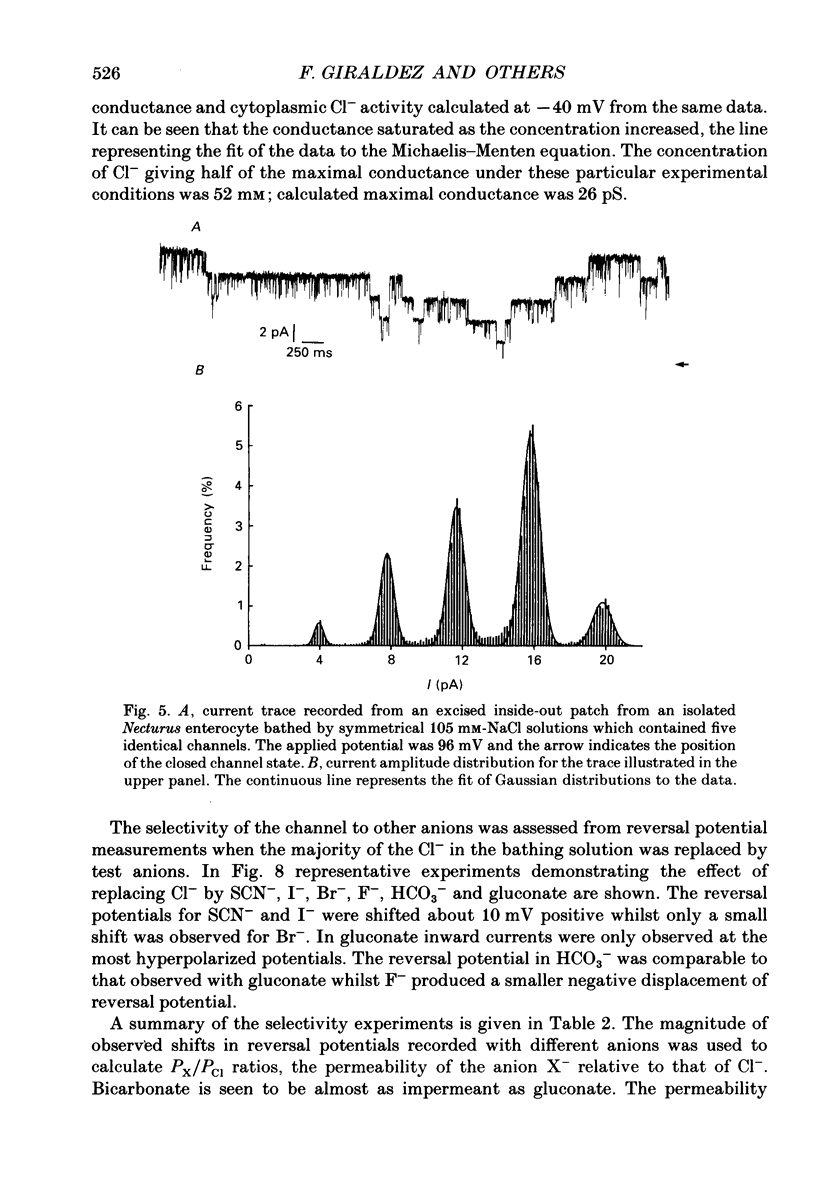
