Abstract
The global pandemic of novel coronavirus pneumonia (COVID-19) has resulted in millions of deaths over the past three years. As one of the most commonly affected extra-pulmonary organs, numerous studies have reported varying degrees of liver injury in a significant proportion of patients with COVID-19, particularly in severe and critically ill patients. Early prediction of liver dysfunction in hospitalized patients would facilitate the clinical management of COVID-19 and improve clinical prognosis, but reliable and valid predictive models are still lacking.
Methods
We collected data from 286 patients with RT-PCR confirmed COVID-19 admitted to various ICUs from the case system. These patients were randomly divided into a training cohort (50%) and a validation cohort (50%). In the training cohort, we first used ROC curves to measure the predictive efficiency of each of the variables for the development of liver damage during hospitalization in patients with COVID-19, followed by LASSO regression analysis to screen the variables for predictive models and logistic regression analysis to identify relevant risk factors. A nomogram based on these variables was created following the above model. Finally, the efficiency of the prediction models in the training and validation cohorts was assessed using AUC, consistency index (C index), calibration curves and Decision Curve Analysis.
Results
Out of a total of 80 parameters for COVID-19 patients admitted to the ICUs, 10 were determined to be significantly associated with the occurrence of liver dysfunction during hospitalization. Based on these predictors, further prediction models were used to construct and develop a nomogram that was offered for practical clinical application. The C-index of the column line graphs for the training and validation cohorts was 0.956 and 0.844 respectively. in addition, the calibration curves for the model showed a high degree of agreement between the predicted and actual incidence of liver dysfunction in patients with COVID-19.
Conclusion
By developing a predictive model and associated nomogram, we predicted the incidence of liver dysfunction during hospitalization in patients with COVID-19 in the ICU. The model’s predictive performance was determined in both the training and validation cohorts, contributing to the clinical management of COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10684-1.
Keywords: COVID-19 pneumonia, Liver dysfunction, Nomogram, Risk prediction model
Introduction
The global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global health crisis [1]. In addition to the respiratory symptoms of malaise, fever, cough, and dyspnea, COVID-19 can also cause varying degrees of damage to other systemic organs such as the liver, kidneys, heart, brain, immune system, urinary system, and reproductive system [2]. Numerous studies have found that patients with COVID-19 are highly susceptible to liver function abnormalities, the incidence of which varies widely across different regions of the world [3–7]. In the plasma of these patients, concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), reflecting hepatocellular damage; alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT), reflecting bile duct damage or cholestasis; total bilirubin (TBil), reflecting hepatic clearance and bile secretion capacity, and prothrombin time (PT) and albumin (Alb), reflecting synthetic capacity, showed varying degrees of abnormality [8]. The liver injury associated with COVID-19 is often characterized by elevated ALT and AST levels, while AST abnormalities occur earlier, more frequently, and more significantly, with the strongest correlation with mortality [9, 10]. In addition, studies have shown that liver injury is strongly correlated with the severity of COVID-19 and that patients admitted to the intensive care unit are more likely to suffer from symptoms of liver injury such as elevated transaminases, which usually means a longer stay in the ICU, as well as higher rates of intubation (65%), renal replacement therapy (33%) and morbidity and mortality (42%) during hospitalization [9, 11–13].
The factors that affect COVID-2019 related liver injury may be multifaceted. According to relevant clinical studies, the risk factors include not only baseline characteristics such as old age, male, high body mass index (BMI), potential underlying liver diseases, but also inflammatory indicators such as high serum C-reactive protein (CRP), procalcitonin (PCT), and white blood cell count, It also includes disease severity and drugs (Azvudine, Paxlovid, glucocorticoids, etc.) [6, 13–16]. Therefore, we collected clinical data from a certain number of critically ill patients with COVID-19 to develop a valid predictive model for assessing the incidence of liver injury in COVID-19 patients admitted to the intensive care unit, which would help clinicians to identify the onset of liver injury earlier and intervene in a timely manner to improve the prognosis.
Methods
Study population
Patients admitted to the respiratory ICU, neurology ICU, and general ICU wards between November 2022 and February 2023 were systematically reviewed through a medical record review. This study included all COVID-19 patients from our hospital who met the following criteria:
(1) Age 18 years or older;
(2) Positive nucleic acid test for SARS-CoV-2;
(3) Clinical symptoms ranging from mild to severe COVID-19 (as defined by WHO guidelines);
(4) Availability of liver function-related data during hospitalization, including liver enzymes (ALT, AST), bilirubin, and coagulation function indicators.
All patients received standard clinical care during the study period, with regular liver function monitoring.
Exclusion Criteria:
(1) Presence of other severe comorbidities (e.g., malignancy, acute heart failure, etc.);
(2) Prior immunotherapy other than interferon or antiviral drugs (e.g., Azvudine, Paxlovid);
(3) Incomplete liver function test data during hospitalization;
(4) Underwent liver biopsy or other interventions during hospitalization that led to missing liver function data.
A total of 286 patients were included based on the interim guidelines for the diagnosis of COVID-19 by the World Health Organization [17] and the above inclusion and exclusion criteria. All patients recruited had positive pharyngeal specimens for SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) tests. We clarified these patients’ survival status and disease therapies at the time of collection to ensure the integrity of the clinical data. This study was approved by the Ethics Committee of Tianjin Medical University General Hospital (IRB2022-YX-268–01).
Data collection and pre-processing
All patients had pharyngeal swabs collected for nucleic acid testing of the SARS-CoV-2 upon admission and those who tested positive were included in this study. Baseline population characteristics (age, gender, body mass index), clinical data (vital signs on admission, comorbidities, and laboratory tests during hospitalization), and treatment and medication data were then collected in detail for these patients. Laboratory tests include routine blood tests (eg. white blood cells, lymphocytes, neutrophils), indicators of inflammation (C-reactive protein and procalcitonin), arterial blood gas analysis, coagulation, liver and kidney function, and cardiac enzymes. Time-sensitive variables, such as lactate levels and oxygenation indices, were measured at predefined intervals: 0 h (at admission), 6 h, 12 h, 24 h, and subsequent time points as clinically indicated. This ensures a clear timeline for data collection and consistency across the study cohort.For infection, other common pathogenic bacteria and sites of infection were recorded; for treatment, the use of mechanical ventilation, continuous renal replacement therapy, and prone position mechanical ventilation was recorded, and for medication, the type and dosage of vasoactive drugs, sedative drugs, glucocorticoids, and antiviral drugs used during the patient’s hospital stay were recorded. We recorded and verified the data by different personnel to reduce data bias, and preprocessed all variables, including handling missing values, outliers, and standardizing or normalizing variables with different scales or large discrepancies. All enrolled patients were randomly assigned to the training and validation cohorts. Patients in the validation cohort were then divided into a normal group and an occurrence of liver injury group by the maximum value of liver enzymes during the patient’s hospitalization, and the remaining variables were incorporated to construct predictive models and plot nomogram, which were finally validated in the validation cohort and evaluated the clinical practice value through decision curve analysis.
Statistical analysis
Patients were divided into two groups based on the presence or absence of liver dysfunction during hospitalization. Liver dysfunction was defined using a combination of ALT/AST levels and other hepatic parameters such as total bilirubin, albumin, and INR. The severity of liver dysfunction was stratified into three categories based on ALT/AST levels and abnormalities in bilirubin/INR:
Mild: ALT/AST ≤ 2 × ULN.
