Abstract
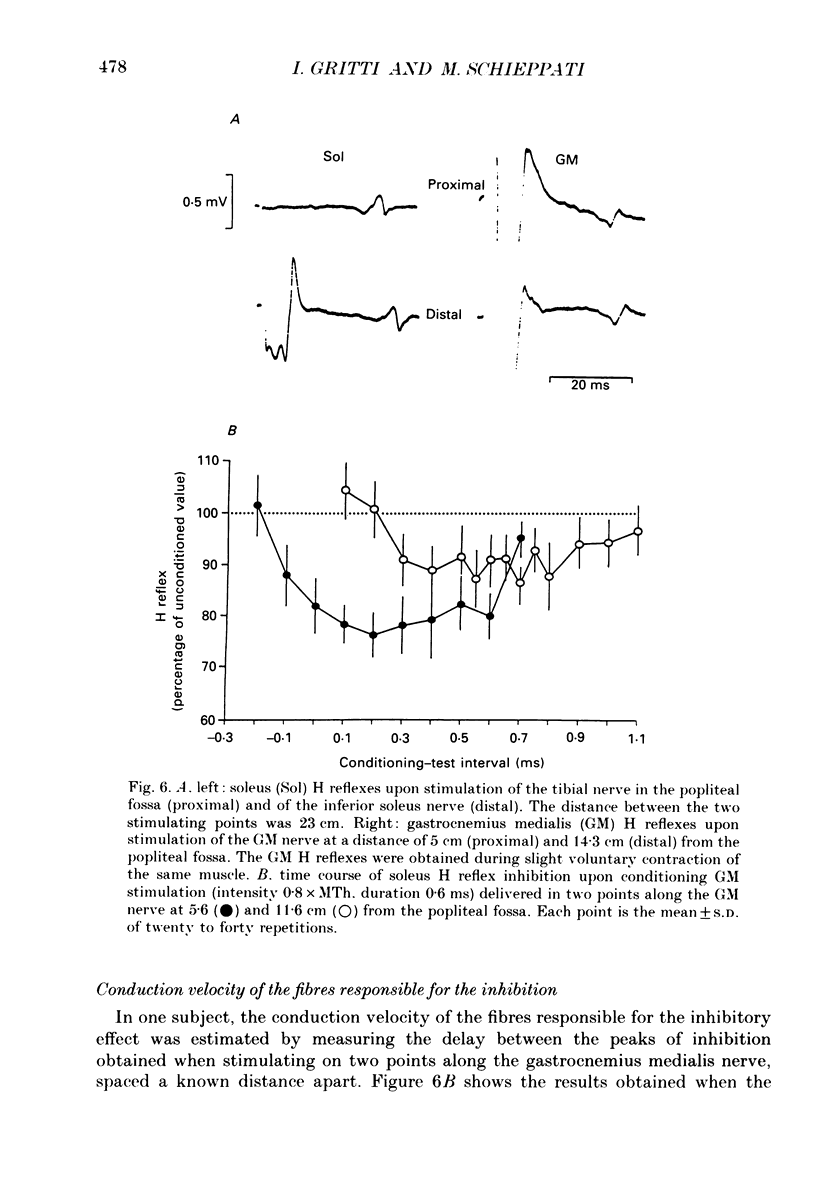
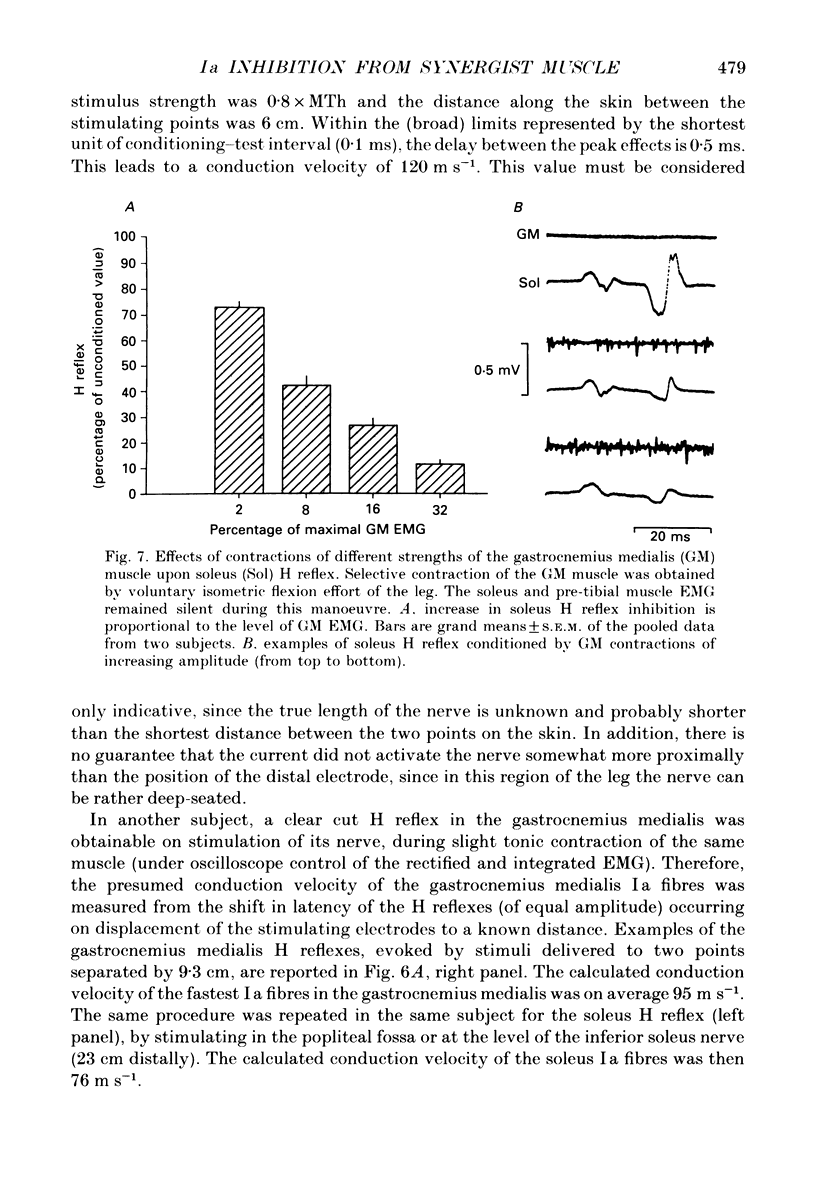
1. The possibility that the Ia afferent fibres from the gastrocnemius medialis muscle could be responsible for a decrease in excitability of the soleus motor pool was investigated. 2. The soleus H reflex, evoked by tibial nerve stimulation in the popliteal fossa, was conditioned by a single stimulus to the gastrocnemius medialis nerve at various stimulus intensities and conditioning-test intervals. Care was taken to avoid spread of current from the conditioning stimulus to the tibial nerve, and the results obtained by surface stimulation were compared with those obtained by stimulation through a needle whose tip was positioned closer to the nerve. 3. Stimulation of the gastrocnemius medialis nerve induced two short-lasting periods of inhibition in the soleus H reflex, peaking at about 0 and 5 ms of conditioning-test delay. The early inhibition could begin at a stimulus strength as low as 0.5 x MTh (the Motor Threshold). The later inhibition appeared on greater stimulus strength than the earlier. 4. Prolonged vibration of the Achilles tendon abolished the capability of the conditioning stimulus to induce the short-latency inhibition of the soleus H reflex. 5. By stimulating the gastrocnemius medialis nerve at two points separated by a known distance, the conduction velocity of the fibres responsible for the early inhibition was estimated, and found to be around 100 m s-1. 6. Isometric leg flexion, accomplished by tonic activation of gastrocnemius medialis and lateralis but not soleus, was able to induce an inhibition of the soleus H reflex even at very low levels of gastrocnemius electromyographic activity. 7. These findings strongly suggest the existence of an inhibitory effect of primary spindle afferent fibres from the gastrocnemius medialis muscle onto the soleus motor pool. This is not unexpected, since the gastrocnemius medialis muscle can be either agonist or antagonist to the soleus muscle in the performance of different movements.
