling materials and points are used for this purpose in combination with a sealer [18]. AH Plus, an epoxy resin based sealer and Endosequence BC, a calcium silicate based sealer are two types of sealers used for the filling of root canals. EndoSequence BC Sealer exhibited significantly shorter setting time and lower flowability at 140 °C compared to room temperature. This raised questions about the appropriateness of BC Sealer for warm vertical root canal filling techniques. Recently, a new calcium silicate-based sealer called EndoSequence BC Sealer HiFlow (BC Sealer HiFlow, Brasseler USA) has been produced with a focus on warm filling techniques [19]. According to the manufacturer, when heated, BC Sealer HiFlow exhibits lower viscosity compared to BC Sealer, and it is stated to be more radiopaque, making it more appropriate for the warm vertical root canal obturation method [20]. Various techniques have been proposed for filling of root canals, one of which is warm vertical compaction. The warm vertical compaction method, suggested by Schilder in 1967, allows for three-dimensional, dense, and dimensionally stable root canal fillings, accommodating accessory canals [21].
Adhesion, defined as the union and bonding of two surfaces, is crucial both statically and dynamically in endodontics. Static adhesion involves eliminating spaces that allow liquid passage between the root canal filling and canal wall, while dynamic adhesion ensures resistance to dislodgment of the root canal filling material [22, 23]. Shear bond, tensile bond, and push-out tests can be used to assess the adhesion of root canal sealers to dentin. The push-out test also evaluates the type of fracture after testing, categorized as adhesive, cohesive, or mixed. After carrying out a push-out test, specimens usually are examined under magnification using a stereomicroscope to evaluate fracture types. The literature review reveals that no study has yet evaluated bond strength of AH Plus and BC Sealer HiFlow using warm vertical compaction. At the same time, no study has examined the bond strength of these sealers when used with different irrigation procedures. This study was conducted to fill this gap in the literature.
The aim of this study was to evaluate the effect of EDTA and HEDP solutions used during irrigation of root canals on the bond strength of epoxy resin based AH Plus sealer and calcium silicate based EndoSequence BC Sealer HiFlow by using a push-out test. The null hypotheses of the study were that there is no difference in dentin bond strength regarding different final irrigation sequences and there is no difference in dentin bond strength regarding the two sealers used, AH plus and EndoSequence BC Sealer HiFlow.
Materials and methods
The study was approved by the Yeditepe University’s Clinical Research Ethics Committee with the reference number 20,231,791. The manuscript of this laboratory study has been written according to Preferred Reporting Items for Laboratory studies In Endodontology (PRILE) 2021 guidelines [24]. In this study, the total sample size determined using the G-POWER program was 144.
Preparation of specimens
The research was conducted on 144 extracted single-rooted lower premolar human teeth. Teeth having similar lengths, complete apical development, and no root resorption and defects, and extracted for periodontal or orthodontic reasons were used. Prior to the extractions, informed consent was received from the patients. The teeth were preserved at room temperature in distilled water containing 0.1% thymol crystal until the time of use.
Inspection and distribution of specimens
During the selection and preparation of the samples, teeth were examined under an operating microscope (X10 magnification) for residual tissue/defects or fractures/cracks. Calculus and soft tissues on the teeth included in the study were removed using a periodontal curette (#3–4 Gracey, Nordent, USA). Teeth with fractures and cracks were not included in the study. Radiographs were taken at bucco-lingual and mesio-distal projection to verify that the teeth had single canals. To ensure root length standardization, teeth were cut with a diamond fissure bur (ISO 806314, 014, Meisinger, Germany) under water cooling at 16 mm from the apex. Roots with an oval canal shape when viewed coronally were excluded. Only teeth having round cross-sections were included. A no. 10 K-type file (Dentsply Maillefer, Ballaigues, Switzerland) was used to check for any obstruction in the apical root regions; roots with an obstructed apical region were excluded. The roots of sample teeth were instrumented using an ISO 10 K-file (Mani, Japan) so that the tip of the file protruded from the apical foramen. This ensured that all specimens met the inclusion criteria and were suitable for further procedures.
Randomized assignment
To ensure a balanced distribution of the specimens into the test groups, the software of randomizer.org was used to randomly allocate the teeth.
Grouping of specimens
Sample teeth were divided into 4 main groups, each with 36 teeth, according to the final irrigation protocol to be applied. 36 teeth in each main group were randomly divided into 2 subgroups of n = 18, according to the sealers to be used (AH Plus or Endosequence BC Sealer HiFlow).
Root canal preparation
A #10 K-file tip was advanced beyond the apical foramen, and the working length was determined to be 15 mm, 1 mm shorter than the obtained length. The root canals were shaped with the Protaper Next rotary file system up to X4. Irrigation procedures were performed using a 30-gauge needle, which was 2 millimeters shorter than the working length. After the completion of irrigation procedures specific to each group, sodium hypochlorite for final irrigation was activated with Endoactivator for 60 s, and the irrigation phase was completed. All teeth were dried with sterile paper points and prepared for canal filling procedures. The following irrigation protocols were followed in each group:
Group 1
Between files, 2 ml. of 5.25% sodium hypochlorite were used to irrigate the root canals. After shaping, all canals were treated with 5 ml. of sodium hypochlorite solution. Subsequently, 5 ml. of 17% EDTA were used for irrigation for 1 min. Between the solutions, 2 ml. of distilled water were used, and after irrigating with 5 ml. of sodium hypochlorite solution, activation was performed for 60 s.
Preparation of HEDP mixtures for groups 2 and 3
The capsule containing 0.9 mg etidronic acid in HEDP was prepared for use in Group 2 by mixing the powder with 10 milliliters of distilled water, and in Group 3, it was mixed with 10 ml of 5.25% sodium hypochlorite. Mixing was performed in a sterile container for an average of 1 min, and the content of the capsule was stirred until it completely dissolved in the solution.