Moderate: ALT/AST 2–5 × ULN.
Severe: ALT/AST > 5 × ULN or INR > 1.5.
The prevalence of each severity category was calculated and reported.
We used the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables to compare differences between the groups. Prior to regression modeling, we performed univariate analysis (e.g., t-tests or chi-square tests) to initially screen for variables significantly associated with the outcome (i.e., liver dysfunction), thereby narrowing down the candidate variables.We assessed the predictive validity of each variable for the occurrence of liver injury during hospitalization in COVID-19 patients using ROC curves and ranked and visualized the AUC values of all variables using bar graphs. In addition, we calculated the correlations between variables (e.g., Pearson’s correlation coefficient) to identify potentially redundant variables. For highly correlated variables (e.g., correlation coefficient > 0.8), only one variable was retained to avoid multicollinearity affecting the regression model. In order to avoid the occurrence of model estimation distortion or difficulty in estimating accuracy due to the possible precise or highly correlated relationships between continuous variables in the study. We used the cor function to calculate the correlation coefficients between 48 continuous variables and conducted a significance test (significance level set at 0.95). Finally, use the Corrplot package to draw the correlation heat map between variables.
To eliminate the dimensional influence between different evaluation indicators, we used the Min–Max function to standardize continuous variables, addressing the comparability issue between data indicators. After standardization, the Least Absolute Shrinkage and Selection Operator (LASSO) regression was employed as a method for variable selection by incorporating L1 regularization (penalizing model coefficients) [17]. LASSO regression was chosen for its ability to handle multicollinearity and to shrink the coefficients of less important variables to zero, thereby automatically selecting the most relevant features. This approach is particularly advantageous in the context of high-dimensional data, as it reduces the risk of overfitting and improves the model’s generalizability to new data.
The process of variable selection using LASSO regression was conducted as follows:
Data preprocessing: All potential predictor variables were standardized to ensure comparability across different scales.
Cross-validation: We employed tenfold cross-validation to identify the optimal penalty parameter (λ), which balances model simplicity and predictive accuracy. Variables with non-zero coefficients at the optimal λ were selected for inclusion in the model.
Feature selection: The selected variables were then incorporated into a logistic regression model to predict the probability of liver dysfunction in critically ill COVID-19 patients.
Subsequently, the logistic regression model was used to construct a nomogram for visualizing the predicted probability of liver dysfunction events. To ensure model reliability, we randomly divided the patient data into a training set and a test set in a 1:1 ratio. The model’s performance was evaluated in both datasets.
The process of Model Evaluation was conducted as follows:
Discrimination: The model’s discrimination was assessed using receiver operating characteristic (ROC) curves and the area under the curve (AUC). An AUC below 0.6 was interpreted as low discrimination, 0.6 to 0.75 as moderate discrimination, and above 0.75 as high discrimination. Additionally, the Harrell concordance index (C-index) was calculated to further evaluate the model’s ability to distinguish between patients with and without liver dysfunction.
Calibration: Calibration curves were generated to assess the agreement between predicted probabilities and observed outcomes in the validation cohort, providing an evaluation of the model’s predictive accuracy.
Clinical Practice: Decision Curve Analysis (DCA) is utilized to assess the clinical utility of predictive models for realistic decision making. The overall effectiveness of the model in guiding clinical decision making was quantified by comparing the net benefits of different decision strategies over a range of threshold probabilities, reflecting the balance between the benefits of true positives and the costs of false positives and false negatives.
Moreover,a sensitivity analysis was conducted by excluding patients with known liver disease to evaluate potential bias introduced by their inclusion.These steps ensure a rigorous approach to variable selection, model construction, and performance evaluation, improving the clarity and reproducibility of our methodology.
All statistical analyses were performed using R software (version 4.2.2; R Foundation for Statistical Computing), with a significance level set at p < 0.05.
Results
Population baseline data and clinical information of enrolled patients with COVID-19
The median age of this study population is 81 years old (IQR, 73–89 years old), with 220 males (77%) and 66 females (23%). There was no difference in baseline characteristics such as age, gender, and body mass index between the liver injury group and the normal group(p > 0.05); In terms of complications, hypertension (147; 51%) is the most common coexisting disease, followed by diabetes (38%), while chronic kidney disease, tumor, immune disease, and chronic obstructive pulmonary disease account for 14%, 11%, 10% and 8% of the total patients, respectively. In terms of outcomes, the median length of stay in the ICU for all patients was 13 days (8.25–22 days), with a 28-day mortality rate of 43%.,the liver dysfunction group had higher levels of both parameters than the normal group (Table 1). Among the cohort, the prevalence of liver dysfunction was stratified as follows: mild (55%), moderate (27.2%), and severe (17.8%). Patients with severe liver dysfunction were more likely to exhibit abnormalities in bilirubin and INR, suggesting significant hepatic impairment.
