Abstract
Background
This study aims to compare the outcomes of two-level anterior cervical discectomy and fusion (ACDF) procedures using stand-alone cages versus cage and plate fixation in patients diagnosed with cervical disc herniation (CDH).
Materials and methods
This retrospective analysis included 60 patients who underwent two-level ACDF procedures. Patients were divided into two groups: one treated with stand-alone cages and the other with cage and plate fixation. Data on surgical duration, blood loss, fusion stability, and complication rates were collected. Clinical outcomes, including neck pain and functional status, were assessed using standard scoring systems.
Results
Plate fixation provided superior fusion stability but was associated with longer surgery durations, higher intraoperative blood loss, and increased complication rates. Stand-alone cages reduced intraoperative trauma but demonstrated higher subsidence rates and prolonged fusion times. Both techniques resulted in significant improvements in neck pain and disability scores.
Discussion
While both approaches are effective for managing cervical disc herniation, each has distinct advantages and limitations. Surgical technique selection should be individualized, considering patient-specific anatomical factors, functional demands, and the risk-benefit profile of each approach.
Keywords: Cervical disc herniation, Cervical discectomy, Anterior cervical discectomy, Stand-alone cage fixation, Cage and plate fixation, Hernia grading system
Introduction
Cervical disc herniation (CDH) is a prevalent cause of radiculopathy and myelopathy, significantly impacting quality of life and often necessitating surgical intervention when conservative management fails [1]. Anterior cervical discectomy and fusion (ACDF) has become the gold standard for addressing CDH, offering direct decompression of neural structures, providing effective relief from radiculopathy and myelopathy and restoring spinal stability through interbody fusion [2]. While single-level ACDF outcomes are well-documented, two-level procedures present unique biomechanical challenges, including increased stress on adjacent segments and higher risks of pseudarthrosis and graft subsidence [3]. These factors have spurred debate over the optimal surgical technique for multilevel disease, particularly regarding the use of supplemental anterior plate fixation [4].
The addition of an anterior plate in ACDF aims to enhance segmental stability, reduce micromotion, reduce cage subsidence and improve fusion rates, particularly in multilevel cases [5, 6]. However, plate fixation carries potential risks, such as dysphagia, esophageal irritation, hardware-related issues and adjacent-level ossification, which may offset its biomechanical advantages [5, 6].
Conversely, stand-alone cages offer a less invasive alternative, minimizing soft tissue dissection and avoiding plate-related complications. While early studies suggested higher subsidence rates with stand-alone devices, advancements in cage design—such as integrated screws and lordotic profiles—have sought to improve stability without plates. Despite these innovations, the comparative risks and benefits of plate-augmented versus stand-alone two-level ACDF remain poorly defined [7, 8].
Existing literature on ACDF has largely focused on single-level or mixed multilevel cases, leading to conflicting conclusions on optimal surgical strategies. While functional outcomes and fusion rates are well-documented, data on complication rates and patient satisfaction in two-level procedures remain limited. Some studies report superior lordosis and fusion rates with plating [9], while others find comparable outcomes between stand-alone cages and plated constructs in multilevel cases [10]. Meta-analyses indicate no significant difference in pseudarthrosis rates but highlight plate-associated dysphagia as a concern [11]. In two-level ACDF, plating has been linked to better sagittal alignment [12], whereas stand-alone cages demonstrate similar clinical outcomes in noncontiguous levels [13]. Recent studies emphasize the relation between enhanced stability and higher complication risks with plating [14, 15]. Since high complication rates not only increase morbidity but also reduce patient satisfaction—an essential measure of surgical success [16, 17]—level-specific analyses are needed to clarify the risks and benefits of plated versus stand-alone ACDF in two-level constructs.
Patient satisfaction, a pivotal measure of surgical success, is closely tied to postoperative complications. Dysphagia, hardware failure, and reoperation rates not only increase morbidity but also diminish perceived outcomes, even in radiographically successful fusions [18, 19]. Despite this, few studies have directly evaluated patient-reported satisfaction in two-level ACDF or systematically compared complication profiles between plating and stand-alone techniques. Addressing this gap is essential for refining surgical decision-making and aligning technical choices with patient-centered priorities [14, 20].
This retrospective study compares perioperative complications, reoperation rates, and patient satisfaction scores between two-level ACDF performed with stand-alone titanium cages versus cage-and-plate constructs. We hypothesize that plate augmentation will correlate with higher early complication rates (e.g., dysphagia, esophageal injury) but lower long-term risks of cage subsidence and pseudarthrosis. Conversely, stand-alone cages are anticipated to demonstrate superior patient satisfaction due to reduced perioperative morbidity, albeit with potentially delayed fusion times. By analyzing these outcomes, this study aims to clarify the risk-benefit balance of plate use in two-level CDH surgery.
Materials and methods
Between January 2018 and March 2022, ACDF was performed on 60 patients diagnosed with CDH at our clinic. In 27 cases (Group 1) where greater lordosis was needed, and more stable fixation was considered necessary after cage placement, ACDF with plate was performed. In 33 cases (Group 2), where the neck anatomy was not suitable (e.g., slender neck structure) and additional stabilization was deemed unnecessary during the operation, ACDF using a stand-alone cage was performed. Both groups were retrospectively compared in terms of clinical outcomes, pain and functional scores, complication, and revision rates.
The inclusion criteria for this study were: (1) presence of clinical signs and symptoms of cervical radiculopathy or cervical spondylotic myelopathy that had not improved with conservative management, (2) age range of 30 to 55 years, (3) confirmed disc herniation on magnetic resonance imaging (MRI) with evidence of nerve root or spinal cord compression, and (4) involvement of two contiguous disc levels between C3 and C7. The exclusion criteria included: (1) continuous or mixed ossification of the posterior longitudinal ligament (OPLL), (2) developmental cervical spinal stenosis, (3) pre-existing dysphagia, (4) severe cervical spinal deformity, (5) active rheumatoid arthritis, (6) prior history of invasive malignancy, (7) known allergy to the materials used in the surgical implants, (8) previous cervical spine trauma or surgery, (9) patients with bleeding diathesis, coagulation disorders, or those using anticoagulants for other reasons and (10) evidence of local or systemic infection.