Group 2
Between files, 2 ml. of 5.25% NaOCl were used to irrigate the root canals. Final irrigation was performed with 5 ml. of sodium hypochlorite, followed by 5 ml of HEDP and distilled water mixture. Between the two solutions, 2 ml of distilled water were used, and then irrigation was performed with 5 ml of sodium hypochlorite solution, followed by activation for 60 s.
Group 3
Between files, 2 ml of HEDP + NaOCl were used to irrigate the root canals. After shaping, for final irrigation, 5 ml of HEDP + NaOCl were applied to the root canals, followed by activation for 60 s.
Group 4
Between files, 2 ml of 5.25% NaOCl were used to irrigate the root canals. After shaping, all canals were treated with 5 ml of NaOCl solution, and activation was performed for 60 s.
The root canals of the sample teeth were dried, and root canal fillings were completed using the warm vertical compaction method. Teeth were kept in an incubator at 37 °C with 100% humidity and in the dark for 7 days to ensure complete setting of the root canal filling paste. After 1 week, 3 horizontal sections of 1 mm were obtained from apical, middle and coronal. Sectioning was performed using an IsoMet device (IsoMet5000, Buehler, IL, USA) under water cooling with 0.3 mm thick diamond discs rotating at a low speed of 300–350 rpm. Thickness of sections was measured with a digital caliper. Sections were taken at 3, 7 and 11 mm from the apex.
Measurement of bond strength
The bond strengths of the prepared samples were tested using the Instron device. With the prepared setup, force was applied at a constant speed of 1 mm per minute until failure occurred in the bond between the root canal filling and dentin. Three different sizes of tips (0.3, 0.6 and 0.8 mm) were used to match the root canal filling in size. The Newton value given by the device at the moment of failure was recorded, and then the values were divided by the contact area of the sections to the root canal wall to obtain the bond strength in megapascals. The following formula was used to calculate the push-out bond strength value in megapascals (MPa):
Push-out bond strength (MPa) = Maximum load (N) / Root canal filling connection area (mm²).
After the tests, the debonded samples were examined under a stereomicroscope (Leica MZ6, Leica; Germany) at x10 magnification to evaluate the type of failure.
Results
In this study, statistical analyses were performed with NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA) package program. In addition to descriptive statistical methods (mean, standard deviation), the distribution of variables was examined with the Shapiro-Wilk normality test, one-way analysis of variance and Three-Way ANOVA were used in intergroup comparisons of normally distributed variables, Tukey multiple comparison test was used in subgroup comparisons, independent t test was used in comparisons of paired groups, and chi-square test was used in comparisons of qualitative data. The results were evaluated at a significance level of p < 0.05.
Table 1 presents the force at which the bond between the filling and dentin failed. There was a statistically significant difference observed in the push-out averages between Solution * Sealer groups (p = 0.0001). No statistically significant difference was observed in the push-out averages between Solution * Region groups (p = 0.862). A statistically significant difference was observed in the push-out averages between Sealer * Region groups (p = 0.045). There was no statistically significant difference observed in the push-out averages among Solution * Sealer * Region groups (p = 0.221).
Table 1.
The force in MPa at which the bond between the filling and dentin failed
| Solution | Sealer | Coronal | Middle | Apical | Mean | p |
|---|---|---|---|---|---|---|
|
NaOCl- EDTA (Group 1) |
AH Plus | 1.16 ± 0.24 | 1.46 ± 0.38 | 1.76 ± 0.37 | 1.46 ± 0.41 | 0.001 |
| HiFlow | 1.08 ± 0.23 | 1.37 ± 0.27 | 1.34 ± 0.36 | 1.26 ± 0.32 | ||
| Mean | 1.12 ± 0.24 | 1.42 ± 0.33 | 1.55 ± 0.42 | 1.36 ± 0.38 | ||
|
NaOCl- HEDP (Group 2) |
AH Plus | 0.88 ± 0.29 | 1.27 ± 0.53 | 1.41 ± 0.26 | 1.18 ± 0.43 | 0.685 |
| HiFlow | 0.84 ± 0.24 | 1.27 ± 0.36 | 1.34 ± 0.46 | 1.15 ± 0.42 | ||
| Mean | 0.86 ± 0.26 | 1.27 ± 0.45 | 1.37 ± 0.37 | 1.17 ± 0.43 | ||
|
HEDP (NaOCl) (Group 3) |
AH Plus | 0.75 ± 0.17 | 1.08 ± 0.37 | 1.20 ± 0.37 | 1.01 ± 0.37 | 0.001 |
| HiFlow | 1.08 ± 0.26 | 1.29 ± 0.47 | 1.54 ± 0.55 | 1.30 ± 0.48 | ||
| Mean | 0.91 ± 0.27 | 1.19 ± 0.43 | 1.37 ± 0.49 | 1.16 ± 0.45 | ||
|
NaOCl (Group 4) |
AH Plus | 0.66 ± 0.20 | 1.05 ± 0.24 | 1.27 ± 0.29 | 0.99 ± 0.35 | 0.256 |
| HiFlow | 0.77 ± 0.15 | 1.02 ± 0.21 | 0.96 ± 0.24 | 0.91 ± 0.23 | ||
| Mean | 0.71 ± 0.18 | 1.03 ± 0.22 | 1.12 ± 0.31 | 0.95 ± 0.30 |
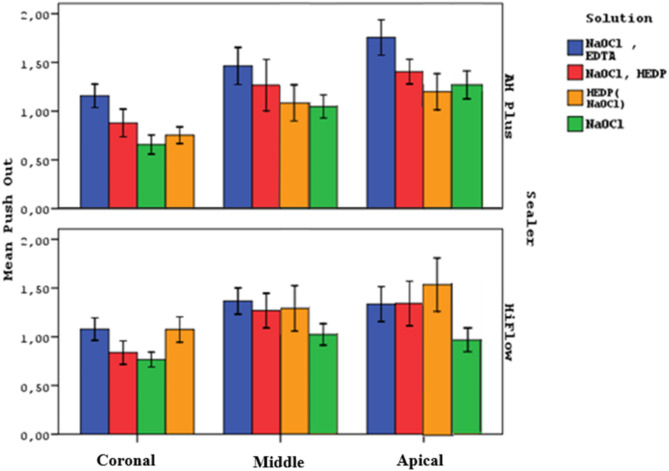
Figure 1 shows the push-out values in the coronal, middle and apical regions of all groups.