Table 1.
Demographic, clinical, and treatment characteristics of the full study cohort before randomization, grouped by liver dysfunction status
| Variables | Total (n = 286) | No Liver dysfunction group (n = 106) | Liver dysfunction group (n = 180) | p |
|---|---|---|---|---|
| Age(years), Median (Q1,Q3) | 81 (73, 89) | 80.5 (73, 88) | 82 (74, 90) | 0.585 |
| Gender, n (%) | 0.041 | |||
| Female | 66 (23) | 32 (30) | 34 (19) | |
| Male | 220 (77) | 74 (70) | 146 (81) | |
| BMI, Median (Q1,Q3) | 23.44 (20.81, 26.18) | 23.41 (21.26, 27.04) | 23.59 (20.76, 25.97) | 0.731 |
| DAY_28.Mortality, n (%) | 0.031 | |||
| No | 164 (57) | 70 (66) | 94 (52) | |
| Yes | 122 (43) | 36 (34) | 86 (48) | |
| Icu_staytime(days), Median (Q1,Q3) | 13 (8.25, 22) | 11 (7.25, 15) | 15 (9, 24.25) | < 0.001 |
| Mechanical_ventilation, n (%) | < 0.001 | |||
| No | 180 (63) | 87 (82) | 93 (52) | |
| Yes | 106 (37) | 19 (18) | 87 (48) | |
| Mechanical_ventilation_time, Median (Q1,Q3) | 0 (0, 6) | 0 (0, 0) | 0 (0, 9) | < 0.001 |
| CRRT, n (%) | < 0.001 | |||
| No | 253 (88) | 103 (97) | 150 (83) | |
| Yes | 33 (12) | 3 (3) | 30 (17) | |
| Infection site, n (%) | ||||
| Intestinal.infection | < 0.001 | |||
| No | 256 (90) | 104 (98) | 152 (84) | |
| Yes | 30 (10) | 2 (2) | 28 (16) | |
| Urinary.infection | 0.654 | |||
| No | 281 (98) | 105 (99) | 176 (98) | |
| Yes | 5 (2) | 1 (1) | 4 (2) | |
| Catheter.related.infection | 0.007 | |||
| No | 263 (92) | 104 (98) | 159 (88) | |
| Yes | 23 (8) | 2 (2) | 21 (12) | |
| Skin.and.soft.tissue.infection | 0.036 | |||
| No | 272 (95) | 105 (99) | 167 (93) | |
| Yes | 14 (5) | 1 (1) | 13 (7) | |
| Abdomen.cavity.infection | 0.222 | |||
| No | 275 (96) | 104 (98) | 171 (95) | |
| Yes | 11 (4) | 2 (2) | 9 (5) | |
| Coexisting illness, n (%) | ||||
| Hypertension | 0.46 | |||
| No | 139 (49) | 48 (45) | 91 (51) | |
| Yes | 147 (51) | 58 (55) | 89 (49) | |
| Diabetes | 0.98 | |||
| No | 177 (62) | 65 (61) | 112 (62) | |
| Yes | 109 (38) | 41 (39) | 68 (38) | |
| Chronic.obstructive.pulmonary.disease | 0.173 | |||
| No | 263 (92) | 101 (95) | 162 (90) | |
| Yes | 23 (8) | 5 (5) | 18 (10) | |
| Chronic.renal.insufficiency | 0.412 | |||
| No | 246 (86) | 94 (89) | 152 (84) | |
| Yes | 40 (14) | 12 (11) | 28 (16) | |
| Tumor.disease | 0.359 | |||
| No | 254 (89) | 97 (92) | 157 (87) | |
| Yes | 32 (11) | 9 (8) | 23 (13) | |
| Immunology.disease | 0.002 | |||
| No | 256 (90) | 103 (97) | 153 (85) | |
| Yes | 30 (10) | 3 (3) | 27 (15) | |
| Microbiology type, n (%) | ||||
| Acinetobacter.baumannii | 1 | |||
| No | 248 (87) | 92 (87) | 156 (87) | |
| Yes | 38 (13) | 14 (13) | 24 (13) | |
| Klebsiella.pneumoniae | 0.054 | |||
| No | 227 (79) | 91 (86) | 136 (76) | |
| Yes | 59 (21) | 15 (14) | 44 (24) | |
| Pseudomonas.aeruginosa | < 0.001 | |||
| No | 211 (74) | 98 (92) | 113 (63) | |
| Yes | 75 (26) | 8 (8) | 67 (37) | |
| Staphylococcus.aureus | < 0.001 | |||
| No | 253 (88) | 103 (97) | 150 (83) | |
| Yes | 33 (12) | 3 (3) | 30 (17) | |
| Escherichia.coli | 0.3 | |||
| No | 282 (99) | 106 (100) | 176 (98) | |
| Yes | 4 (1) | 0 (0) | 4 (2) | |
| Fungi | < 0.001 | |||
| No | 180 (63) | 87 (82) | 93 (52) | |
| Yes | 106 (37) | 19 (18) | 87 (48) | |
| Vitals, Median (Q1,Q3) | ||||
| HR(bpm) | 92 (76, 112) | 90 (78, 103.5) | 93.5 (75, 118) | 0.187 |
| RR(rpm) | 21 (18, 28) | 20 (18, 25) | 22 (18, 30) | 0.012 |
| SBP(mmHg) | 132 (110, 145) | 137 (122.75, 147.75) | 127 (104.75, 145) | 0.004 |
| DBP(mmHg) | 70 (59, 80) | 74 (66, 83.5) | 67.5 (53.5, 79) | < 0.001 |
| T(℃) | 37.2 (36.5, 38.1) | 37 (36.5, 37.8) | 37.4 (36.58, 38.3) | 0.01 |
| SPO2(%) | 90 (84, 93) | 92 (88.25, 95) | 89 (78.75, 93) | < 0.001 |
| Medication use | ||||
| Azvudine, n (%) | < 0.001 | |||
| No | 232 (81) | 102 (96) | 130 (72) | |
| Yes | 54 (19) | 4 (4) | 50 (28) | |
| Paxlovid, n (%) | < 0.001 | |||
| No | 235 (82) | 100 (94) | 135 (75) | |
| Yes | 51 (18) | 6 (6) | 45 (25) | |
| Prone.position.Mechanical_ventilation, n (%) | 0.014 | |||
| No | 234 (82) | 95 (90) | 139 (77) | |
| Yes | 52 (18) | 11 (10) | 41 (23) | |
| Propofol, n (%) | 0.007 | |||
| No | 235 (82) | 96 (91) | 139 (77) | |
| Yes | 51 (18) | 10 (9) | 41 (23) | |
| Dexmedetomidine, n (%) | < 0.001 | |||
| No | 225 (79) | 95 (90) | 130 (72) | |
| Yes | 61 (21) | 11 (10) | 50 (28) | |
| Midazolam, n (%) | < 0.001 | |||
| No | 206 (72) | 89 (84) | 117 (65) | |
| Yes | 80 (28) | 17 (16) | 63 (35) | |
| Vasoactive.drug.dose(mg), Median (Q1,Q3) | 21.15 (0, 355.75) | 5.5 (0, 258.3) | 30 (0, 453.9) | 0.019 |
| Vasoactive.drug.dose_24h(mg), Median (Q1,Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.838 |
| Morphine, n (%) | 0.008 | |||
| No | 242 (85) | 98 (92) | 144 (80) | |
| Yes | 44 (15) | 8 (8) | 36 (20) | |
| Esketamine, n (%) | 0.068 | |||
| No | 270 (94) | 104 (98) | 166 (92) | |
| Yes | 16 (6) | 2 (2) | 14 (8) | |
| Glucocorticoids.dose, Median (Q1,Q3) | 160 (40, 443.75) | 185 (40, 400) | 127.5 (40, 451.25) | 0.475 |
| Dopamine, n (%) | 0.033 | |||
| No | 204 (71) | 84 (79) | 120 (67) | |
| Yes | 82 (29) | 22 (21) | 60 (33) | |
| Norepinephrine, n (%) | 0.038 | |||
| No | 196 (69) | 81 (76) | 115 (64) | |
| Yes | 90 (31) | 25 (24) | 65 (36) | |
| Epinephrine, n (%) | 0.008 | |||
| No | 181 (63) | 78 (74) | 103 (57) | |
| Yes | 105 (37) | 28 (26) | 77 (43) | |
| Laboratory findings, Median (Q1, Q3) | ||||