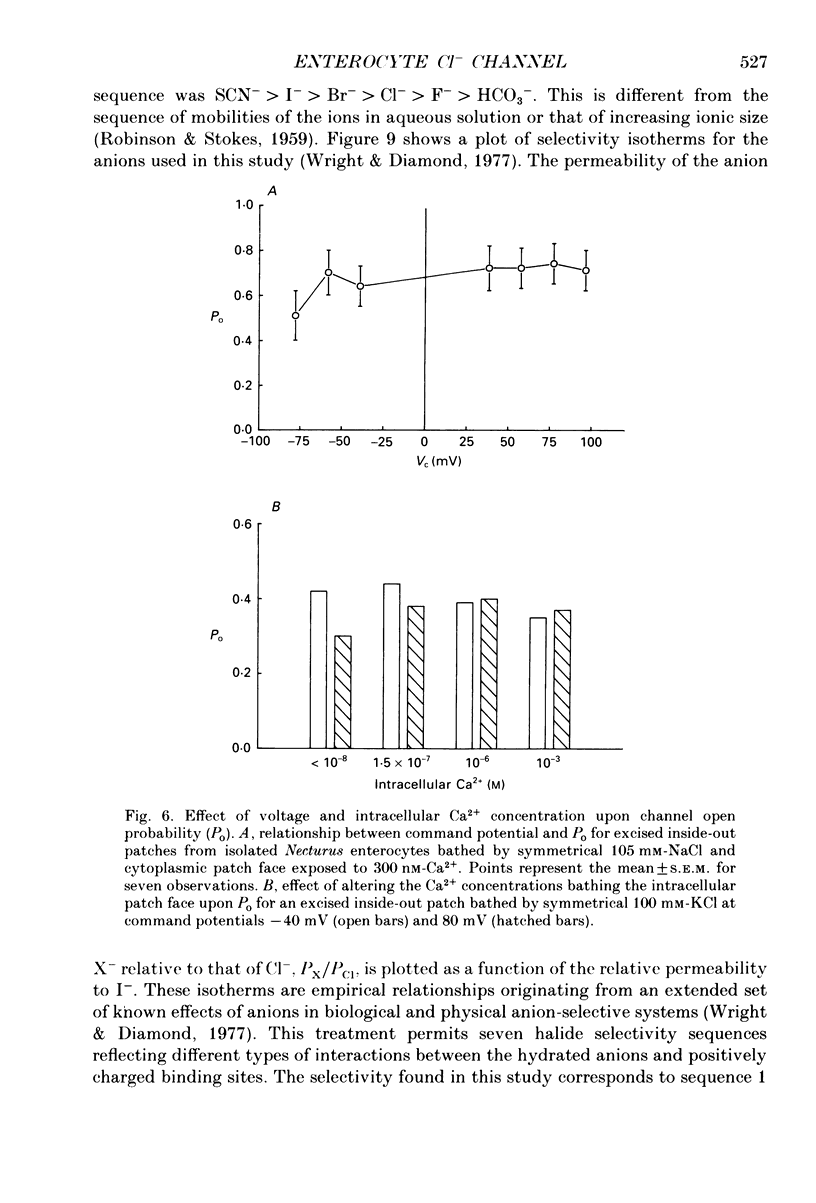
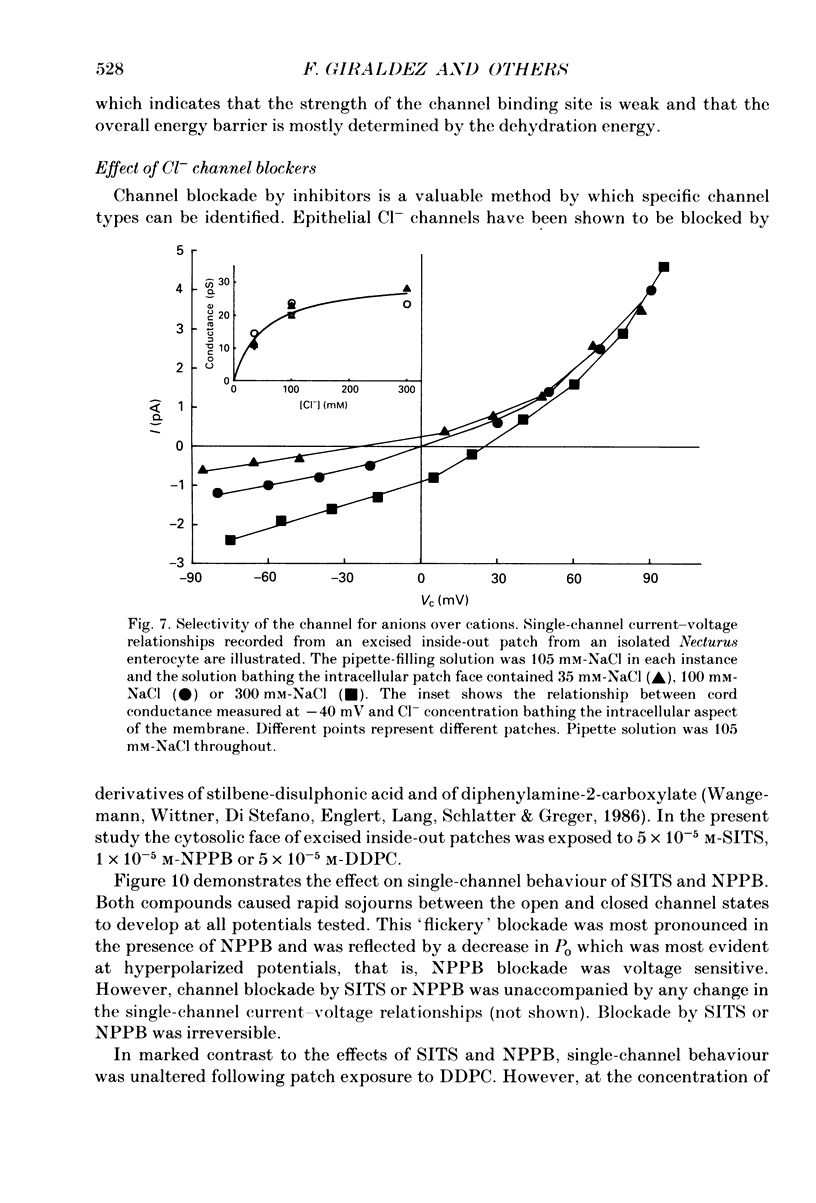