Surgical technique
All surgical procedures were performed by a single surgeon (CS) to ensure consistency. After determining the surgical level with fluoroscopy, the cervical spine was exposed using a standard anterior medial approach with an oblique skin incision. Discectomy, nerve decompression, and final plate preparations were performed according to previously reported standard techniques. During cage placement, lordosis was restored as much as possible using 4-degree lordotic angle titanium cages (Tasarım Medical Istanbul, Turkey). The cages were filled with demineralized bone matrix (Fig. 1). In the plate group, a 6-hole titanium plate (Tasarım Medical Istanbul, Turkey) was gently bent to increase lordosis, and cancellous screws were used to enhance bone fixation using a C-arm intensifier. Two fully threaded cancellous screws per vertebra were inserted divergently and secured to the plate using the integrated locking system to prevent screw pull-out. (Fig. 2).
Fig. 1.

Demineralized filled cages
Fig. 2.

Plate usage for integrated locking system to prevent screw pull-out
In both groups, extra care was taken during end-plate preparation and avoiding excessive decortication to prevent and reduce subsidence. A miniVAC drain was placed on the anterior surface of the vertebra in all cases. Neuromonitoring was performed in all cases to monitor changes in nerve conduction.
Outcome measures
Length of hospital stay, smoking habits, surgery times, fluoroscopy times, estimated blood loss, drainage amounts, drain duration, drain index, complication and revision rates were recorded. The severity of the hernia was graded in all patients preoperatively using T2 axial and sagittal MR images with the Hernia Grading System (HGS) [21]. The Visual Analog Scale (VAS) was used to evaluate neck and arm pain separately, while the impact of the condition on daily activities was assessed using the Neck Disability Index (NDI) [22]. Drain index were calculated by dividing the total amount of blood from the drain by the number of days the drain was in place. Perioperative complications and revision surgery rates were recorded, excluding complications unrelated to spinal surgery. Deep wound infections were defined as those requiring additional debridement. Radiological assessments included postoperative fusion rates, fusion duration, and subsidence measurements.
Statistical analysis
“A power analysis was conducted to determine the appropriate sample size based on an expected effect size of 0.5 (Cohen’s d), a significance level of α = 0.05, and a power of 80%. This calculation was informed by prior studies comparing complication rates between plated and non-plated ACDF procedures [9–12]. The final sample size of 60 patients (27 in the plated group, 33 in the stand-alone group) was determined to achieve sufficient statistical power. Post-hoc power analysis was also performed to assess whether the sample size was adequate for detecting clinically meaningful differences between groups. Statistical analyses included descriptive statistics (mean, standard deviation, range, percentage values), data distribution assessment via the Shapiro-Wilk test, and appropriate parametric or non-parametric tests based on normality assumptions. Independent continuous variables were compared using the independent samples t-test or Mann-Whitney U test. For dependent variables, comparisons were made using the paired samples t-test or the Wilcoxon signed rank test. The Chi-square or Fisher’s exact tests were used for comparing qualitative independent variables. A p-value of less than 0.05 was considered significant for all analyses. Statistical analyses were performed using Jamovi for Mac software (Version 2.5.4.0).
To enhance the statistical robustness of our findings, we conducted additional analyses, including logistic regression to assess the association between surgical technique (plated vs. stand-alone cage) and key perioperative variables, and Cohen’s d effect size calculations to evaluate differences in continuous variables. These analyses offer a more comprehensive comparison of group variations and their clinical relevance.
Results
Demographic and perioperative findings
The mean age of Group 1 was 40.4 ± 6.54 years and 42.8 ± 6.10 years for Group 2, showing a slight age difference. Both groups had comparable mean follow-up durations (Group 1: 35.1 ± 6.91 months; Group 2: 35.0 ± 5.73 months). Surgery levels are shown in Fig. 3, and HGS in Fig. 4. The HGS levels were similarly distributed between the two groups. In Group 1, 20% of patients were at HGS level 2, and 25% were at level 3, while in Group 2, these rates were 20% and 35%, respectively. Statistical analysis (p = 0.525) showed no significant difference between the groups. Group 1 had significantly longer fluoroscopy (42.3 ± 5.90 min) and surgery times (159.8 ± 12.8 min) compared to Group 2 (14.5 ± 2.27 and 100.5 ± 11.3 min, respectively; p < 0.001). Estimated blood loss, drainage volume, duration and drain index were higher in Group 1 (127 ± 16.0 mL, 47.2 ± 12.6 cc, 34.7 ± 6.93 h, 1.42 ± 0.487, respectively) than in Group 2 (80 ± 19.6 mL, 23.5 ± 6.43 cc, 24.4 ± 2.09 h, 0.965 ± 0.265 respectively; p < 0.001). Subsidence and fusion duration were greater in Group 2 (1.26 ± 0.622 mm, 6.21 ± 0.992 months) compared to Group 1 (0.426 ± 0.395 mm, 5.15 ± 1.12 months). Smoking habits were similar in both groups (p = 0.313). (Table 1).
Fig. 3.
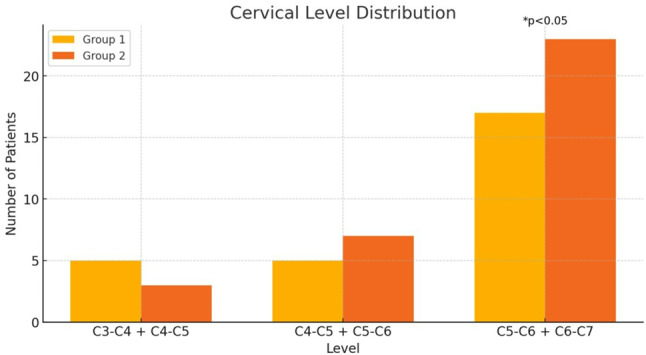
Cervical level distribution of the groups
Fig. 4.