| Sodium bicarbonate(mmol/L) | 24.9 (21.2, 30.5) | 25.5 (21.92, 31.73) | 24.6 (20.27, 30) | 0.226 |
| Hematocrit_min(%) | 30 (24, 36) | 32 (28.25, 37) | 28.5 (22, 35) | < 0.001 |
| Hemoglobin_min(g/L) | 99 (74, 117.75) | 106 (94, 121.75) | 89.5 (58.75, 114) | < 0.001 |
| Platelets_min(X10^9/L) | 138.5 (92, 195.5) | 170.5 (131, 240.75) | 115.5 (71.5, 169) | < 0.001 |
| Wbc_max(X10^9/L) | 13.46 (9.2, 19.05) | 11.88 (8.04, 16.04) | 14.63 (9.73, 20.92) | 0.002 |
| Bicarbonate_min(mmol/L) | 38.35 (24.77, 42.95) | 39.35 (35, 43.72) | 37.1 (22.06, 41.83) | 0.002 |
| Bicarbonate_max(mmol/L) | 46.75 (41.6, 50.8) | 47.3 (43.6, 51.08) | 46.05 (37.5, 50.3) | 0.08 |
| Bun_max(mg/dl) | 9.5 (9.5, 16.2) | 9.5 (9.5, 9.65) | 9.5 (9.5, 23) | 0.226 |
| Calcium_min(mg/dL) | 0.99 (0.92, 1.13) | 1 (0.95, 1.14) | 0.97 (0.9, 1.1) | 0.008 |
| Creatinine_max(umol/L) | 107 (70.25, 190.75) | 87 (65, 169.5) | 121 (74, 204) | 0.013 |
| Glucose_min(mmol/L) | 5.1 (4.1, 7.18) | 5.3 (4.3, 7.57) | 5 (4, 6.75) | 0.116 |
| Glucose_max(mmol/L) | 13.35 (8.93, 19.08) | 12.85 (9.1, 18.4) | 13.55 (8.88, 19.6) | 0.532 |
| Sodium_min(mmol/L) | 133 (128.48, 136) | 133.2 (130.1, 136.2) | 132.4 (128, 135.27) | 0.168 |
| Sodium_max(mmol/L) | 142.85 (137.72, 150.38) | 140.7 (137.43, 146.28) | 143.45 (138.07, 152.38) | 0.028 |
| Potassium_min(mmol/L) | 3.34 (3.03, 3.7) | 3.41 (3.23, 3.8) | 3.28 (2.98, 3.63) | 0.003 |
| Potassium_max(mmol/L) | 4.7 (4.16, 5.3) | 4.4 (4.1, 4.96) | 4.81 (4.23, 5.45) | 0.002 |
| Fibrinogen_min(g/L) | 3.26 (2.48, 4.23) | 3.54 (2.84, 4.5) | 3.08 (2.37, 3.96) | 0.001 |
| Inr_max | 1.19 (1.09, 1.44) | 1.15 (1.05, 1.26) | 1.26 (1.11, 1.63) | < 0.001 |
| Pt_max(s) | 12.8 (11.7, 15.5) | 12.6 (11.5, 14) | 13.3 (11.8, 17.02) | 0.029 |
| Ptt_max(s) | 32.15 (28.3, 37.1) | 30.2 (27.4, 34.48) | 34.35 (29.9, 39.32) | < 0.001 |
| PCT_max(ng/L) | 0.6 (0.14, 5.04) | 0.42 (0.06, 4.76) | 0.67 (0.2, 5.06) | 0.096 |
| BNP(pg/ml) | 225.5 (68.72, 566.75) | 194.5 (75.17, 518.25) | 232.5 (67.45, 576.75) | 0.732 |
| CK(U/L) | 109 (53.25, 340.75) | 72 (45, 164.25) | 161.5 (68, 651) | < 0.001 |
| CK_MB(U/L) | 14 (10, 22) | 12 (8, 19) | 15.1 (11, 25) | < 0.001 |
| CTnT(ug/L) | 0.05 (0.02, 0.37) | 0.03 (0.02, 0.1) | 0.1 (0.02, 1.54) | < 0.001 |
| CRP_max(mmol/L) | 10.2 (5.61, 19.17) | 7.86 (4.1, 12.85) | 12.35 (7.39, 23) | < 0.001 |
| Lac_0h(mmol/L) | 1.7 (1.22, 2.2) | 1.6 (1.2, 2.18) | 1.7 (1.3, 2.3) | 0.21 |
| Lac_6h(mmol/L) | 1.7 (1.3, 2.3) | 1.5 (1.2, 2.3) | 1.75 (1.3, 2.32) | 0.107 |
| Lac_12h(mmol/L) | 1.6 (1.3, 2.4) | 1.5 (1.2, 2.1) | 1.7 (1.37, 2.5) | 0.035 |
| Lac_18h(mmol/L) | 1.6 (1.3, 2.3) | 1.55 (1.2, 2.18) | 1.6 (1.3, 2.4) | 0.213 |
| Lac_24h(mmol/L) | 1.8 (1.3, 2.4) | 1.5 (1.2, 2.18) | 1.9 (1.4, 2.6) | 0.003 |
| Oxygenation.index_0h(mmHg) | 167.82 (102.99, 256.16) | 189.78 (125.7, 271.99) | 161.5 (98.73, 242.47) | 0.03 |
| Oxygenation.index_24h(mmHg) | 151.09 (98.15, 203.82) | 147.44 (105.48, 199.02) | 151.92 (97.87, 205.31) | 0.888 |
| Oxygenation.index_72h(mmHg) | 145.96 (100.45, 204.36) | 149.2 (108.52, 218.02) | 140.83 (92.6, 201.1) | 0.203 |
| Oxygenation.index_day5(mmHg) | 152.34 (99.21, 215.92) | 183.72 (112.53, 252.86) | 141.07 (86.9, 191.89) | < 0.001 |
Continuous variables are expressed as median and interquartile range (IQR), while categorical variables are presented as frequencies and percentages (%)
Abbreviations: CRRT Continuous renal replacement therapy, HR Heart rate, RR Respiratory rate, SBP Systolic blood pressure, DBP Diastolic blood pressure, WBC White blood cell, INR International normalized ratio, PTP Rothrombin Time, APTT Activated Partial Thromboplastin Time, PCT Procalcitonin, CRPC-reactive protein, BNPB-type natriuretic peptide, CK CreatineKinase, TNT Troponin T
Co-infection site and microbial type
Regarding the sites of co-infection, the incidence of gastrointestinal infections (16%), catheter-associated infections (12%), and skin and soft tissue infections (13%) was higher in the liver dysfunction group compared to the non-liver dysfunction group. However, there was no difference between the two groups in the incidence of urinary tract infections and intra-abdominal infections. Additionally, the liver dysfunction group was more likely to be infected with Pseudomonas aeruginosa (37%), Staphylococcus aureus (17%), and fungi (48%).
Treatment measures and medication use
We focused on the use of commonly used treatment techniques in the ICU for all patients, including mechanical ventilation (37%), continuous kidney replacement therapy (12%), and prone position ventilation to improve oxygenation in acute respiratory distress syndrome (18%). The utilization rate of these treatment methods in the liver dysfunction group is higher than that in the normal group.
The medication usage during the patient’s hospitalization was recorded, including vasoactive drugs: dopamine (29%), norepinephrine (31%), and adrenaline (37%); Sedative drugs: propofol (18%), midazolam (28%), and dexmedetomidine (21%); The median total doses of vasoactive drugs and glucocorticoids used were 21.15 mg (0,355.75 mg) and 160 mg (40, 443.75 mg), respectively. Due to the inclusion of critically ill patients in this study, the use rates of antiviral drugs Azvudine (19%) and Paxlovid (18%) were not high.
Across the cohort, abnormal laboratory findings included a decrease in hematocrit [30% (IQR, 24, 36%)], an increase in white blood cell count [13.46 * 10 ^ 9/L (IQR, 9.2, 19.05 * 10 ^ 9/L)], Activated partial thromboplastin time, as well as prothrombin time, were prolonged at 32.15 s (IQR, 28.3, 37.1 s) and 12.8 s (IQR, 11.7, 15.5 s), respectively. The level of inflammatory biomarkers in serum also increased, with 10.2 mg/dl of C-reactive protein (IQR, 5.61, 19.17 mg/dl) and 0.6 ng/ml of Calcitonin (IQR, 0.14, 5.04 ng/ml).