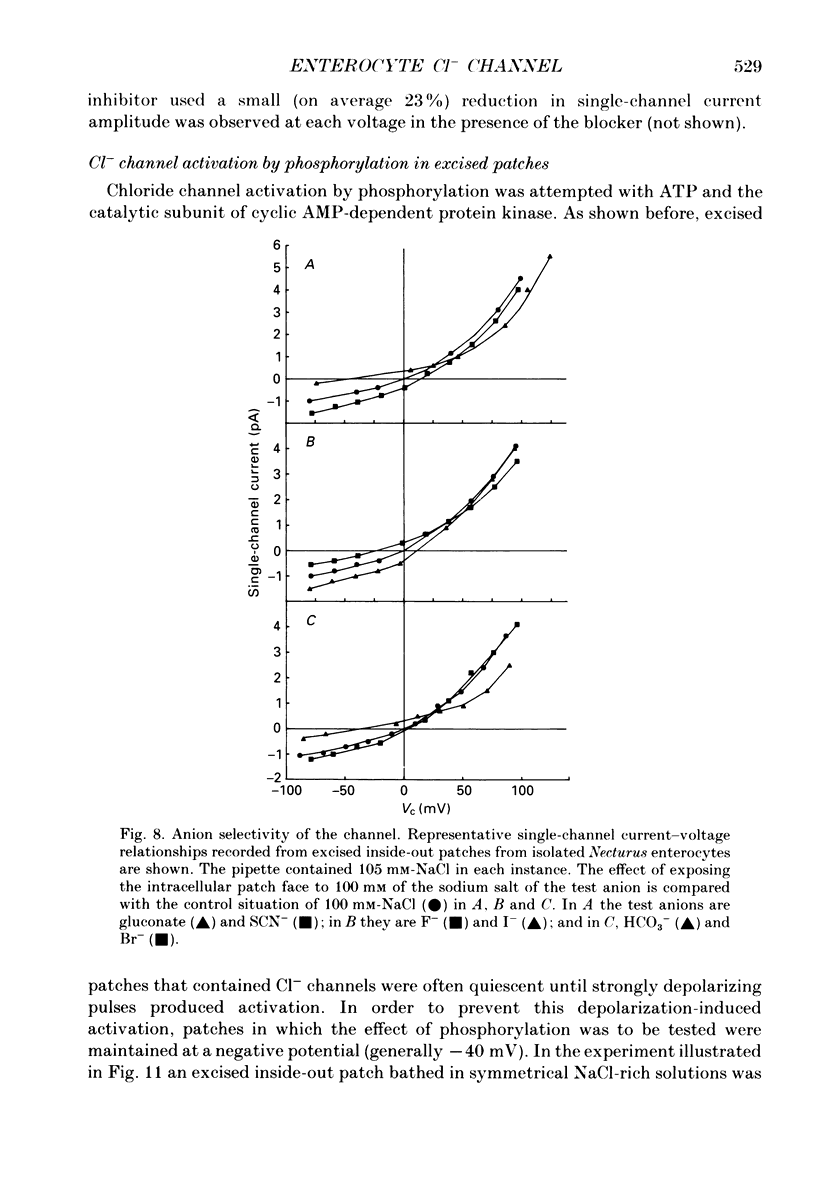