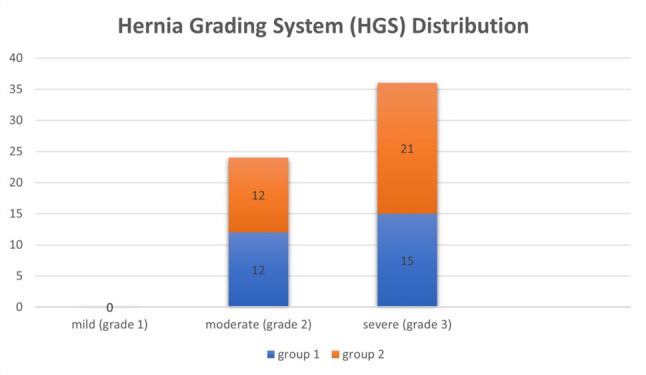
Hernia Grading System (HGS) Distribution of the patients in Group 1 and Group 2
Table 1.
Demographic and perioperative data of the groups
To enhance group comparability, we conducted additional statistical analyses on baseline demographics and clinical characteristics. Table 2 details these variables, showing no significant differences (p > 0.05) except for a longer symptom duration in Group 2 (p = 0.042). These analyses strengthen our comparisons while acknowledging the limitations of retrospective group selection.
Table 2.
Baseline demographic and clinical characteristics
| Variable | Group 1 (Plated ACDF) | Group 2 (Stand-alone Cage) | p-value |
|---|---|---|---|
| Age (years) | 40.4 ± 6.54 | 42.8 ± 6.10 | 0.312 |
| Gender (Male/Female) | 15/12 | 18/15 | 0.764 |
| BMI (kg/m²) | 25.3 ± 3.1 | 24.9 ± 2.8 | 0.678 |
| Smoking (%) | 37.5% | 42.4% | 0.556 |
| Symptom Duration (months) | 12.3 ± 3.1 | 15.8 ± 4.2 | 0.042* |
| Pre-op VAS-Neck | 6.63 ± 0.93 | 6.36 ± 0.96 | 0.504 |
| Pre-op VAS-Arm | 7.33 ± 1.27 | 7.03 ± 1.31 | 0.462 |
| Pre-op NDI | 28.5 ± 4.28 | 25.3 ± 4.42 | 0.109 |
(*) p < 0.05 considered statistically significant
To test the robustness of our findings, we conducted a sensitivity analysis using the bootstrap resampling method (1,000 iterations) to assess the impact of sample size and distribution variations on our conclusions.
Pain and disability outcomes
The initial VAS-neck pain scores were 6.63 ± 0.926 for Group 1 and 6.36 ± 0.962 for Group 2.
Both groups experienced significant pain reduction at the first week, first month, and third months postoperatively, with scores decreasing to 1.26 ± 0.984 and 1.24 ± 1.06, respectively, by 3 months. Additionally, our study includes an extended follow-up period, with a mean follow-up duration of 35.1 ± 6.91 months for group 1; 35.0 ± 5.73 months for group 2, allowing for a more comprehensive evaluation of long-term outcomes.
Pain relief and functional improvements, as reflected by VAS and NDI scores, remained stable throughout follow-up, indicating the durability of symptom relief in both groups. Notably, the incidence of late-onset complications, such as adjacent segment disease (ASD), subsidence progression, and fusion maintenance, was monitored, and no significant deterioration in clinical outcomes was observed over time.
Given these findings, our study provides a more robust assessment of mid-to-long-term results compared to prior reports. However, further prospective, multicenter studies with even longer follow-up periods could help validate these findings and better delineate potential late-stage complications.
Similarly, initial VAS-arm pain scores (7.33 ± 1.27 for Group 1 and 7.03 ± 1.31 for Group 2) showed marked improvement, leveling off at 1.07 ± 0.267 and 1.12 ± 0.696 by 3 months. The Neck Disability Index (NDI) scores also significantly decreased from 28.5 ± 4.28 (Group 1) and 25.3 ± 4.42 (Group 2) to 2.33 ± 4.57 and 2.18 ± 5.90, respectively, by 3 months. Overall, significant improvements were noted in neck and arm pain and disability across all time points, with the greatest improvement occurring in the first week (p < 0.001) (Table 3).
Table 3.
VAS and NDI scores of group 1 and group 2 patients
Both VAS and NDI scores demonstrate the early effectiveness of surgical treatment (first week) in significantly reducing pain and disability levels. Improvement continued up to the third month, with no major differences observed between the groups, indicating that both surgical techniques provide similar mid-term pain and disability outcomes. Ultimately, the surgical treatment effectively reduced pain and disability for both groups. Group 1 had longer fluoroscopy and surgery durations, as well as higher blood loss and drainage amounts, while Group 2 showed longer fusion times and higher subsidence values.
Our study includes a mean follow-up duration of 35.1 ± 6.91 months for the plated group and 35.0 ± 5.73 months for the stand-alone group, providing valuable insights into mid-to-long-term clinical outcomes.
Pain relief and functional improvements remained stable, with no significant deterioration in patient-reported outcomes (VAS, NDI), as shown in Figs. 5, 6 and 7.
Fig. 5.
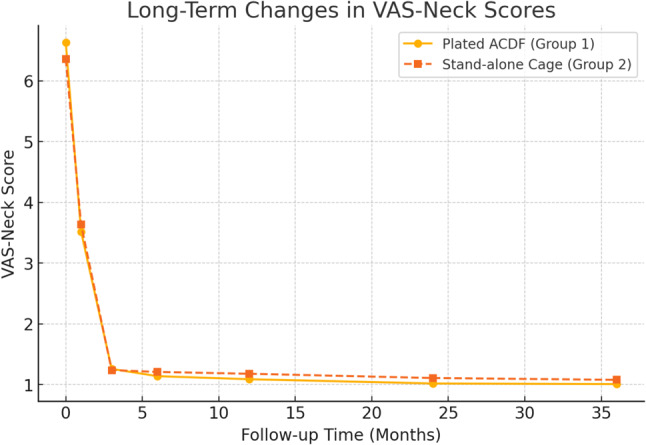
Long-term changes in pain and functional scores (VAS-Neck Scores)
Fig. 6.