Fig. 1.
The push-out values in the coronal, middle and apical regions of all groups
There was a statistically significant difference observed in the push-out averages among the Solution Groups (p = 0.0001). The push-out averages of Group 1 were found to be significantly higher than those of the other groups (p = 0.0001). The push-out averages of Group 4 were significantly lower than those of Group 2 and Group 3 (p = 0.0001). No statistically significant difference was observed between Group 2 and Group 3 (p = 0.997). These differences suggested that the null hypothesis in terms of the effect of irrigation solutions on bond strength had to be rejected.
Statistical analysis of push-out averages across coronal, middle, and apical regions revealed significant differences (p = 0.0001). The apical region exhibited significantly higher push-out values than the coronal and middle regions (p = 0.0001). The middle region also showed significantly higher push-out values than the coronal region (p = 0.0001).
If the relationship between solutions and sealers is examined without dividing them into regions, in Group 1, the push-out averages of AH Plus subgroups were significantly higher than those of the HiFlow subgroups (p = 0.001). In Group 2, without regional division, the averages of AH Plus subgroups were higher than HiFlow subgroups, but there was no statistically significant difference (p = 0.685). In Group 3, without regional division, the averages of AH Plus subgroups were significantly lower than those of HiFlow subgroups (p = 0.001). In Group 4, without regional division, the averages of AH Plus subgroups were higher than HiFlow subgroups, but there was no statistically significant difference (p = 0.256).
There was no statistically significant difference observed in the push out averages between AH Plus and HiFlow groups, when all samples were included in the test, regardless of regions and irrigation regimens (p = 0.883).
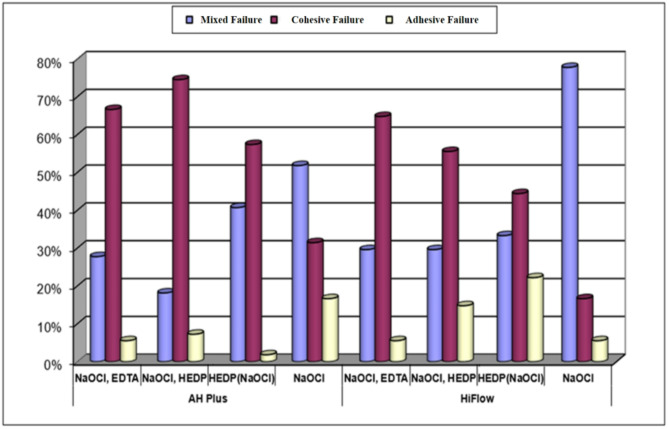
Figure 2 shows the types of failure associated with each group after observation under a stereomicroscope. In AH Plus and HiFlow subgroups; there was a statistically significant difference observed in the distribution of failure types. Cohesive failure was predominant in the 1st, 2nd, and 3rd groups, where a chelating agent (EDTA or HEDP) was utilized. On the other hand, mixed failure was more frequently observed in Group 4 where only sodium hypochlorite was used.
Fig. 2.
Types of failure associated with each group after observation under stereomicroscope
Discussion
Studies investigating the clinical efficacy of irrigation solutions are mostly performed using extracted human teeth and artificial canals. To overcome the difficulty of achieving standardization, some studies have used acrylic blocks or tooth models that mimic human teeth. The use of these materials has been reported to facilitate standardization as it ensures that the size and structural characteristics are the same [25]. However, it has been reported that these synthetic materials cannot fully mimic clinical conditions because they do not contain the unique organic and inorganic structure of dentin, dentinal tubules and there may be differences in the attachment of microorganisms to the surface compared to the natural surface [26]. Therefore, we used extracted human teeth in our study.
Wu et al. (1993) stated that in order to minimize variability in research, teeth from the same group should be selected and their dimensions, canal diameters and canal anatomy should be similar [27]. For this reason, teeth from the same tooth group were used in the study. In order to ensure the standardization of the study, straight, unbeveled, single-rooted lower premolars were preferred to be used [28]. However, we think that the fact that the age and gender of the people to whom the teeth belonged and the stresses to which the teeth were exposed are unknown may constitute a limitation for our study. A study by Thaler et al. (2008) showed a possible correlation between tooth age and root dentin permeability, which may influence distribution and effectiveness of drugs used for root canal disinfection [29].
Today, nickel titanium (Ni-Ti) rotary file systems are widely preferred for root canal shaping. Studies show that compared to stainless steel files, these systems help to shape root canals in a centralized manner, reduce the risk of transportation in narrow and curved canals, shorten duration of the procedure and make the work easier for both patients and dentists [30–32]. For this reason, Protaper Next rotary instruments were used for shaping the teeth within this study. The choice of these instruments was influenced by the need to increase comparability with other studies. In this way, the final diameters of the shaped root canal and apical foramen were standardized, allowing the 6% X4 gutta-percha cone (with an apical diameter of #40), to be used comfortably with an apical fit.