Single-factor analysis of all variables
Figures 1 and 2 represent the ROC curves of all univariate variables included in the study and the corresponding bar chart of AUC values, respectively. In our calculations, platelets had the highest predictive efficiency (AUC = 0.729), followed by serum creatine kinase level, international normalized ratio maximum, partial thromboplastin time maximum, and serum C-reactive protein concentration maximum.
Fig. 1.
ROC curves for each variable in predicting the occurrence of liver dysfunction during hospitalisation in COVID-19 patients
Fig. 2.
AUC values of all variables for predicting the occurrence of liver injury in patients with COVID-19 in the ICU
Correlation tests and covariance analysis of numerical variables
Figure 3 presents the correlation heatmap of continuous variables included in the study. Among them, “Lac_24h” (24-h lactate level) and “Lac_12h” (12-h lactate level) exhibit a strong positive correlation (depicted in dark red with large dots), indicating consistent trends in their values over time.
Fig. 3.
Heat map of correlations between continuous variables(*p < 0.05, **p < 0.01, *** p < 0.001)
Similarly, “CRP_max” (maximum C-reactive protein) and “PCT_max” (maximum procalcitonin) also show a strong positive correlation, possibly reflecting the synergistic changes of inflammatory markers in critically ill patients.
In contrast, a potential negative correlation is observed between “Oxygenation.index_0h” (oxygenation index at admission) and “Lac_0h” (lactate level at admission), suggesting that poorer oxygenation status is associated with higher lactate levels, which may reflect the impact of hypoxia on metabolism. Another negative correlation is noted between “Hemoglobin_min” (minimum hemoglobin) and “Lac_0h,” indicating a possible relationship between low hemoglobin levels and elevated lactate levels.
Some variables show correlations close to zero, such as “BMI” (body mass index) and “CRP_max,” suggesting no significant linear relationship between the two. Certain variables demonstrate similar correlation patterns, such as lactate-related variables (Lac_0h, Lac_6h, Lac_12h) and oxygenation indices (Oxygenation.index_0h, Oxygenation.index_24h, Oxygenation.index_72h). This may indicate a close clinical relationship among these variables.
Predictors of liver dysfunction in COVID-19 pneumonia patients in ICU
We selected lambda.1se as the optimal λ value (0.042) for screening variables. Variables such as mechanical ventilation, duration of mechanical ventilation, intestinal infection, tumor diseases, Pseudomonas aeruginosa, fungi, diastolic blood pressure, oxygen saturation, Azvudine, Paxlovid, minimum platelet count, minimum hematocrit, maximum CRP, and oxygenation index on day 5 were found to be associated with abnormal liver function during hospitalization ( Fig. 4).
Fig. 4.
14 related variables of liver injury in ICU patients with COVID-19 screened by LASSO regression analysis
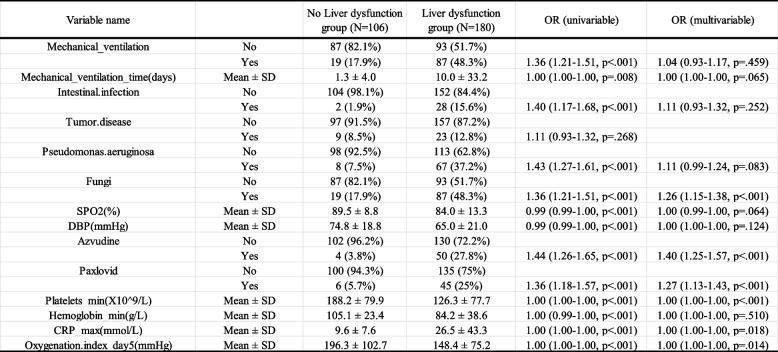
Univariate and multivariate logistic regression analyses were performed on the selected 14 variables. The results demonstrated that mechanical ventilation, duration of mechanical ventilation, gastrointestinal infection, Pseudomonas aeruginosa infection, fungal infection, diastolic blood pressure, oxygen saturation, use of azvudine or paxlovid, lowest platelet count, lowest hematocrit level, highest C-reactive protein (CRP) level, and oxygenation index on day 5 were independent predictors of liver dysfunction in ICU-admitted COVID-19 patients (P < 0.05, Table 2). The effect sizes and confidence intervals for these predictors are visualized in a forest plot. Specifically, patients requiring prolonged mechanical ventilation, experiencing gastrointestinal, Pseudomonas aeruginosa, or fungal infections, receiving azvudine or paxlovid during hospitalization, with lower platelet count and hematocrit levels, higher maximum CRP levels, or a lower oxygenation index on day 5 were more likely to develop liver dysfunction.
Table 2.
Multivariate logistic regression analysis of liver dysfunction during hospitalization of COVID-19 patients in ICU
Abbreviations: OR Odds ratio
Model evaluation and validation
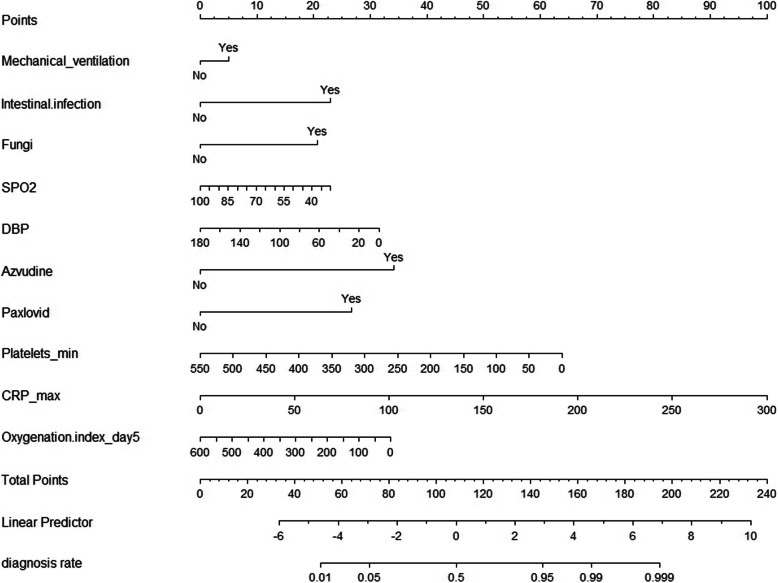
The concordance index (C-index) of the model was calculated as 0.910, indicating excellent predictive performance. To further simplify the model, we excluded statistically insignificant variables and potential confounders that could bias outcome estimation in the regression analysis. The final predictive model included ten variables: mechanical ventilation, intestinal infection, fungal infection, diastolic blood pressure, oxygen saturation, use of azvudine, use of Paxlovid, platelet minimum, CRP maximum, and oxygenation index on day 5. The recalculated C-index of the simplified model was 0.900. The sensitivity analysis demonstrated that after excluding patients with pre-existing liver disease, the key predictors identified in the primary analysis remained consistent. The sensitivity analysis demonstrated that after excluding patients with pre-existing liver disease, the key predictors identified in the primary analysis remained consistent, indicating the robustness of the model(Supplementary Table 1). The model’s discrimination remained strong, with the AUC value of the sensitivity analysis model calculated as 0.9425, which was comparable to the AUC of the full dataset, suggesting no significant impact from excluding this subgroup(Supplementary Fig. 1). Additionally, the calibration curve for the sensitivity analysis model closely aligned with the ideal red line, indicating that the predicted probabilities were well-matched with the actual outcomes(Supplementary Fig. 2). Furthermore, the Hosmer–Lemeshow calibration test yielded a P-value of 0.2663 (> 0.05), confirming that there was no significant difference between the predicted and observed outcomes, and thus, the model demonstrated good calibration. These findings further support the reliability and generalizability of the model even when patients with pre-existing liver disease are excluded. We used the selected variables to construct a nomogram for predicting the likelihood of liver dysfunction in critically ill COVID-19 patients (Fig. 5). In this nomogram, the first row, labeled “Points,” serves as a reference for the score assigned to each variable. For any given patient, the total score is calculated by summing the scores of all predictive variables. This total score is then mapped to the “Total Points” row, which is subsequently converted into a linear predictor (“Linear Predictor”) to estimate the probability of liver dysfunction. Patients were stratified into low-risk, moderate-risk, and high-risk groups based on their nomogram scores. Higher risk scores were significantly associated with worse clinical outcomes: Mortality: High-risk patients had a mortality rate of 55% compared to 33% in low-risk patients (p = 0.023); ICU Length of Stay: High-risk patients had a median ICU stay of 34.3 days compared to 16 days in low-risk patients (p < 0.01). Moreover, the nomogram demonstrated good predictive accuracy for severe liver dysfunction, defined as ALT/AST > 5 × ULN and INR (> 1.5), with an AUC of 0.8118(Fig. 6).