Abstract
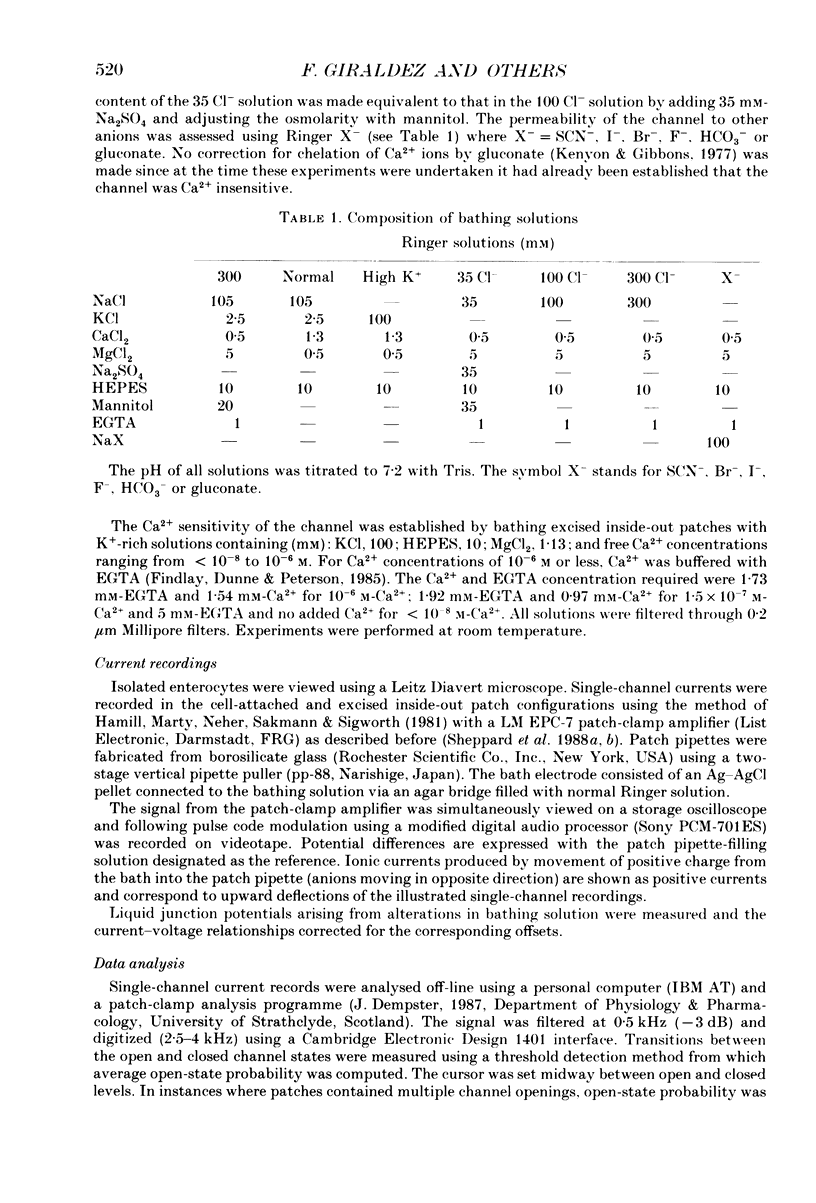