Increased enlargement in the apical region may increase the efficiency of irrigation by providing greater access to the solutions used during irrigation of root canals [33–35]. In our study, root canals were enlarged to an apical size of 40/0.06 in order to be comparable with other similar studies and to ensure adequate contact of irrigation solutions with the canal walls.
Irrigation needles are classified by the “gauge” system, a measurement system used in dentistry. For effective irrigation, a needle with an appropriate apical expansion size in the canal must be selected [36]. Today, the most commonly used irrigation needle in clinical practice is the 30 G (0.30 mm) needle. In this study, the 30 G irrigation needle was adjusted to be 2 mm shorter than working length to achieve irrigation application standard defined by ISO.
EDTA is the most commonly used chelating agent to remove the inorganic part of the smear layer in clinical applications. Saito et al. examined removal of smear layer by applying 17% EDTA for 15 s., 30 s. and 1 min. in their study and stated that the application of EDTA for 1 min. removed more smear layer [37]. Therefore, 1-minute application was preferred in the present study.
In the SEM studies where Niu et al. evaluated the final irrigation protocol, they recommended the use of NaOCl for the removal of exposed organic matrix after the use of EDTA [38]. Similarly, Yamada et al. also suggested the use of NaOCl after EDTA for the removal of surface debris and smear layer [12]. For these reasons, NaOCl was used as the final irrigant in our study and this procedure was applied in all groups for standardization. It has been reported that sequential use of NaOCl and EDTA solutions decreases the pH of NaOCl, decreases its activity [39]. Therefore, in our study, 2 mL of distilled water was used between NaOCl and EDTA solutions in order to prevent interaction between the solutions.
Paulson et al. applied 2.5% NaOCl, 2.5% NaOCl/17% EDTA and 2.5% NaOCl/9% Dual Rinse HEDP irrigation protocols and filled the root canals with Biodentine and performed a push out test. They reported that the bond strength of the 2.5% NaOCl/9% Dual Rinse HEDP group was significantly higher [40]. Etidronic acid was used in this study because it has sufficient binding strength, does not react with NaOCI, can be used as a compound with NaOCI and is recommended as an alternative to EDTA and citric acid.
Ulusoy et al. (2017) reported no significant difference between different concentrations of paracetic acid (PAA) (0.5%, 0.1, 2%) and HEDP (9–18%) used at different concentrations in a study investigating effectiveness of smear layer removal from canal walls [41]. Since there was no difference between 9% and 18% concentrations of HEDP in terms of smear layer removal, 9% HEDP solution was preferred to be used in this study according to manufacturer’s instructions [42].
In addition to irrigation solutions, activation techniques have been used to increase the effectiveness of solutions in order to provide ideal irrigation from past to present [43], [44]. Endoactivator is a sonic device to activate irrigants [45], The sonic waves generated during irrigation activation with this device create an intense hydrodynamic effect in the canal, increasing the penetration of irrigation solution into dentinal tubules [46]. Considering this effect, Endoactivator was used in our study.
Ruddle reported that the application time of the Endoactivator should be 60 s to activate the intracanal solution used and remove existing debris from the canal [47]. In our study, Endoactivator was also applied for 60 s for activation of NaOCl used in final irrigation.
Qu et al. demonstrated that EndoSequence BC Sealer hardened in a significantly shorter period of time and showed lower fluidity at a temperature of 140 °C compared to room temperature. Therefore, the suitability of BC Sealer for warm vertical root canal filling techniques was questioned [19]. Viapiana et al. reported changes in the physical properties of calcium silicate based sealers due to high temperature [48].
Calcium silicate based sealers undergo significant structural changes with temperature during their application by thermoplastic obturation techniques. In calcium silicate sealers, the capacity to form apatite is a desirable property, but temperature increase can affect biomineralization process. Thus, it is important have knowledge on chemo-mechanical properties of calcium hydroxide based sealers and understand how they can be influenced by thermal application [49, 50]. Although thermoplastic methods offer high adaptability, they can adversely affect the physical properties of Calcium silicate based sealers. Therefore, there is a need to produce new calcium silicate based sealers for use with warm filling techniques. In this study, BC Sealer HiFlow, a newly developed Calcium silicate based sealer, was chosen to address this need.
In their study comparing the physical and chemical changes of BC sealer and BC Sealer HiFlow with temperature, Chen et al. reported that BC Sealer HiFlow, which is also intended for use in the warm vertical filling technique, has better flow and viscosity properties than BC sealer at elevated temperatures [51]. As a result of these studies; BC Sealer HiFlow in combination with warm obturation techniques may be a valuable option in root canal treatment [52]. Studies by Chen and Ruiqi Yang [51, 52] suggest the use of BC Sealer HiFlow with warm filling techniques. However, there are not adequate number of studies on the bond strength of this sealer for use with warm filling techniques. For this reason, our study aimed to contribute to clinical use by investigating the bond strength of BC Sealer HiFlow to dentin, which is indicated to be suitable for use with warm vertical compaction techniques.
Bayram et al. reported that removal of smear layer increased the adhesion of the root canal sealer to root canal dentin by penetrating dentinal tubules [53]. It has been reported that calcium silicate-based paste forms an interfacial layer called “mineral infiltration zone” in the root dentin wall. This chemical interaction in the interfacial dentin and micromechanical interaction with tag-like structures provide adhesion between the paste and dentin [54, 55]. In this study, it was found that in all groups where the smear layer was removed with chelating agents, the dentin bond strength of BC Sealer HiFlow, a calcium silicate-based root canal sealer, was higher than within the NaOCl (Group4) group where the smear was not removed. The push-out averages of the NaOCl (Group4) group were significantly lower than those of the NaOCl, HEDP (Group2) and HEDP (NaOCl) (Group3) groups. Our results can be considered to be consistent with the finding reported by Bayram et al. that “root canal paste increases adhesion by penetrating better into the opened dentinal tubules”.