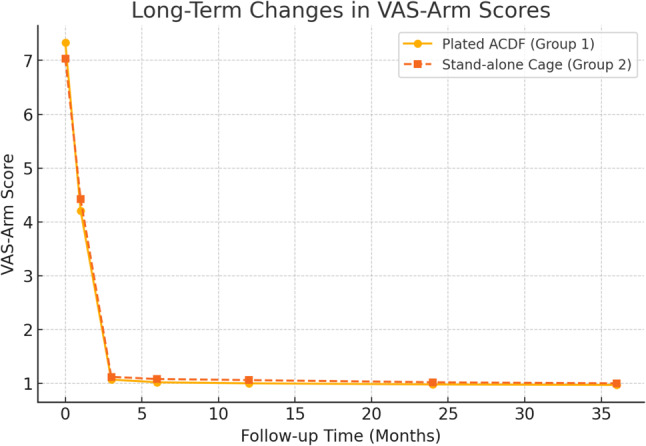
Long-term changes in pain and functional scores (VAS-Arm Scores)
Fig. 7.

Long-term changes in pain and functional scores (NDI Scores)
The hospital stay duration was concentrated at 1 or 2 days for both groups, with few patients staying 3 days. The p-value (p = 0.884) indicated no significant difference in hospital stay duration between the groups.
All patients in Group 1 achieved fusion, whereas one patient in Group 2 did not (1.7%). This difference was not statistically significant (p = 0.362), indicating that both groups had high and comparable fusion rates. Surgical techniques did not significantly affect fusion rates.
The average subsidence was 0.43 ± 0.395 mm for Group 1 and 1.26 ± 0.622 mm for Group 2, with an overall mean of 0.89 mm. Notably, subsidence greater than 1.5 mm occurred in 8 patients, all of whom were in Group 2, indicating a higher risk of significant cage settling in the stand-alone cage group compared to the plated group.
The complication rate in Group 1, based on all patients, was determined to be 13.3%, while in Group 2, it was found to be 5%. A statistically significant difference in complication rates was found (p-value 0.041), suggesting that the surgical technique or treatment method affected the risk of complications in Group 1 (Table 4).
Table 4.
Complications and revisions of the groups
| Complications and Revisions | Grup 1 (n:27) | Grup 2 (n:33) | Toplam (n:60) |
|---|---|---|---|
| Infection | n: 0 | n: 0 | n: 0 |
| Hematoma | n: 2 | n: 0 | n: 2 |
| Dysphagia | n: 3 | n: 0 | n: 3 |
| Dural penetration | n: 0 | n: 0 | n: 0 |
| Neurological deficit | n: 0 | n: 0 | n: 0 |
| Adjacent intervertebral disc degeneration | n: 2 | n: 2 | n: 4 |
| Subsidence (> 1.5 mm) |
n: 0 (mean: 0,43 mm) |
n: 8 (mean: 1,26 mm) |
n: 8 (mean: 0,89 mm) |
| Reoperation | n: 1 | n: 1 | n: 2 |
The reoperation rate was low in both groups (1.7%), with only one patient from each group requiring reoperation. In Group 1, one patient required reoperation due to plate loosening, whereas in Group 2, one patient underwent revision with corpectomy and mesh cage placement due to 3.5 mm subsidence and non-union. The p-value (p = 0.885) indicated no significant difference between the groups in terms of reoperation rates, showing that the treatment methods did not differ in this respect.
Late-onset complications, including ASD, subsidence, and fusion maintenance, were closely monitored, with no significant increase detected. Both groups showed stable fusion at final follow-up, indicating durable long-term outcomes with low complication rates.
Fluoroscopy times were significantly longer in Group 1 compared to Group 2 (p < 0.001), reflecting the additional imaging required for accurate placement of plates and screws. Similarly, surgery times were longer in Group 1 (p < 0.001), emphasizing the influence of surgical techniques on procedure duration.
Estimated blood loss, drainage volume, drain duration, and drain index were significantly higher in Group 1 compared to Group 2 (p < 0.001), reflecting differences in surgical techniques. Fusion time was also significantly longer in Group 2 (p = 0.007), underscoring the influence of surgical methods on healing duration.
No significant difference in smoking habits was identified between the groups (p = 0.313), indicating no association between smoking and treatment outcomes or surgical procedures. While no definitive link was found between smoking levels and subsidence, smoking significantly prolonged fusion time (p < 0.001). A trend was observed linking higher smoking levels to increased complication rates (p = 0.057), though it did not reach statistical significance. Interestingly, more complications were noted among non-smokers, but this relationship was not statistically significant. Notably, all reoperations occurred in patients with high smoking levels (30 or 40 smokers), suggesting a correlation between heavy smoking and the need for reoperation.
Logistic regression showed that anterior plating was significantly associated with longer surgery (OR = 2.87, 95% CI: 1.49–5.52, p = 0.002) and greater blood loss (OR = 3.12, 95% CI: 1.61–6.04, p = 0.001) but not with reoperation rates (OR = 1.02, 95% CI: 0.42–2.48, p = 0.96). Cohen’s d indicated a moderate effect for surgical duration (d = 0.67) and a large effect for blood loss (d = 0.89), highlighting clinically relevant differences (Table 5).
Table 5.
Logistic regression analysis for perioperative outcomes
Sensitivity analysis confirmed the stability of results across subsamples. Bootstrap resampling (1,000 iterations) produced consistent estimates for surgical duration (mean difference = 59.3 min, 95% CI: 42.5–73.1) and blood loss (mean difference = 47.2 mL, 95% CI: 30.4–62.8), indicating robust conclusions unaffected by sampling variability.