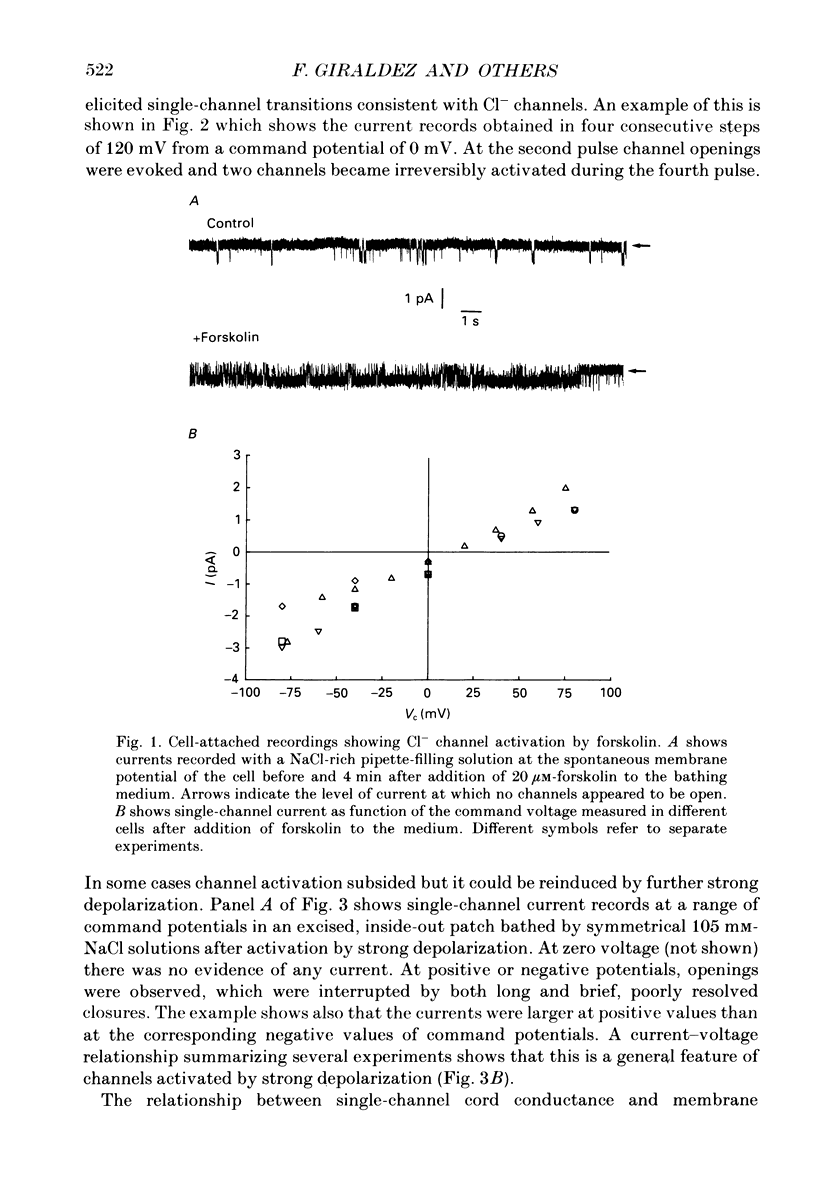