Tuncel et al. (2015) examined the effect of 17% EDTA, 9% HEBP and 1% PAA solutions on iRoot SP and AH Plus paste in their study on the effect of chelating agents on dentin bond strength. They reported that chelating agents increased the bond strength for both sealers, but this difference was not statistically significant [56]. In this study, the binding strength of NaOCl, EDTA (Group 1); NaOCl, HEDP (Group 2) and HEDP (NaOCl) (Group 3) groups with chelating agents were found to be significantly higher than the binding strength of NaOCl (Group 4) without chelating agents. Both studies are in agreement that chelation agents increase bond strength. The fact that the binding strength was found to be significantly higher in our study compared to the results of Tuncel et al. may be due to the large number of samples used within the present study.
Patil et al. studied the efficacy of different solutions to remove smear layer from the apical third of the root canal. The groups consisted of Chloroquick (Innovationsendo, Nashik, India), a new commercial form of HEDP consisting of a mixture of 5% NaOCl and 18% HEDP, 5.25% NaOCl with surfactant added, and 17% EDTA, MTAD and saline solution with surfactant added. It was concluded that the combination of 5.25% NaOCl with surfactant and 17% EDTA with surfactant is superior to MTAD and Chloroquick solutions for smear layer removal [57].
Srivasta et al. (2020) reported that teeth that were irrigated using 17% EDTA + green tea extract solution showed higher bond strength than the control group in their study in which they evaluated the push out bond strength of calcium silicate based based BioRoot RCS (Septodont, Saint-Maur-des-Fosses, France) [58]. In this study, in agreement with the findings of Buldur et al. [59] and Srivasta et al., the bond strength of the BC Sealer HiFlow group irrigated with EDTA exhibited the highest values together with the BC Sealer / HEDP (mixed with NaOCl) solution group. According to our results, the use of calcium silicate based BC Sealer HiFlow following final irrigation with EDTA or HEDP (mixed with NaOCl) may be recommended in terms of bond strength.
The studies indicate that the best adhesion of AH Plus to dentin was observed in research where EDTA was used during root canal irrigation [60–62]. In our study, chelating agents were also used within the final irrigation sequence, and in the group where 17% EDTA was used as the chelating agent (Group 1), the adhesion of AH Plus to root dentin was significantly higher than within other groups. The results of this study are consistent with the findings of Neelakantan et al. [63].
In line with studies by Neelekantan et al. [63]., the higher bond strength of BC Sealer HiFlow in Group 3 in our study, as opposed to the higher bond strength of AH Plus in other groups, could be explained by the unique effects of BC Sealer HiFlow on calcium silicate based sealers. However, a clear explanation of the impact of these irrigants on different calcium silicate based sealers has not been made yet, as there is a lack of information about the actual chemical reactions of these substances. Further studies are needed to delve into this matter.
Irrigation solutions aside, no significant difference was observed between the push-out averages of the AH Plus Sealer and BC Sealer HiFlow groups (p = 0.883). In contrast to studies demonstrating better bonding strength of AH Plus root canal sealer compared to silicate-based sealers, the lack of a significant difference in this study may be attributed to various factors that could diminish the adhesive superiority of resin-based sealers [64]. AH Plus can bind to the organic content of dentin, but this effect does not occur during the final irrigation with NaOCl [65]. In our study, the removal of exposed collagen matrix during the final irrigation with NaOCl and the formation of an oxygen inhibition zone may have negatively affected the adhesion of the AH Plus sealer, leading to a decrease in bond strength. This could explain why the bond strength of the AH Plus sealer did not surpass that of BC Sealer HiFlow in our study. The reasons for using NaOCl as the final irrigation solution have been discussed earlier.
Mishra et al. in their studies stated that the highest bond strength was in the apical 1/3 region, while the coronal 1/3 showed the lowest bond strength values. The researchers reported that the better penetration of the sealer into dentinal tubules through the down-packing process led to better compaction of the obturation material in the apical 1/3 of the root canal [66]. In our study, when each group was evaluated individually, significantly higher megapascal values were obtained apically compared to coronally for all groups.
NaOCl, EDTA (Group 1a and 1b), NaOCl, HEDP (Group 2a and 2b), and HEDP (NaOCl) (Group 3a and 3b) groups showed a high presence of cohesive failure. According to the results of the study conducted by Donnermeyer et al. (2018), the majority of cohesive failures in samples subjected to push-out tests were attributed to the high level of adhesion of the root canal filling sealer to dentin [64].
Conclusion
Irrigation using chelating agents such as EDTA and HEDP mixed with gave higher adhesion values compared to teeth where only NaOCl was used as an irrigant. EDTA and HEDP mixed with NaOCl can be advocated bot for resin based and calcium silicate based sealers.
Acknowledgements
N/A.
Abbreviations
- EDTA
Ethylenediaminetetraacetic acid
- HEDP
Hydroxyethylidene diphosphonic acid
- NaOCl
Sodium hypochlorite
- n
Number
- °C
Degrees celcius
- ml
Milliliters
- rpm
Revolutions per minute
- mg
Milligrams
- %
Percent
- MPa
Megapascals
- NCSS
Number cruncher statistical system
- G
Gauge
- N
Newton
- X10
Times 10
- NiTi
Nickel titanium
- ISO
International standards organization
- PAA
Paracetic acid
Author contributions
GEE Perfomed the experimentation and participated in the writing of the text. RFK participated in the writing of the text. JT participated in the writing of the text. All authors reviewed the manuscript.