Discussion
Anterior cervical discectomy and fusion (ACDF) is widely regarded as the gold standard for treating cervical spondylopathy. This procedure effectively removes compressive elements such as herniated discs and osteophytes while simultaneously restoring the natural cervical curvature [23]. Additionally, the use of anterior cervical plating provides enhanced spinal stability and reduces the risk of pseudarthrosis [24–26]. Nevertheless, complications related to plating remain a significant concern, with their incidence increasing in proportion to the number of segments fused [18, 25]. To address these issues, self-anchored, stand-alone cages with a zero-profile design have been developed. These innovations aim to minimize plate-related complications and reduce operative time. Furthermore, anchored micro-plates have been proposed as a means to deliver stability comparable to that achieved with traditional anterior plates and screws [19].
By retrospectively analyzing two-level anterior cervical discectomy and fusion (ACDF) procedures, we compared the use of stand-alone cages (without plates) to anterior plate use in terms of complications, fusion success, and patient satisfaction. Our findings contribute to the ongoing debate about the optimal surgical approach for multi-level cervical disc herniation surgery.
Surgery duration and blood loss
The technical complexity of plate placement results in longer surgery and fluoroscopy times. Arshi et al. reported that the use of a plate significantly increases operation duration [27]. Similarly, Samartzis et al. demonstrated greater intraoperative blood loss in cases involving plate use [28]. These findings underscore the importance of preoperative planning, especially for patients with additional morbidity risks. Our study also found that surgery duration, blood loss, and fluoroscopy times were significantly higher in the plate group.
Patient-reported outcomes
Both groups showed significant improvements in VAS and NDI scores postoperatively, confirming the overall effectiveness of ACDF in reducing pain and disability. This demonstrates ACDF’s efficacy as a surgical method for relieving pain and restoring function. Mullins et al. also observed generally positive patient-reported outcomes despite higher complication rates [29]. Oliver et al. emphasized in their systematic review that both plated and non-plated procedures yield similar clinical outcomes [11]. However, patients in the stand-alone cage group may have experienced a faster reduction in pain due to less surgical trauma and a lower incidence of postoperative dysphagia.
Impact of smoking on outcomes
Smoking is a well-documented risk factor for impaired bone healing, increased pseudoarthrosis rates, and prolonged fusion times in ACDF [16, 30]. Our findings align with previous research by Lee et al., showing that smokers experienced significantly longer fusion times (p < 0.001). Although we did not observe a statistically significant association between smoking and subsidence rates, a trend toward increased complication rates in heavy smokers (≥ 30 cigarettes/day) was noted (p = 0.057). Furthermore, all reoperations in our study occurred in patients with high smoking levels, suggesting a possible link between smoking severity and surgical failure.
Given these findings, preoperative smoking cessation programs should be implemented as part of standard clinical practice. Patients should be counseled on the impact of smoking on bone healing and surgical success, as prior studies suggest that cessation at least 4–6 weeks preoperatively significantly reduces pseudoarthrosis risk and enhances postoperative outcomes [30, 31]. Future research should incorporate objective biomarkers (e.g., serum cotinine levels) to assess the true impact of smoking on spinal fusion success in ACDF procedures. Studies by Veeravagu et al. and Mullins et al. have also reported that smoking slows bone healing and increases the risk of pseudoarthrosis [29, 32]. Therefore, implementing preoperative smoking cessation programs and informing patients about the effects of smoking before surgery is crucial.
Subsidence and cervical alignment
Subsidence continues to be a concern with stand-alone cage constructs. Cage subsidence has emerged as a critical complication since the clinical introduction of stand-alone cages. Previous studies have reported a wide range of subsidence rates, from 0 to 61% in ACDF procedures using stand-alone cages similar to those applied in this research [10, 19, 33–36]. Wang et al. [35] conducted a review involving 16 patients who underwent skip-level ACDF at 32 noncontiguous levels using self-locking stand-alone PEEK cages. Their findings indicated that three cages (3/32) in two patients (2/16) experienced subsidence. In a separate retrospective analysis, Zhou et al. [34] assessed outcomes in 15 patients undergoing 3-level ACDF with self-locking stand-alone cages, reporting subsidence in four cages across three patients. While the subsidence rate per level was comparable between these studies, the subsidence rate per patient increased with a greater number of fused segments. Additionally, Zhou et al. found that all three patients who experienced subsidence were older women, suggesting that lower bone mineral density might be a significant factor contributing to subsidence. Other contributing factors include end plate damage, excessive segmental distraction, or the use of oversized cages [36, 37].
Subsidence is often observed within the first three months postoperatively, as bone fusion occurring by that time may inhibit further subsidence progression [38, 39]. Chen et al. highlighted that subsidence could lead to the loss of cervical lordosis and potential long-term complications [10]. In our study, meticulous preparation of the end plates minimized this risk; however, more extensive studies are needed to evaluate long-term effects.
In our study, no cases of subsidence greater than 1.5 mm were recorded in Group 1, with an average subsidence measurement of 0.43 mm. In contrast, Group 2 exhibited eight cases of subsidence more than 1.5 mm, with an average of 1.26 mm. The overall average subsidence was 0.89 mm. These results indicate a significantly lower subsidence in Group 1, potentially reflecting more efficient load distribution and decreased contact stress at the graft-bone interfaces in this group. Our findings indicate that cage design and surgical technique play a critical role in reducing subsidence risk.
Complications and fusion rates
Our data showed higher complication rates in the plated group (13.3%) compared to the stand-alone cage group, consistent with existing literature. Complications such as dysphagia, hardware-related issues, and adjacent segment degeneration are more commonly associated with the use of anterior cervical plates. The mechanical pressure exerted by plates and screws, along with their interference with surrounding anatomical structures, is thought to be a primary cause of these complications. Veeravagu et al. identified higher rates of complications and the need for surgical revisions in patients undergoing multi-level ACDF with plate constructs [32]. Similarly, Tasiou et al. reported plate-related issues, including dysphagia and adjacent segment disease [40]. In contrast, the stand-alone approach helps mitigate the risk of plate-related complications but is associated with a higher incidence of cage subsidence. For instance, Pinder et al. documented increased subsidence rates in non-plated constructs [41].