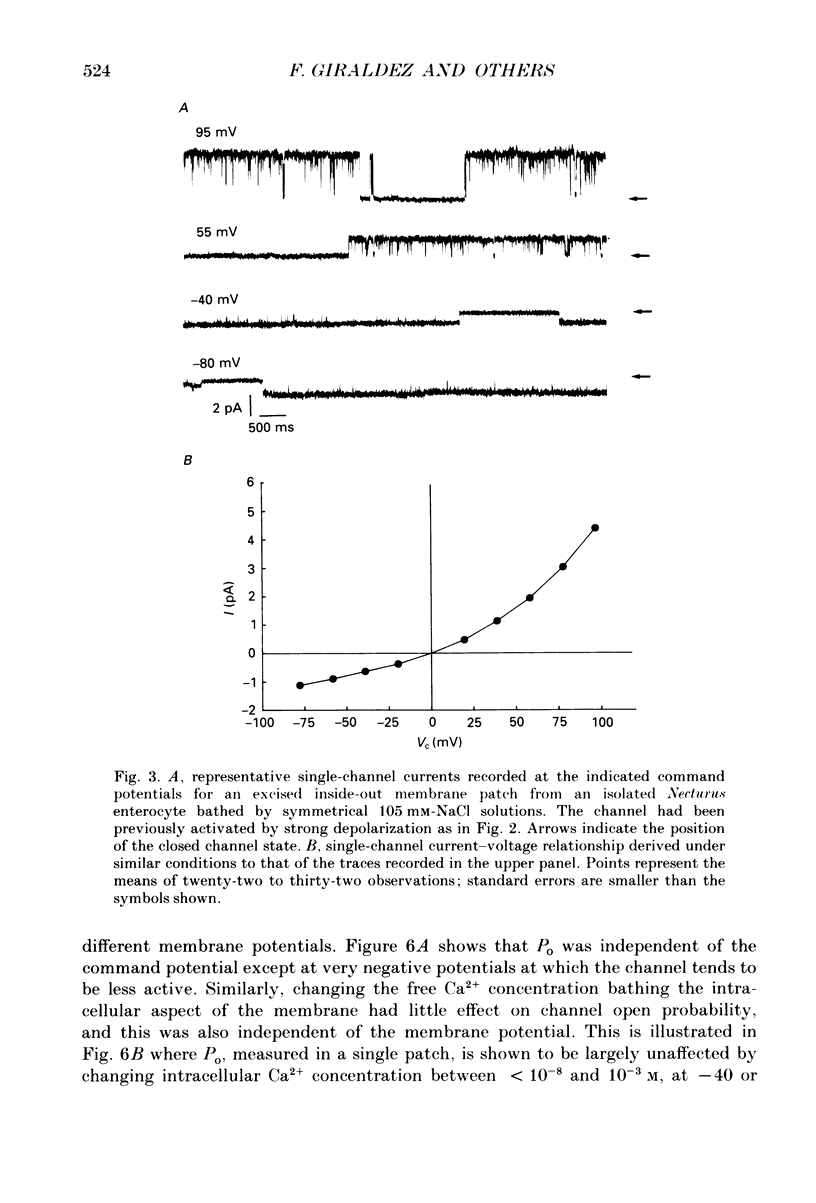
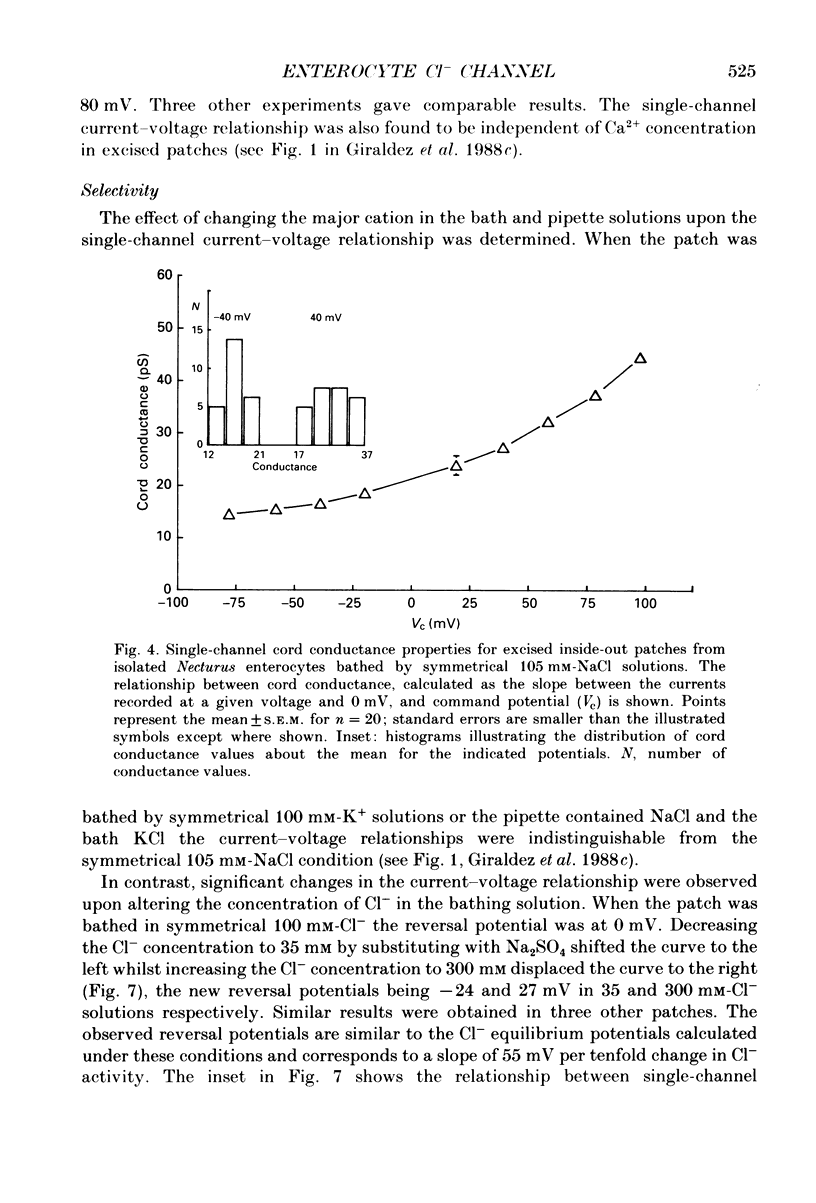