Fig. 5.
The final nomogram consisting of Mechanical ventilation, Intestinal infection, Fungal infection, DBP, Oxygen saturation, use of Azvudine, use of Paxlovid, Platelet minimum, CRP maximum, and Oxygenation index on day 5 is shown. Abbreviation: DBP, Diastolic blood pressure; CRP, C-reaction protein
Fig. 6.
The ROC curves for the prediction of severe liver dysfunction by nomogram scores
To validate the model’s performance, all participants were randomly assigned to a training and validation cohorts at a 1:1 ratio. To validate the performance of the model, we plotted the ROC curves for the training and test cohorts (Fig. 7) and calculated AUC values of 0.9284 and 0.8316, respectively, which indicated that the model had high predictive ability. Finally, the calibration plot is used to show the predicted probability of liver injury in 180 COVID-19 patients during hospitalization in the ICU and the actual observed incidence ratio according to the calculated score of the model (Fig. 8). The results show good consistency between the predicted probability of event occurrence and actual observations.
Fig. 7.
The ROC curves for the prediction model. A and B represent the ROC curves for predicting the incidence of liver dysfunction in the training and validation cohorts, respectively. Abbreviation: ROC, receiver operating characteristic curve
Fig. 8.
Calibration plot of the prediction model. The dotted line represents the performance of the nomogram, while the solid line corrects any bias in the nomogram. The dashed line is the reference line where the nomogram is located
The decision curve analysis revealed that the predictive model for liver dysfunction provided superior clinical utility compared to the “Treat All” and “Treat None” strategies(Fig. 9). Across the threshold probability range of 0.1 to 0.8, the model consistently demonstrated higher net benefit, with its optimal performance observed at a threshold probability of 0.5. Notably, the model outperformed alternative strategies in reducing unnecessary interventions for patients at low risk while ensuring appropriate care for high-risk individuals. These findings highlight the potential of the model to improve decision-making in ICU management for COVID-19 patients. However, the model’s performance declined slightly at thresholds above 0.7, suggesting limited utility in distinguishing very high-risk patients.
Fig. 9.
Decision Curve Analysis (DCA) for Predicting Liver Dysfunction in Critically Ill COVID-19 Patients
Discussion
As of March 10, 2023, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected nearly 676 million people worldwide, resulting in over 6.88 million deaths [18]. As research into the disease has progressed, it has become clear that in addition to attacking the lungs and causing varying degrees of respiratory symptoms, the virus can also involve multiple extra-pulmonary organs and cause systemic multi-organ damage. This phenomenon is more frequent in severely and critically ill patients and is associated with longer hospital stays and an increased risk of death [2, 19, 20]. Abnormal liver function is one of the most common extrapulmonary complications among patients with COVID-19. Not only are the abnormal levels of serum transaminase more common than other liver function indicators but also more related to the severity and prognosis of the disease [21].
Relevant clinical studies have shown that the occurrence of COVID-2019-associated liver injury may be influenced by a number of factors, and thus we expected to use relevant clinical indicators to predict the occurrence of liver function abnormalities in patients with COVID-2019. In this study, we screened important factors related to the occurrence of the COVID-2019 associated abnormal liver function with LASSO regression analysis. Next, the 14 variables obtained were included in the logistic regression model. Ultimately mechanical ventilation, intestinal infection, fungal infection, diastolic blood pressure, oxygen saturation, use of azvudine, use of paxlovid, highest CRP level, and oxygenation index on day 5 were identified and used to plot the nomogram of the model. The model was evaluated for discrimination and calibration in terms of the probability of abnormal liver function using the C-index and calibration plots, respectively, and the model proved to have good performance. The best-constructed prediction model was then validated in a validation dataset and the resulting AUC values suggest that the model is generalizable and provides benefit to clinical practice.
In this study, we identified several oxygenation-related indicators as significant predictive variables, highlighting the role of hypoxia and respiratory failure caused by COVID-19 as critical factors contributing to hypoxic liver injury in critically ill patients [22]. Furthermore, systemic stress, shock or heart failure, vascular endothelial damage, coagulopathy, and microthrombus formation can all lead to reduced hepatic blood flow and impaired organ perfusion [23, 24], culminating in ischemic and hypoxic liver injury characterized by a marked elevation in serum transaminase levels [25]. Notably, diastolic blood pressure, which plays a key role in maintaining mean arterial pressure, may serve as a more robust predictor of organ injury. The findings of this study align with previous research demonstrating the significance of hypoxia, inflammation, and organ perfusion parameters in predicting liver injury among critically ill patients. For instance, Yang [26] reported similar associations between mechanical ventilation parameters and liver dysfunction, which corroborates our results. However, this study uniquely integrates these variables into a predictive nomogram, providing a visual and individualized tool for assessing liver dysfunction risk. The nomogram developed in this study has significant clinical implications. By providing an individualized risk estimate, it offers a practical tool to assist clinicians in early identification of patients at high risk of liver injury, thereby enabling timely interventions. This could potentially improve outcomes by guiding decisions on hemodynamic optimization, ventilation strategies, and other supportive measures. Moreover, the nomogram simplifies the application of a complex logistic regression model, making it accessible for bedside use in intensive care units. Future integration of the nomogram into clinical decision support systems could further enhance its utility and impact. Unlike earlier studies that primarily focused on univariate predictors, our approach highlights the value of a comprehensive model incorporating multiple clinical and laboratory parameters.
Adverse conditions such as shock and hypoxia significantly amplify oxidative stress in the liver, triggering inflammation activation and cascade amplification, which further exacerbate liver injury [27]. Moreover, the frequent and prolonged use of mechanical ventilation and high levels of positive end-expiratory pressure (PEEP) in ICU settings may obstruct venous return, causing hepatic congestion and contributing to liver damage [28].The systemic inflammatory response induced by SARS-CoV-2 infection may also contribute to liver injury in severe COVID-19 patients. Elevated levels of infection-related markers such as C-reactive protein (CRP), procalcitonin (PCT), and erythrocyte sedimentation rate (ESR), along with pro-inflammatory cytokines such as TNF-α and IL-6, have been observed in COVID-19 patients. [29–31].The immune-mediated cytokine storm drives the release of large quantities of pro-inflammatory cytokines, exacerbating the non-specific immune inflammatory response in the liver and resulting in secondary liver injury. [2, 32].