Full text
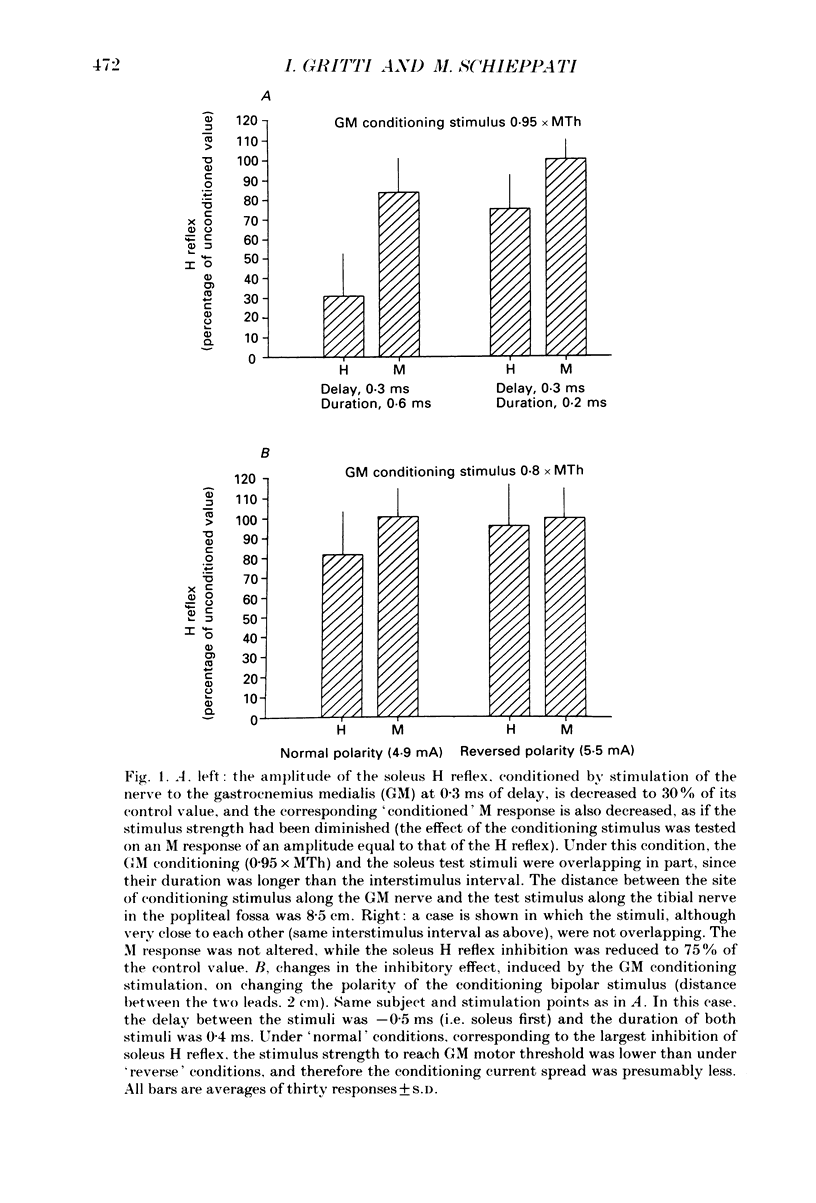
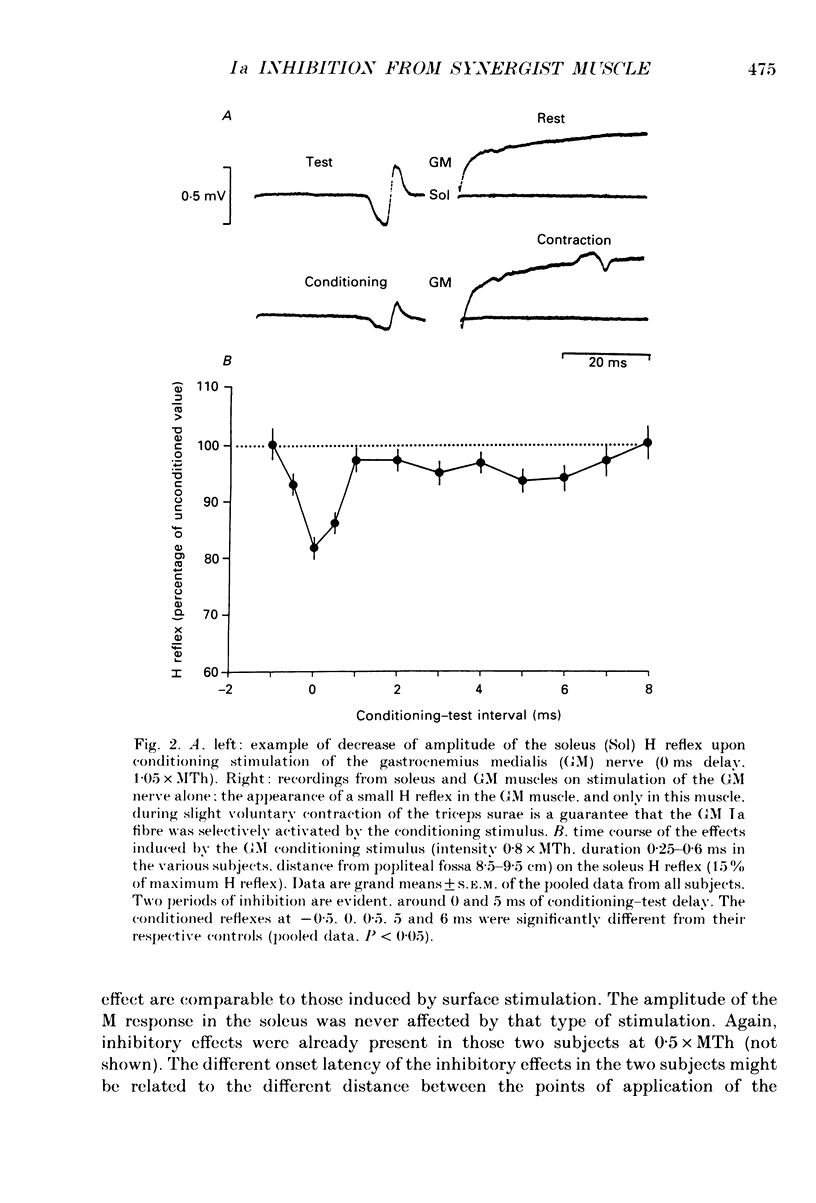
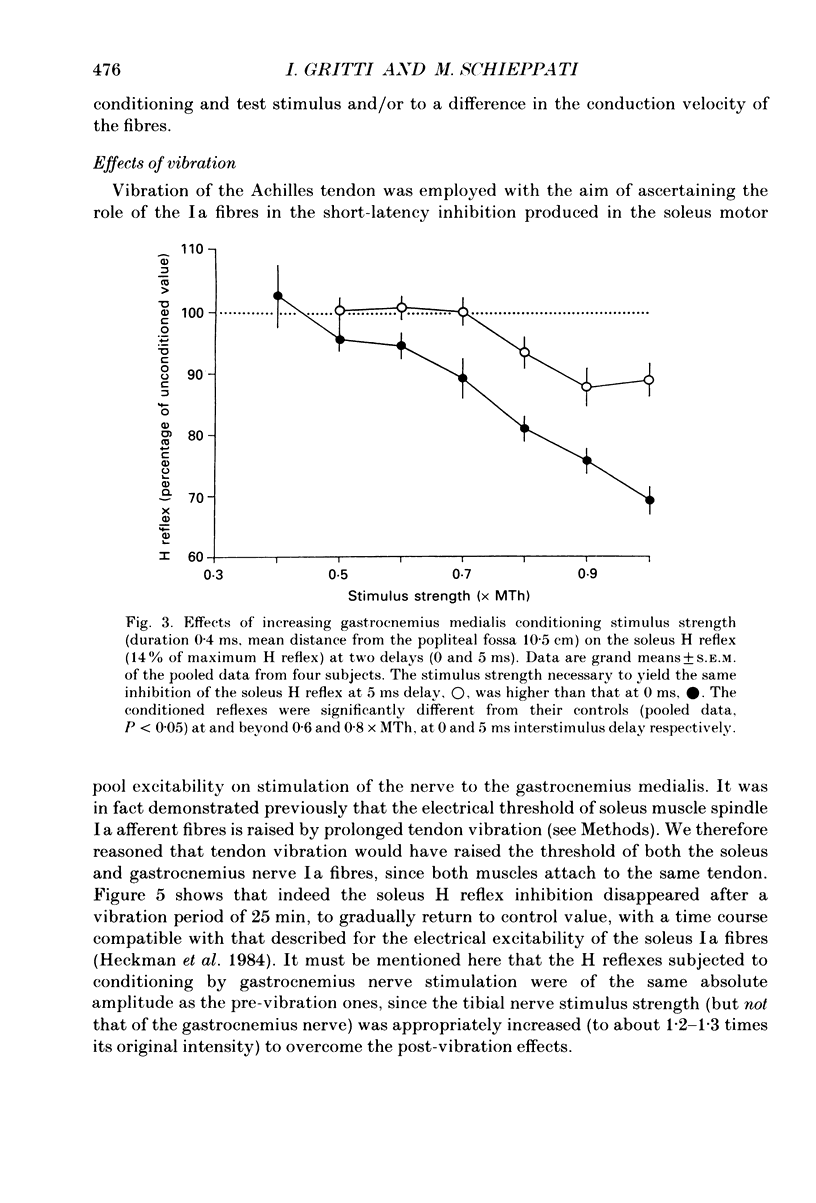
PDF




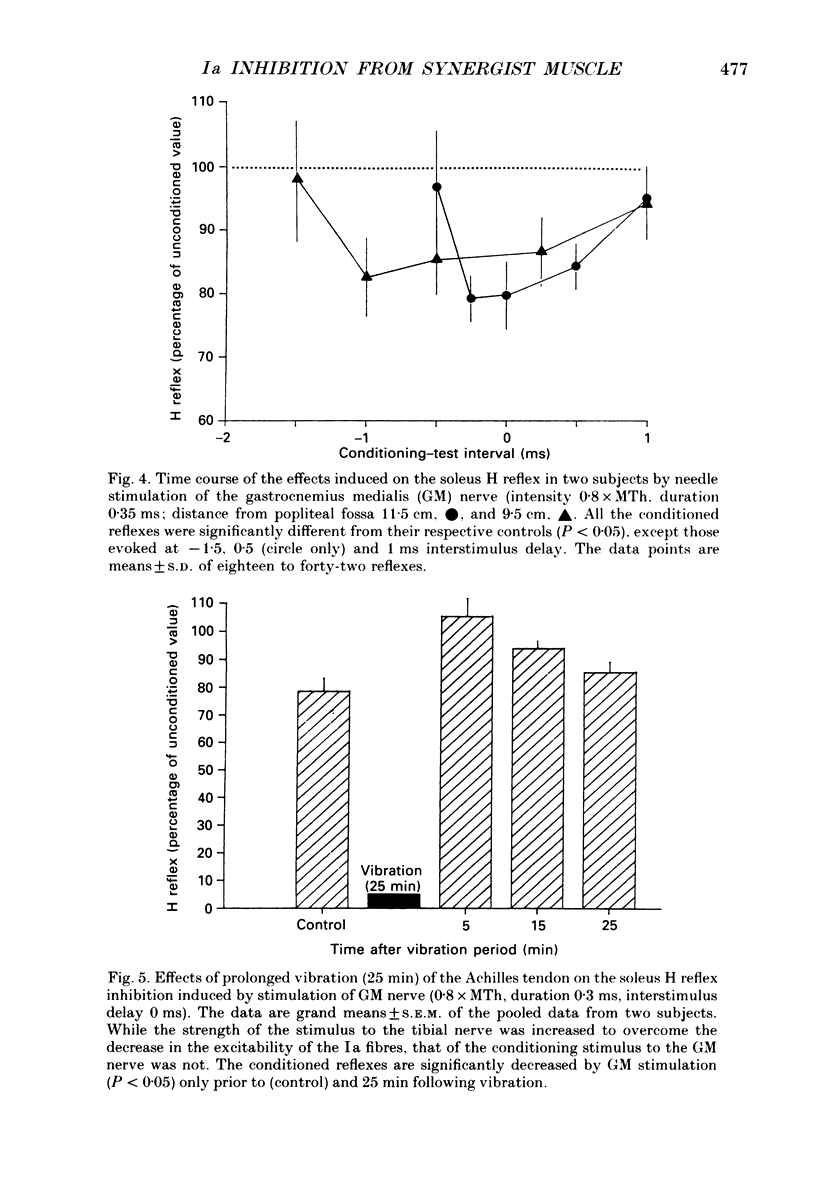





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouaziz Z., Bouaziz M., Hugon M. Modulation of soleus electromyogram during electrical stimulation of medial gastrocnemius nerve in man. Electromyogr Clin Neurophysiol. 1975 Jan-Apr;15(1):31–41. [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984 Sep;52(3):435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983 Jun;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Katz R., Pierrot-Deseilligny E. A re-evaluation of the pattern of group I fibre projections in the human lower limb on using randomly alternated stimulations. Exp Brain Res. 1984;56(1):193–195. doi: 10.1007/BF00237457. [DOI] [PubMed] [Google Scholar]
- Gerilovsky L., Gydikov A., Radicheva N. Changes in the shape of the extraterritorial potentials of tonic motor units, M- and H-responses of triceps surae muscles at different muscle lengths and under conditions of voluntary activation. Exp Neurol. 