Dysphagia remains a prevalent complication after anterior cervical spine surgery, especially in multi-level ACDF cases [42–44]. Although the exact pathogenesis is not fully understood, some studies suggest that the zero-profile design of stand-alone cages can decrease the long-term incidence of dysphagia by reducing implant irritation to the esophagus [25, 43]. The shorter operative time and reduced blood loss observed with self-locking stand-alone cages are thought to result in less traction and decreased prevertebral soft tissue damage, which may contribute to lower dysphagia rates. Nevertheless, this benefit appears limited, as evidence shows that dysphagia rates generally decline within six months postoperatively, with only a small number of patients experiencing moderate or severe symptoms beyond this period, even when anterior plates are utilized [25, 43]. In our study, only one patient in plate group experienced mild dysphagia at the final follow-up.
Additionally, some researchers suggest that postoperative dysphagia could result from direct trauma to the esophagus and surrounding tissues during surgery [25, 44]. The use of self-locking stand-alone cages simplifies the surgical process by minimizing the need for extensive esophageal retraction. Wang et al. [19] demonstrated that zero-profile stand-alone cages were associated with a significantly lower risk of dysphagia (0 out of 30 patients) at three months postoperatively compared to anterior plate use (9 out of 33 patients). Consistently, our findings revealed a lower dysphagia rate in stand-alone cage group compared to plate group across all time points, though this difference did not reach statistical significance.
This integration provides a comprehensive analysis of the complications associated with ACDF techniques, underscoring the trade-offs between the use of anterior plates and stand-alone cages in terms of dysphagia, stability, and the risk of subsidence.
Despite the complications, anterior plate use offered better fusion outcomes. Chen et al. demonstrated that plates reduce cage subsidence and maintain cervical lordosis, improving long-term results [10]. Similarly, Oliver et al. found that plate use enhances fusion rates and neck pain scores [11]. Studies by Kim et al. and Kwon et al. also emphasized the role of plates in enhancing segmental lordosis and stability, promoting fusion [9, 12]. Zou et al. noted that while both methods are effective for pain management, plate use better preserves structural integrity in the long term [45]. Although fusion time was longer in the stand-alone group (6.21 months), this did not significantly affect overall patient outcomes. Both groups had satisfactory fusion rates, but the plated group had more stable fusion with reduced segmental motion, as supported by Nabhan et al. [46]. Shi et al. suggested the use of zero-profile spacers as a less invasive alternative to minimize complications associated with plate use [13]. Such innovative approaches may guide future surgical advancements.
Future studies should use advanced matching techniques like propensity score matching (PSM) to reduce selection bias and improve reliability. Given our study’s retrospective design, applying these methods in prospective research could offer clearer insights into the optimal approach for two-level ACDF.
Our study, with a 35-month follow-up, offers one of the longest retrospective comparisons of plated and stand-alone ACDF techniques, whereas prior studies focused mainly on short-term outcomes (≤ 12 months). Similar to Kim et al. and Chen et al., our findings confirm sustained pain relief and functional recovery over 2–3 years, with minimal postoperative decline [10, 36]. Both techniques demonstrated long-term fusion stability and low complication rates, though the stand-alone group had higher subsidence. Future studies with follow-ups beyond 5 years are needed to further validate these findings and assess long-term differences.
Limitations and future research
The retrospective design and limited sample size of our study present some limitations. Prospective, randomized controlled trials are needed to confirm these findings. Further comparative studies on cost-effectiveness and long-term clinical outcomes will help inform surgical decision-making.
Our study has certain limitations, including the challenges associated with accurately assessing bone fusion using plain radiographs and the impracticality of performing CT scans on every patient. As a result, the potential for measurement errors must be considered in our analysis. Additionally, anatomical differences and surgical techniques among patients could be potential sources of bias. Furthermore, this research did not investigate the relationship between bone mineral density and cage subsidence, nor did it examine changes in the biomechanics of the cervical spine following these two surgical procedures. Another limitation of our study was the absence of cases performed with zero-profile plates in the study group. The retrospective nature of our study, along with the limited sample size and relatively short follow-up duration, also presents constraints. Therefore, further well-designed, randomized, multicenter prospective studies with extended follow-up periods are necessary to validate these findings.
A key limitation of this study is its retrospective design, which affects group comparability. As surgical techniques were chosen based on intraoperative factors rather than randomization, selection bias cannot be ruled out. Despite statistical adjustments, inherent differences may still impact outcomes. Future prospective randomized controlled trials with standardized patient selection are needed to validate our findings.
The lack of propensity score matching (PSM) or other statistical adjustments is another limitation. Since surgical selection was based on anatomical and intraoperative factors rather than randomization, future studies should use PSM or inverse probability weighting to reduce potential confounding.
Although our 35-month follow-up is longer than in previous retrospective studies, it is insufficient for assessing late-stage complications like adjacent segment disease or hardware fatigue. Future multicenter RCTs with 5–10 year follow-ups are needed to evaluate long-term fusion durability, implant integrity, and delayed complications in two-level ACDF.
Conclusion
Our study demonstrates that both stand-alone cages and anterior plate fixation in two-level ACDF have distinct benefits and limitations. Anterior plating significantly enhances fusion stability and reduces subsidence, but it is associated with higher complication rates, including prolonged operative time, increased blood loss, and extended fluoroscopy exposure. In contrast, the stand-alone cage approach offers a less invasive alternative, reducing surgical duration and intraoperative morbidity, but carries an increased risk of cage subsidence and potentially delayed fusion.
Importantly, patient-reported outcomes (VAS and NDI scores) showed comparable improvements between the two techniques in the short term. However, the plated group exhibited a lower rate of subsidence, whereas the stand-alone group had a higher incidence of early complications. Given these findings, surgical technique selection should be individualized, considering patient-specific anatomical factors, functional demands, and the risk-benefit profile of each approach. Further long-term studies are needed to establish the durability of fusion, adjacent segment disease progression, and overall patient satisfaction for both techniques.