We also observed that COVID-19 patients in the ICU were at risk of co-infection, with gastrointestinal infections being the most frequent (10.49%), a higher proportion than in the general ward [33]. The pathogens of co-infection are diverse, with studies showing that the most common co-bacterial infections are Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenza, while the most common co-fungal infections are Candida albicans and Aspergillus [34]. The use of more antibiotics or antifungal agents suggests a higher risk of liver damage, suggesting the need for a more comprehensive and integrated assessment and a more cautious dosing strategy [35]. Patients with severe COVID-19 usually present clinical manifestations including acute respiratory distress syndrome, respiratory failure, acute heart failure, and septic shock [29]. As the liver has a high demand for oxygen, it is more susceptible to decreased arterial oxygen saturation and inadequate hepatic perfusion resulting in hepatic ischemia–reperfusion injury and hepatocellular hypoxia. At the same time, hypoxia-induced oxidative stress promotes the release of several inflammatory factors, which further exacerbates hepatic cell death [36]. Therefore, the use of vascular drugs and monitoring of oxygenation indicators in such patients are important predictors of the development of liver injury.While the oxygenation index on day 5 was identified as a strong predictor of liver dysfunction, its inclusion raises concerns regarding the model’s early applicability. This predictor may limit the model’s use for immediate risk stratification and decision-making within the first few days of ICU admission. However, it offers valuable prognostic information for patients whose clinical trajectory has not improved by day 5, helping refine ongoing management strategies.To address this limitation, future research should aim to develop complementary models using predictors available within the first 24–48 h of admission. Such models could enhance the timeliness of clinical interventions while maintaining high predictive accuracy. Combining early and later-stage predictors may create a more flexible and comprehensive approach to risk assessment.
Finally, we focused on COVID-19 treatment drug induced liver injury. As Azvudine and Paxlovid are mainly recommended for use in adult patients with mild to moderate SARS-CoV 2 infection in the first 5 days and accompanied by high-risk factors for progression to severe illness [37–41], their usage rates in this study were relatively low [Azvudine (19%), Paxlovid (18%)], but we still observed that the use of the above two drugs increased the incidence of serum transaminase abnormalities. The association between Azvudine and liver dysfunction may be explained by its metabolism in the liver and its potential to induce mitochondrial toxicity. As a nucleotide analog, Azvudine can interfere with mitochondrial DNA replication by inhibiting mitochondrial DNA polymerase, leading to mitochondrial dysfunction, oxidative stress, and hepatocyte damage [42]. These mechanisms have been reported in studies examining other nucleotide analogs, suggesting a potential class effect [43]. Paxlovid, on the other hand, primarily metabolized by the cytochrome P450 enzyme CYP3A4, may pose a risk of hepatotoxicity due to its pharmacokinetic profile [44]. Co-administration with medications that are also CYP3A4 substrates can lead to competitive inhibition, resulting in elevated plasma concentrations of both drugs and subsequent liver enzyme abnormalities [45]. Protease inhibitors, a key component of Paxlovid, have previously been associated with transient elevations in liver enzymes during antiviral therapy, further supporting the need for vigilance when prescribing this medication [46]. Due to the side effects of liver damage caused by Azvudine and Paxlovid, and limited research on their use in patients with severe COVID-19, it is currently clear that the use of these drugs is not recommended in patients with existing severe liver dysfunction [47]. Given these potential risks, we recommend that clinicians closely monitor liver function in patients receiving Azvudine or Paxlovid, particularly those with pre-existing liver disease, concurrent use of hepatotoxic medications, or other risk factors. Regular liver function testing and dose adjustments based on hepatic tolerance may help mitigate the risk of adverse events and optimize treatment outcomes.
The prevalence of liver dysfunction observed in our study (62.9%) is notably higher than the rates reported in general hospitalized COVID-19 populations, where liver abnormalities are seen in approximately 20% to 50% of patients [6, 13, 48, 49]. This discrepancy likely reflects the critical nature of our study cohort, which consists solely of ICU-admitted patients. Previous studies have shown that critically ill COVID-19 patients are more prone to multiorgan dysfunction, systemic inflammation, and hypoxia, all of which contribute to an increased risk of liver injury [50–52]. Furthermore, while severe liver dysfunction remains rare in general COVID-19 populations, our results highlight its significant prevalence in ICU settings, where factors such as prolonged mechanical ventilation, high positive end-expiratory pressure (PEEP), and hemodynamic instability may exacerbate hepatic injury. These findings underscore the importance of monitoring liver function closely in critically ill COVID-19 patients and tailoring interventions to mitigate the risk of severe dysfunction. The additional analysis demonstrated that the nomogram is effective not only in predicting general liver dysfunction but also in identifying patients at risk of severe liver dysfunction. This capacity to stratify patients by risk severity enhances its clinical utility, particularly in guiding interventions for critically ill COVID-19 patients. Furthermore, the stratified analysis revealed a strong association between nomogram scores and key clinical outcomes, including mortality, ICU length of stay, and progression to liver failure. These findings underscore the nomogram’s value as a comprehensive tool for risk assessment and decision-making in ICU settings.
On December 15, 2022, China’s strategy for COVID-19 transferred from zero tolerance to the prevention of severe COVID-19. However, after policy changes, there was still a rapid increase in the number of infections and hospitalizations, which posed challenges to hospitals and medical workers. Referring to a large number of existing Observational study, we have listed many risk factors for liver injury in COVID-19 patients. Based on the patients admitted to the ICU in a major hospital in Tianjin after December 2022, we have constructed a prediction model for liver dysfunction, which may help clinicians to identify those patients with abnormal liver function early and reduce their progression to severe liver through timely interventions injury or even liver failure through timely intervention, with the aim of improving patient prognosis.
This study has some limitations. First, the single-center design and relatively small sample size limit the generalizability of our findings. The patient cohort, consisting exclusively of critically ill COVID-19 patients, may not represent the broader population of COVID-19 patients with varying disease severities. Second, to ensure the generalizability of the model, we used elevated serum aminotransferases, which are most commonly manifested in COVID-19 patients with abnormal liver function, as an evaluation indicator. This may result in the omission of some patients whose main manifestations are abnormalities in other indicators (such as ALP, GGT, TBil, ALB, etc.), and this model needs further improvement to increase its practicality. Thirdly, this study lacks external validation, which is essential for assessing the generalizability and reliability of the model in broader clinical settings. The absence of validation in diverse patient populations and healthcare environments may limit the immediate applicability of the nomogram in other clinics or hospitals. Future research should prioritize multi-center studies involving larger, more diverse cohorts to rigorously validate and refine the model. Furthermore, integrating dynamic predictors, such as time-dependent variations in clinical parameters, could enhance the model’s predictive performance and its clinical utility across varying contexts.
Above all, this study provides a novel, clinically relevant tool for predicting liver dysfunction in critically ill COVID-19 patients in ICU. External validation and further refinement will be crucial to ensure its broad applicability and impact on patient care.