1977 Jul;56(1):91–101. doi: 10.1016/0014-4886(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972 Mar 24;175(4028):1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C., Stark L. Interactions between voluntary and postural mechanisms of thehuman motor system. J Neurophysiol. 1970 May;33(3):365–381. doi: 10.1152/jn.1970.33.3.365. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Stretch and Hoffmann reflexes during phasic voluntary contractions of the human soleus muscle. Electroencephalogr Clin Neurophysiol. 1978 May;44(5):553–561. doi: 10.1016/0013-4694(78)90122-0. [DOI] [PubMed] [Google Scholar]
- Gravel D., Arsenault A. B., Lambert J. Soleus-gastrocnemius synergies in controlled contractions produced around the ankle and knee joints: an EMG study. Electromyogr Clin Neurophysiol. 1987 Oct-Nov;27(6-7):405–413. [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L. F., Nielsen R. P., Heckman C. J., Hutton R. S. Tendon vibration-induced inhibition of human and cat triceps surae group I reflexes: evidence of selective Ib afferent fiber activation. Exp Neurol. 1986 Nov;94(2):333–347. doi: 10.1016/0014-4886(86)90107-x. [DOI] [PubMed] [Google Scholar]
- Hayward L., Breitbach D., Rymer W. Z. Increased inhibitory effects on close synergists during muscle fatigue in the decerebrate cat. Brain Res. 1988 Feb 2;440(1):199–203. doi: 10.1016/0006-8993(88)91178-x. [DOI] [PubMed] [Google Scholar]
- Heckman C. J., Condon S. M., Hutton R. S., Enoka R. M. Can Ib axons be selectively activated by electrical stimuli in human subjects? Exp Neurol. 1984 Dec;86(3):576–582. doi: 10.1016/0014-4886(84)90090-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudina L. P., Pantseva R. E. Recurrent inhibition of firing motoneurones in man. Electroencephalogr Clin Neurophysiol. 1988 Feb;69(2):179–185. doi: 10.1016/0013-4694(88)90213-1. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Mark R. F., Coquery J. M., Paillard J. Autogenetic reflex effects of slow or steady stretch of the calf muscles in man. Exp Brain Res. 1968;6(2):130–145. doi: 10.1007/BF00239167. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A., Romanò C., Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989 Feb;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A., Schieppati M. Postural adjustments associated with voluntary contraction of leg muscles in standing man. Exp Brain Res. 1988;69(3):469–480. doi: 10.1007/BF00247301. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Katz R., Hultborn H. Functional organization of recurrent inhibition in man: changes preceding and accompanying voluntary movements. Adv Neurol. 1983;39:443–457. [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Robinson K. L., McComas A. J., Belanger A. Y. Control of soleus motoneuron excitability during muscle stretch in man. J Neurol Neurosurg Psychiatry. 1982 Aug;45(8):699–704. doi: 10.1136/jnnp.45.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanò C., Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987 Sep;390:271–284. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28(4):345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]