Funding
No funding has been received in this study and manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All experiments were performed in accordance with relevant guidelines and regulations. Extracted human teeth were used in the study. The Institutional Review Board of Yeditepe University whose ethical approval is printed below deemed it unnecessary to receive consent since all patients whose extracted teeth are used in the study have already given consent in the clinics prior to extraction procedure and all teeth were extracted due to medical reasons.
Consent for publication
The authors indicated in this manuscript give consent for the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang S-E, Bae K-S. Scanning electron microscopy study of the adhesion of Prevotella nigrescens to the dentin of prepared root canals. J Endod. 2002;28(6):433–7. 10.1097/00004770-200206000-00004 [DOI] [PubMed] [Google Scholar]
- 2.Drake DR, Wiemann AH, Rivera EM, Walton RE. Bacterial retention in canal walls in vitro: Effect of smear layer. J Endod. 1994;20(2):78–82. 10.1016/S0099-2399(06)81186-6 [DOI] [PubMed] [Google Scholar]
- 3.Galvan DA, Ciarlone AE, Pashley DH, Kulild JC, Primack PD, Simpson MD. Effect of smear layer removal on the diffusion permeability of human roots. J Endod. 1994;20(2):83–6. 10.1016/S0099-2399(06)81187-8 [DOI] [PubMed] [Google Scholar]
- 4.Pashley DH, Michelich V, Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent. 1981;46(5):531–7. 10.1016/0022-3913(81)90243-2 [DOI] [PubMed] [Google Scholar]
- 5.George S, Kishen A, Song KP. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005;31(12):867–72. 10.1097/01.don.0000164855.98346.fc [DOI] [PubMed] [Google Scholar]
- 6.Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–98. 10.1016/J.JOEN.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Tartari T, Wichnieski C, Bachmann L, et al. Effect of the combination of several irrigants on dentine surface properties, adsorption of chlorhexidine and adhesion of microorganisms to dentine. Int Endod J. 2018;51(12):1420–33. 10.1111/iej.12960 [DOI] [PubMed] [Google Scholar]
- 8.Candeiro GTDM, Correia FC, Duarte MAH, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38(6):842–5. 10.1016/J.JOEN.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 9.Wu D, Ma YZ, Jia J, et al. Removal of the root canal smear layer using Carisolv III and sodium hypochlorite. Med (Baltim). 2020;99(22):e20372. 10.1097/MD.0000000000020372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basrani B, Haapasalo M. Update on endodontic irrigating solutions. Endod Top. 2012;27(1):74–102. 10.1111/ETP.12031 [Google Scholar]
- 11.Çalt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28(1):17–9. 10.1097/00004770-200201000-00004 [DOI] [PubMed] [Google Scholar]
- 12.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: part 3. J Endod. 1983;9(4):137–42. 10.1016/S0099-2399(83)80032-6 [DOI] [PubMed] [Google Scholar]
- 13.Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions: a scanning electron microscopic study: part 2. J Endod. 1982;8(11):487–92. 10.1016/s0099-2399(82)80073-3 [DOI] [PubMed] [Google Scholar]
- 14.Tartari T, Guimarães BM, Amoras LS, Duarte MAH, Silva e Souza PAR, Bramante CM. Etidronate causes minimal changes in the ability of sodium hypochlorite to dissolve organic matter. Int Endod J. 2015;48(4):399–404. 10.1111/IEJ.12329 [DOI] [PubMed] [Google Scholar]
- 15.Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42(4):335–43. 10.1111/J.1365-2591.2008.01514.X [DOI] [PubMed] [Google Scholar]
- 16.Boessler C, Peters OA, Zehnder M. Impact of lubricant parameters on rotary instrument torque and force. J Endod. 2007;33(3):280–3. 10.1016/J.JOEN.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Ballal NV, Gandhi P, Shenoy PA, et al. Safety assessment of an etidronate in a sodium hypochlorite solution: randomized double-blind trial. Int Endod J. 2019;52(9):1274–82. 10.1111/IEJ.13129 [DOI] [PubMed] [Google Scholar]
- 18.Michaud RA, Burgess J, Barfield RD, Cakir D, McNeal SF, Eleazer PD. Volumetric expansion of gutta-percha in contact with eugenol. J Endod. 2008;34(12):1528–32. 10.1016/J.JOEN.2008.08.025 [DOI] [PubMed] [Google Scholar]
- 19.Qu W, Bai W, Liang YH, Gao XJ. Influence of warm Vertical Compaction technique on Physical properties of Root Canal Sealers. J Endod. 2016;42(12):1829–33. 10.1016/J.JOEN.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 20.EndoSequence®. BC Sealer™ Product Category| Endodontics.