Conclusion
By establishing the prediction model and nomogram of abnormal liver function during hospitalization for COVID-19 patients in ICU, we can find abnormal liver function as soon as possible and prevent the possibility of further liver failure. The performance of this model has been validated, which is helpful for the clinical practice of medical workers.
Supplementary Information
Supplementary Material 1. Figure 1. ROC Curve for Sensitivity Analysis: Excluding Patients with Pre-existing Liver Disease.
Supplementary Material 2. Figure 2. Calibration Curve for Sensitivity Analysis: Excluding Patients with Pre-existing Liver Disease.
Supplementary Material 3. Table1. Comparison of Key Predictors Between Full Model and Sensitivity Analysis.
Abbreviations
- CRRT
continuous renal replacement therapy
- HR
heart rate
- RR
respiratory rate
- SBP
systolic blood pressure
- DBP
Diastolic blood pressure
- WBC
white blood cell
- INR
International normalized ratio
- PT
Prothrombin Time
- APTT
Activated Partial Thromboplastin Time
- PCT
procalcitonin
- CRP
C-reactive protein
- BNP
B-type natriuretic peptide
- CK
Creatine Kinase
- TNT
Troponin T
- LASSO
Least Absolute Shrinkage and Selection Operator
Authors’ contributions
ZW wrote the main manuscript text and LZ prepared figures. All authors reviewed the manuscript.
Funding
This work was supported by the grant from the Tianjin “the Belt and Road” Joint Laboratory Project, 24PTLYHZ00200); Tianjin Science and Technology Innovation Talent Training Project, 24JRRCRC00160);Tianjin Health Research Project, TJWJ2024XK006); Tianjin Medical University General Hospital Clinical Research Program, 22ZYYLCCG05); China International Medical Exchange Foundation Cardiovascular Multidisciplinary Integrated Thinking Research Fund Project, N-2021–15-8).
Data availability
The data that support the findings of this study are available on request from the corresponding author, [KX],upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (Approval Number: IRB2022-YX-268–01) on December 29, 2022. All procedures involving human participants adhered to the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Given the retrospective nature of this study and the use of anonymized medical records, the Medical Ethics Committee approved a waiver of written informed consent for patients with COVID-19. The waiver was granted as the research involved no more than minimal risk to the participants, and the data were anonymized to ensure privacy and confidentiality.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/.
- 2.Ning Q, Wu D, Wang X, Xi D, Chen T, Chen G, Wang H, Lu H, Wang M, Zhu L, et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct Target Ther. 2022;7(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed). 2020;368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed]
- 8.Bertolini A, van de Peppel IP, Bodewes F, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology (Baltimore, MD). 2020;72(5):1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology (Baltimore, MD). 2020;72(2):389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clinical gastroenterology and hepatology. 2020;18(10):2378-2379.e2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology (Baltimore, Md). 2020;72(3):807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, et al. Clinical characteristics and risk factors of liver injury in COVID-19: a retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14(5):723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver international : official journal of the International Association for the Study of the Liver. 2020;40(6):1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi X, Liu C, Jiang Z, Gu Y, Zhang G, Shao C, Yue H, Chen Z, Ma B, Liu D, et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73(2):455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibshirani R. Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological). 1996;58(1):267–88. 10.1111/j.2517-6161.1996.tb02080.x.
- 18.The COVID-19 Map. https://coronavirus.jhu.edu/map.html.
- 19.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45(8): 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40(6):1278–81. [DOI] [PubMed] [Google Scholar]
- 23.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med. 2000;109(2):109–13. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X-J, Cheng X, Yan Z-Z, Fang J, Wang X, Wang W, Liu Z-Y, Shen L-J, Zhang P, Wang P-X, et al. An ALOX12–12-HETE–GPR31 signaling axis is a key mediator of hepatic ischemia–reperfusion injury. Nat Med. 2018;24(1):73–83. [DOI] [PubMed] [Google Scholar]
- 28.Chen P, Zhou B. Clinical Characteristics of COVID-19 in Patients With Liver Injury. Clin Gastroenterol Hepatol. 2020;18(12):2846–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, Pistello M, Guarracino F, Ghiadoni L, Forfori F, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76(4):1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57(4): 106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfizer Inc. Fact sheet for healthcare providers: emergency use authorization for Paxlovidtm [PDF]. U.S. Food and Drug Administration. 2021. Revised November 2024. Retrieved from https://labeling.pfizer.com/ShowLabeling.aspx?id=17109. Accessed 20 Dec 2024.
- 39.Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. The Journal of infection. 2023;87(2):e24-e27. [DOI] [PMC free article] [PubMed]
- 40.Zhang JL, Li YH, Wang LL, Liu HQ, Lu SY, Liu Y, Li K, Liu B, Li SY, Shao FM, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, Wang H, Cui G, Liu Y, Wang J, et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2020;7(19):e2001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar N, Delu V, Ulasov I, Kumar S, Singh RK, Kumar S, Shukla A, Patel AK, Yadav L, Tiwari R, et al. Pharmacological Insights: Mitochondrial ROS Generation by FNC (Azvudine) in Dalton’s Lymphoma Cells Revealed by Super Resolution Imaging. Cell Biochem Biophys. 2024;82(2):873–83. [DOI] [PubMed] [Google Scholar]
- 43.Kumar N, Delu V, Shukla A, Singh RK, Ulasov I, Fayzullina D, Kumar S, Patel AK, Yadav L, Tiwari R, et al. Safety assessment of a nucleoside analogue FNC (2’-deoxy-2’- β-fluoro-4’-azidocytidine ) in Balb/c Mice: acute toxicity study. Asian Pac J Cancer Prev. 2023;24(6):2157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loos NHC, Beijnen JH, Schinkel AH. The mechanism-based inactivation of CYP3A4 by Ritonavir: what mechanism? Int J Mol Sci. 2022;23(17):9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sathish JG, Bhatt S, DaSilva JK, Flynn D, Jenkinson S, Kalgutkar AS, Liu M, Manickam B, Pinkstaff J, Reagan WJ, et al. Comprehensive Nonclinical Safety Assessment of Nirmatrelvir Supporting Timely Development of the SARS-COV-2 Antiviral Therapeutic, Paxlovid™. Int J Toxicol. 2022;41(4):276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, Pozniak A, Boffito M, Waters L, Burger D, et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin Pharmacol Ther. 2022;112(6):1191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamb YN. Nirmatrelvir Plus Ritonavir: First Approval. Drugs. 2022;82(5):585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74(6):1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen LY, Chu HK, Bai T, Tu SJ, Wei Y, Li ZL, Hu LL, Zhu R, Zhang L, Han CQ, et al. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis. 2020;21(9):512–8. [DOI] [PubMed] [Google Scholar]
- 51.Huang H, Chen S, Li H, Zhou XL, Dai Y, Wu J, Zhang J, Shao L, Yan R, Wang M, et al. The association between markers of liver injury and clinical outcomes in patients with COVID-19 in Wuhan. Aliment Pharmacol Ther. 2020;52(6):1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Figure 1. ROC Curve for Sensitivity Analysis: Excluding Patients with Pre-existing Liver Disease.
Supplementary Material 2. Figure 2. Calibration Curve for Sensitivity Analysis: Excluding Patients with Pre-existing Liver Disease.
Supplementary Material 3. Table1. Comparison of Key Predictors Between Full Model and Sensitivity Analysis.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, [KX],upon reasonable request.