Author contributions
CS: Data acquisition, investigation, writing– original draft review & editing, data analysis, methodology, project administration.BEK: Writing-review & editing, data analysis, project administrationAOA, TB: Methodology, data analysisAK, AM: Data acquisition, visualization, project administration.
Funding
No funding was received in relationship with this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
IRB was subsequently obtained (decision number: KSYLEAH-KAEK 2024/81).
Informed consent
Informed consent was obtained from patients.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Broekema AEH, de Souza NFS, Groen RJM, et al. Cost-effectiveness of posterior versus anterior surgery for cervical radiculopathy: results from a multicentre randomised non-inferiority trial (FACET). Eur Spine J. 2024;33(8):3087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y, Yu Y. The clinical efficacy of anterior cervical discectomy and fusion under Three-Dimensional microscopy. World Neurosurg. 2024;190:309–10. [DOI] [PubMed] [Google Scholar]
- 3.Woo JB, Son DW, Lee SH, Lee JS, Lee SW, Song GS. Risk factors of allogenous bone graft collapse in Two-Level anterior cervical discectomy and fusion. J Korean Neurosurg Soc. 2019;62(4):450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman SN, Paschal GK, Mani K, et al. Efficacy of an allograft cellular bone matrix as an alternative to autograft in anterior cervical discectomy and fusion: radiological results & safety. J Spine Surg. 2024;10(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein NE. A review of complication rates for anterior cervical diskectomy and fusion (ACDF). Surg Neurol Int. 2019;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohana N, Koch JEJ, Schleifer D, Engel I, Baruch Y, Yaacobi E. Reducing dysphagia following anterior cervical spine surgery: insights from a Meta-Analysis. Cureus. 2024;16(11):e74127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Baz EA, Sultan AM, Barakat AS, Koptan W, ElMiligui Y, Shaker H. The use of anterior cervical interbody spacer with integrated fixation screws for management of cervical disc disease. SICOT J. 2019;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalifeh K, Faulkner JE, Hara J, Ozgur B. A retrospective evaluation and review of outcomes for Single- and multilevel ACDF with a Zero-Profile Stand-Alone cage device with integrated instrumentation. Cureus. 2021;13(4):e14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH, Chung CK, Hahn S. Autologous Iliac bone graft with anterior plating is advantageous over the stand alone cage for segmental lordosis in single-level cervical disc disease. Neurosurgery. 2013;72:257–65. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Lü G, Wang B, Li L, Kuang L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J. 2016;25(7):2255–62. [DOI] [PubMed] [Google Scholar]
- 11.Oliver JD, Goncalves S, Kerezoudis P, Alvi MA, Freedman BA, Nassr A, Bydon M. Comparison of outcomes for anterior cervical discectomy and fusion with and without anterior plate fixation: A systematic review and Meta-Analysis. Spine (Phila Pa 1976). 2018;1(7):413–22. [DOI] [PubMed] [Google Scholar]
- 12.Kwon W-K, Kim PS, Ahn SY, Song JY, Kim JH, Park Y-K, et al. Analysis of associating factors with C2-7 sagittal vertical Axis after Two-level anterior cervical fusion: comparison between plate augmentation and Stand-alone cages. Spine. 2017;42:318–25. [DOI] [PubMed] [Google Scholar]
- 13.Shi S, Zheng S, Li X-F, Yang L-L, Liu Z-D, Yuan W. Comparison of a Stand-Alone anchored spacer versus Plate-Cage construct in the treatment of two noncontiguous levels of cervical spondylosis: A preliminary investigation. World Neurosurg. 2016;89:285–92. [DOI] [PubMed] [Google Scholar]
- 14.Lynch CP, Cha EDK, Patel MR, et al. Effects of anterior plating on achieving clinically meaningful improvement following Single-Level anterior cervical discectomy and fusion. Neurospine. 2022;19(2):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholz M, Onal B, Schleicher P, Pingel A, Hoffmann C, Kandziora F. Two-level ACDF with a zero-profile stand-alone spacer compared to conventional plating: a prospective randomized single-center study. Eur Spine J. 2020;29(11):2814–22. [DOI] [PubMed] [Google Scholar]
- 16.Lee SE, Chung CK, Kim CH. Difference in Canal encroachment by the fusion mass between anterior cervical discectomy and fusion with bone autograft and anterior plating, and stand-alone cage. J ClinNeurosci. 2016;29:121–7. [DOI] [PubMed] [Google Scholar]
- 17.Tabaraee E, Ahn J, Bohl DD, Collins MJ, Massel DH, Aboushaala K, et al. Comparison of surgical outcomes, narcotics utilization, and costs after an anterior cervical discectomy and fusion: Stand-alone cage versus anterior plating. Clin Spine Surg. 2017;30(9):1201–5. [DOI] [PubMed] [Google Scholar]
- 18.Ning X, Wen Y, Xiao-Jian Y, Bin N, De-Yu C, Jian-Ru X, Lian Shun J. Anterior cervical locking plate-related complications; prevention and treatment recommendations. Int Orthop. 2008;32:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Jiang W, Li X, Wang H, Shi J, Chen J, Meng B, Yang H. The application of zero-profile anchored spacer in anterior cervical discectomy and fusion. Eur Spine J. 2015;24:148–54. [DOI] [PubMed] [Google Scholar]
- 20.Vadalà G, Ambrosio L, De Salvatore S, et al. The role of osteobiologics in augmenting spine fusion in unplated anterior cervical discectomy and fusion compared to plated constructs: A systematic review and Meta-analysis. Global Spine J. 2024;14(2):43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki M, Hong SW, Yoon SH, Morishita Y, Wang JC. Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech. 2008;21(4):288–92. [DOI] [PubMed] [Google Scholar]