- 21.Schilder H, Hargreaves KM. Filling root canals in three dimensions. 1967. J Endod. 2006;32(4):281–90. 10.1016/J.JOEN.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Ørstavık D, Erıksen HM, Beyer-Olsen EM. Adhesive properties and leakage of root canal sealers in vitro. Int Endod J. 1983;16(2):59–63. 10.1111/J.1365-2591.1983.TB01297.X [DOI] [PubMed] [Google Scholar]
- 23.Gopikrishna V, Venkateshbabu N, Krithikadatta J, Kandaswamy D. Evaluation of the effect of MTAD in comparison with EDTA when employed as the final rinse on the shear bond strength of three endodontic sealers to dentine. Aust Endod J. 2011;37(1):12–7. 10.1111/J.1747-4477.2010.00261.X [DOI] [PubMed] [Google Scholar]
- 24.Nagendrababu V, Peter|, Murray E et al. PRILE 2021 guidelines for reporting laboratory studies in endodontology: a consensus-based development. 2021. 10.1111/iej.13542 [DOI] [PubMed]
- 25.Swimberghe RCD, Coenye T, De Moor RJG, Meire MA. Biofilm model systems for root canal disinfection: a literature review. Int Endod J. 2019;52(5):604–28. 10.1111/IEJ.13050 [DOI] [PubMed] [Google Scholar]
- 26.Davis JM, Maki J, Bahcall JK. An in vitro comparison of the antimicrobial effects of various endodontic medicaments on Enterococcus faecalis. J Endod. 2007;33(5):567–9. 10.1016/j.joen.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 27.Wu M, journal PW-I endodontic. 1993 undefined. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. Wiley Online Libr Wu, PR WesselinkInternational Endod journal, 1993•Wiley Online Libr. 1993;26(1):37–43. 10.1111/j.1365-2591.1993.tb00540.x [DOI] [PubMed]
- 28.Sert S, Aslanalp V, Tanalp J. Investigation of the root canal configurations of mandibular permanent teeth in the Turkish population. Int Endod J. 2004;37(7):494–9. 10.1111/J.1365-2591.2004.00837.X [DOI] [PubMed] [Google Scholar]
- 29.Thaler A 1, Ebert J, Petschelt A, Pelka M. Int Endod J. 2008;41(12):1115–22. 10.1111/j.1365-2591.2008.01486.x [DOI] [PubMed] [Google Scholar]
- 30.Glosson CR, Haller RH, Brent Dove S, del Rio CE. A comparison of root canal preparations using Ni-Ti hand, Ni-Ti engine-driven, and K-Flex endodontic instruments. J Endod. 1995;21(3):146–51. 10.1016/S0099-2399(06)80441-3 [DOI] [PubMed] [Google Scholar]
- 31.Short JA, Morgan LA, Baumgartner JC. A comparison of canal centering ability of four instrumentation techniques. J Endod. 1997;23(8):503–7. 10.1016/S0099-2399(97)80310-X [DOI] [PubMed] [Google Scholar]
- 32.ElAyouti A, Chu AL, Kimionis I, Klein C, Weiger R, Löst C. Efficacy of rotary instruments with greater taper in preparing oval root canals. Int Endod J. 2008;41(12):1088–92. 10.1111/J.1365-2591.2008.01475.X [DOI] [PubMed] [Google Scholar]
- 33.Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod. 2004;30(2):110–2. 10.1097/00004770-200402000-00012 [DOI] [PubMed] [Google Scholar]
- 34.Kok D, Duarte MAH, Da Rosa RA, Wagner MH, Pereira JR, Só MVR. Evaluation of epoxy resin sealer after three root canal filling techniques by confocal laser scanning microscopy. Microsc Res Tech. 2012;75(9):1277–80. 10.1002/JEMT.22061 [DOI] [PubMed] [Google Scholar]
- 35.Akcay M, Arslan H, Durmus N, Mese M, Capar ID. Dentinal tubule penetration of AH plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: a confocal microscopic study. Lasers Surg Med. 2016;48(1):70–6. 10.1002/LSM.22446 [DOI] [PubMed] [Google Scholar]
- 36.Boutsioukis C, Lambrianidis T, Vasiliadis L. Clinical relevance of standardization of endodontic irrigation needle dimensions according to the ISO 9,626:1991 and 9,626:1991/Amd 1:2001 specification. Int Endod J. 2007;40(9):700–6. 10.1111/j.1365-2591.2007.01280.x [DOI] [PubMed] [Google Scholar]
- 37.Saito K, Webb TD, Imamura GM, Goodell GG. Effect of shortened irrigation times with 17% ethylene diamine tetra-acetic acid on smear layer removal after rotary canal instrumentation. J Endod. 2008;34(8):1011–4. 10.1016/J.JOEN.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 38.Niu W, Yoshioka T, Kobayashi C, Suda H. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J. 2002;35(11):934–9. 10.1046/J.1365-2591.2002.00594.X [DOI] [PubMed] [Google Scholar]
- 39.Rossi-Fedele G, Doramac EJ, Guastalli AR, Steier L, Poli De Figueiredo JA. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J Endod. 2012;38(4):426–31. 10.1016/J.JOEN.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 40.Paulson L, Ballal NV, Bhagat A. Effect of Root dentin conditioning on the Pushout Bond Strength of Biodentine. J Endod. 2018;44(7):1186–90. 10.1016/j.joen.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 41.Ulusoy Öİ, Zeyrek S, Çelik B. Evaluation of smear layer removal and marginal adaptation of root canal sealer after final irrigation using ethylenediaminetetraacetic, peracetic, and etidronic acids with different concentrations. Microsc Res Tech. 2017;80(7):687–92. 10.1002/JEMT.22851 [DOI] [PubMed] [Google Scholar]
- 42.Zehnder M. Etidronate (dual Rinse HEDP) for root canal irrigation in clinical use. Quintessenz Zahnmedizin.2019;70(8):896-905. https://qos.quintessenz.de/qd_2019_08_s896_qos_en.pdf
- 43.Rödig T, Bozkurt M, Konietschke F, Hülsmann M. Comparison of the Vibringe system with syringe and passive ultrasonic irrigation in removing debris from simulated root canal irregularities. J Endod. 2010;36(8):1410–3. 10.1016/J.JOEN.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 44.Tronstad L, Barnett F, Schwartzben L, Frasca P. Effectiveness and safety of a sonic vibratory endodontic instrument. Endod Dent Traumatol. 1985;1(2):69–76. 10.1111/J.1600-9657.1985.TB00564.X [DOI] [PubMed] [Google Scholar]