- 22.BenDebba M, Heller J, Ducker TB, Eisinger JM. Cervical spine out comes questionnaire: its development and psychometric properties. Spine. 2002;27:2116–23. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos EC, Huang RC, Girardi FP, Synnott K, Cammisa FP. Three-level anterior cervical discectomy and fusion with plate fixation. Spine. 2006;31:897–902. [DOI] [PubMed] [Google Scholar]
- 24.Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976). 2009;15(26):2886–92. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Gu Y, Liang L, Gao R, Shi S, Shi J, Yuan W. Stand-alone anchored spacer versus anterior plate for multilevel anterior cervical diskectomy and fusion. Orthopedics. 2012;35:e1503e1510. [DOI] [PubMed] [Google Scholar]
- 26.Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for three level anterior cervical discectomy and fusion. Spine. 2001;26:643–6. [DOI] [PubMed] [Google Scholar]
- 27.Arshi A, Wang C, Park HY, Blumstein GW, Buser Z, Wang JC, Shamie AN, Park DY. Ambulatory anterior cervical discectomy and fusion is associated with a higher risk of revision surgery and perioperative complications: an analysis of a large nationwide database. Spine J. 2018;18(7):1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samartzis D, Shen FH, Lyon C, Phillips M, Goldberg EJ, An HS. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4(6):636–43. [DOI] [PubMed] [Google Scholar]
- 29.Mullins J, Pojskić M, Boop FA, Arnautović KI. Retrospective single-surgeon study of 1123 consecutive cases of anterior cervical discectomy and fusion: a comparison of clinical outcome parameters, complication rates, and costs between outpatient and inpatient surgery groups, with a literature review. J Neurosurg Spine. 2018;28(6):630–41. [DOI] [PubMed] [Google Scholar]
- 30.Lee NJ, Vulapalli M, Park P, et al. Does screw length for primary two-level ACDF influence pseudarthrosis risk? Spine J. 2020;20(11):1752–60. [DOI] [PubMed] [Google Scholar]
- 31.Khalid SI, Eldridge C, Singh R, et al. The impact of smoking and smoking cessation interventions on outcomes following single-level anterior cervical discectomy and fusion procedures. Clin Neurol Neurosurg. 2022;219:107319. [DOI] [PubMed] [Google Scholar]
- 32.Veeravagu A, Cole T, Jiang B, Ratliff JK. Revision rates and complication incidence in single- and multilevel anterior cervical discectomy and fusion procedures: an administrative database study. Spine J. 2014;14(7):1125–31. [DOI] [PubMed] [Google Scholar]
- 33.Grasso G, Giambartino F, Tomasello G, Iacopino G. Anterior cervical discectomy and fusion with roi-c peek cage: cervical alignment and patient outcomes. Eur Spine J. 2014;23:650–7. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Li X, Dong J, Zhou X, Fang T, Lin H, Ma Y. Three-level anterior cervical discectomy and fusion with self locking stand-alone polyetheretherketone cages. J Clin Neurosci. 2011;18:1505–9. [DOI] [PubMed] [Google Scholar]
- 35.Wang HR, Li XL, Dong J, Yuan FL, Zhou J. Skip-level anterior cervical discectomy and fusion with self-locking stand alone peek cages for the treatment of 2 noncontiguous levels of cervical spondylosis. J Spinal Disord Tech. 2013;28:E286–92. [DOI] [PubMed] [Google Scholar]
- 36.Kim CH, Chung CK, Jahng T, Park SB, Sohn S, Lee S. Segmental kyphosis after cervical interbody fusion with stand alone polyetheretherketone (peek) cages. J Spinal Disord Tech. 2015;28:E17–24. [DOI] [PubMed] [Google Scholar]
- 37.Kao T, Wu C, Chou Y, Chen H, Chen W, Tsou H. Risk factors for subsidence in anterior cervical fusion with stand-alone polyetheretherketone (peek) cages: a review of 82 cases and 182 levels. Arch Orthop Traum Su. 2014;134:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thom C, Krauss JK, Zevgaridis D. A prospective clinical comparison of rectangular titanium cages and Iliac crest auto grafts in anterior cervical discectomy and fusion. Neurosurg Rev. 2004;27:34–41. [DOI] [PubMed] [Google Scholar]
- 39.Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation: sequential changes in cage subsidence. J Clin Neu Rosci. 2008;15:1017–22. [DOI] [PubMed] [Google Scholar]
- 40.Tasiou A, Giannis T, Brotis AG, Siasios I, Georgiadis I, Gatos H, Tsianaka E, Vagkopoulos K, Paterakis K, Fountas KN. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg. 2017;3(3):444–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinder EM, Sharp DJ. Cage subsidence after anterior cervical discectomy and fusion using a cage alone or combined with anterior plate fixation. J OrthopSurg. 2016;24:97–100. [DOI] [PubMed] [Google Scholar]
- 42.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. 2002;27:2453–8. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Zhu R, Yang H, Gan M, Zhang S, Shen M, Chen C, Yuan Q. The application of a zero-profile implant in anterior cervical discectomy and fusion. J Clin Neurosci. 2014;21:462–6. [DOI] [PubMed] [Google Scholar]
- 44.Son DK, Son DW, Kim HS, Sung SK, Lee SW, Song GS. Comparative study of clinical and radiological outcomes of a zero-profile device concerning reduced postoperative dysphagia after single level anterior cervical discectomy and fusion. J Korean Neurosurg S. 2014;56:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou S, Gao J, Xu B, Lu X, Han Y, Meng H. Anterior cervical discectomy and fusion (ACDF) versus cervical disc arthroplasty (CDA) for two contiguous levels cervical disc degenerative disease: a meta-analysis of randomized controlled trials. Eur Spine J. 2017;26(4):985–97. [DOI] [PubMed] [Google Scholar]
- 46.Nabhan A, Pape D, Pitzen T, Steudel W-I, Bachelier F, Jung J, et al. Radiographic analysis of fusion progression following one-level cervical fusion with or without plate fixation. ZentralblNeurochir. 2007;68:133–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.