- 45.Gu Y, Perinpanayagam H, Kum DJW, et al. Effect of different agitation techniques on the penetration of Irrigant and Sealer into Dentinal Tubules. Photomed Laser Surg. 2017;35(2):71–7. 10.1089/PHO.2016.4125 [DOI] [PubMed] [Google Scholar]
- 46.Al-Obaida MI, Moukaddem R, Allahem Z, AbdulWahed AA, AlOnaizan FA, Al-Madi EM. Comparison of bacterial removal from dentinal tubules with different irrigant agitation techniques: an in vitro study. Saudi Dent J. 2019;31(4):431–6. 10.1016/J.SDENTJ.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruddle C. Endodontic disinfection: Tsunami irrigation. Saudi Endod J. 2015;5:1. 10.4103/1658-5984.149080 [Google Scholar]
- 48.Viapiana R, Guerreiro-Tanomaru J, Tanomaru-Filho M, Camilleri J. Interface of dentine to root canal sealers. J Dent. 2014;42(3):336–50. 10.1016/J.JDENT.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 49.Antunes TBM, Janini ACP, Pelepenko LE, et al. Heating stability, physical and chemical analysis of calcium silicate-based endodontic sealers. Int Endod J. 2021;54(7):1175–88. 10.1111/IEJ.13496 [DOI] [PubMed] [Google Scholar]
- 50.Camilleri J. Sealers and warm gutta-percha obturation techniques. J Endod. 2015;41(1):72–8. 10.1016/J.JOEN.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 51.Chen B, Haapasalo M, Mobuchon C, Li X, Ma J, Shen Y. Cytotoxicity and the Effect of temperature on physical properties and Chemical composition of a New Calcium Silicate-based Root Canal Sealer. J Endod. 2020;46(4):531–8. 10.1016/J.JOEN.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 52.Yang R, Tian J, Huang X, et al. A comparative study of dentinal tubule penetration and the retreatability of EndoSequence BC Sealer HiFlow, iRoot SP, and AH Plus with different obturation techniques. Clin Oral Investig. 2021;25(6):4163–73. 10.1007/S00784-020-03747-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayram HM, Bayram E, Kanber M, Celikten B, Saklar F. Effect of different chelating solutions on the push-out bond strength of various root canal sealers. Biomed Res. 0(0). https://www.alliedacademies.org/articles/effect-of-different-chelating-solutions-on-the-pushout-bond-strength-of-various-root-canal-sealers.html. Accessed December 23, 2023.
- 54.Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res. 2012;91(5):454–9. 10.1177/0022034512443068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaup M, Dammann CH, Schäfer E, Dammaschke T. Shear bond strength of Biodentine, ProRoot MTA, glass ionomer cement and composite resin on human dentine ex vivo. Head Face Med. 2015;11(1). 10.1186/S13005-015-0071-Z [DOI] [PMC free article] [PubMed]
- 56.Tuncel B, Nagas E, Cehreli Z, Uyanik O, Vallittu P, Lassila L. Effect of endodontic chelating solutions on the bond strength of endodontic sealers. Braz Oral Res. 2015;29(1):1–6. 10.1590/1807-3107BOR-2015.VOL29.0059 [DOI] [PubMed] [Google Scholar]
- 57.Patil PH, Gulve MN, Kolhe SJ, Samuel RM, Aher GB. Efficacy of new irrigating solution on smear layer removal in apical third of root canal: a scanning electron microscope study. J Conserv Dent. 2018;21(2):190–3. 10.4103/JCD.JCD_155_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava A, Yadav DS, Murali Rao H, Arun A, Siddique R. Evaluation of push-out bond strength of BioRoot RCS and AH Plus after using different irrigants: an in vitro study. J Conserv Dent. 2020;23(1):26–31. 10.4103/JCD.JCD_223_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buldur B, Oznurhan F, Kaptan A. The effect of different chelating agents on the push-out bond strength of proroot mta and endosequence root repair material. Eur oral Res. 2019;53(2):88–93. 10.26650/eor.20191618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sly MM, Moore BK, Platt JA, Brown CE. Push-out bond strength of a new endodontic obturation system (Resilon/Epiphany). J Endod. 2007;33(2):160–2. 10.1016/J.JOEN.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 61.Rahman Hashem AA, Ghoneim AG, Lutfy RA, Fouda MY. The effect of different irrigating solutions on bond strength of two root canal-filling systems. J Endod. 2009;35(4):537–40. 10.1016/J.JOEN.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 62.De-Deus G, Di Giorgi K, Fidel S, Fidel RAS, Paciornik S. Push-out bond strength of Resilon/Epiphany and Resilon/Epiphany self-etch to root dentin. J Endod. 2009;35(7):1048–50. 10.1016/J.JOEN.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 63.Neelakantan P, Varughese AA, Sharma S, Subbarao CV, Zehnder M, De-Deus G. Continuous chelation irrigation improves the adhesion of epoxy resin-based root canal sealer to root dentine. Int Endod J. 2012;45(12):1097–102. 10.1111/J.1365-2591.2012.02073.X [DOI] [PubMed] [Google Scholar]
- 64.Donnermeyer D, Dornseifer P, Schäfer E, Dammaschke T. The push-out bond strength of calcium silicate-based endodontic sealers. Head Face Med. 2018;14(1). 10.1186/S13005-018-0170-8 [DOI] [PMC free article] [PubMed]
- 65.Neelakantan P, Subbarao C, Subbarao CV, De-Deus G, Zehnder M. The impact of root dentine conditioning on sealing ability and push-out bond strength of an epoxy resin root canal sealer. Int Endod J. 2011;44(6):491–8. 10.1111/J.1365-2591.2010.01848.X [DOI] [PubMed] [Google Scholar]
- 66.Mishra P, Sharma A, Mishra S, Gupta M. Push-out bond strength of different endodontic obturation material at three different sites - In-vitro study. J Clin Exp Dent. 2017;9(6):e733–7. 10.4317/jced.53647 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.