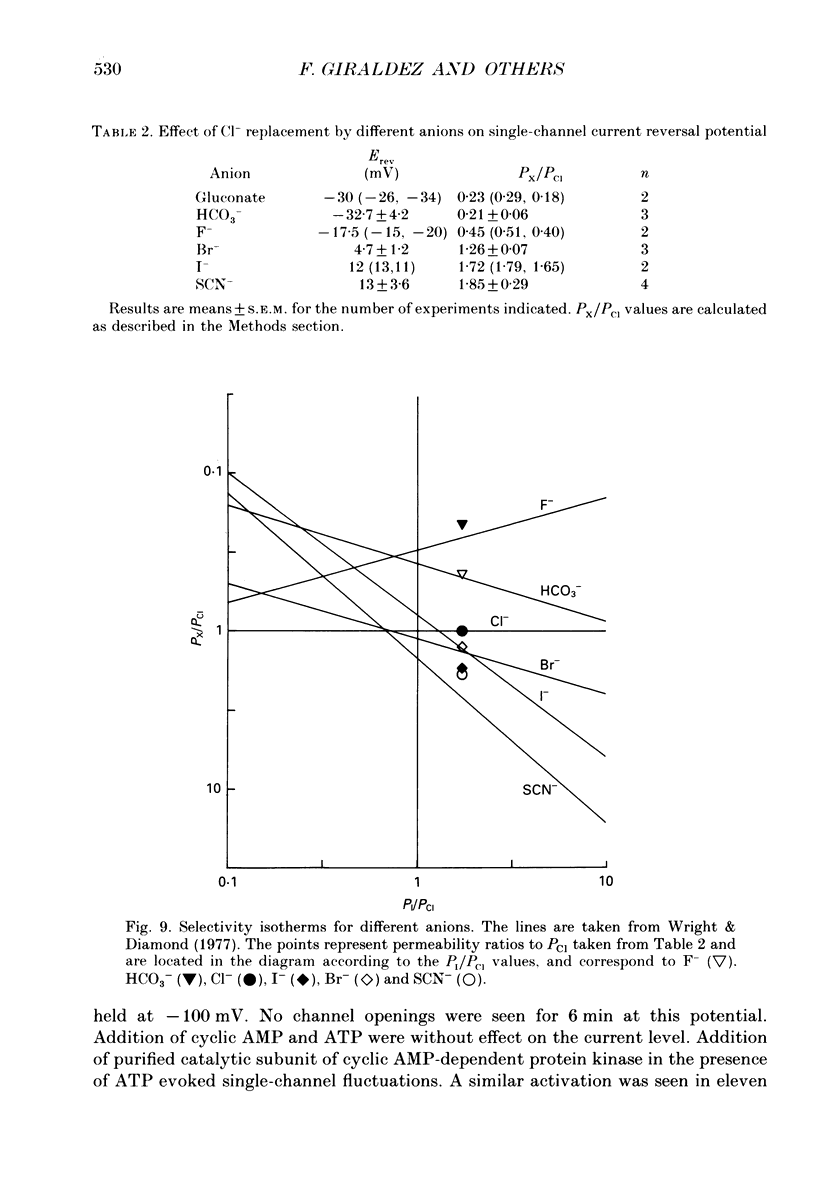
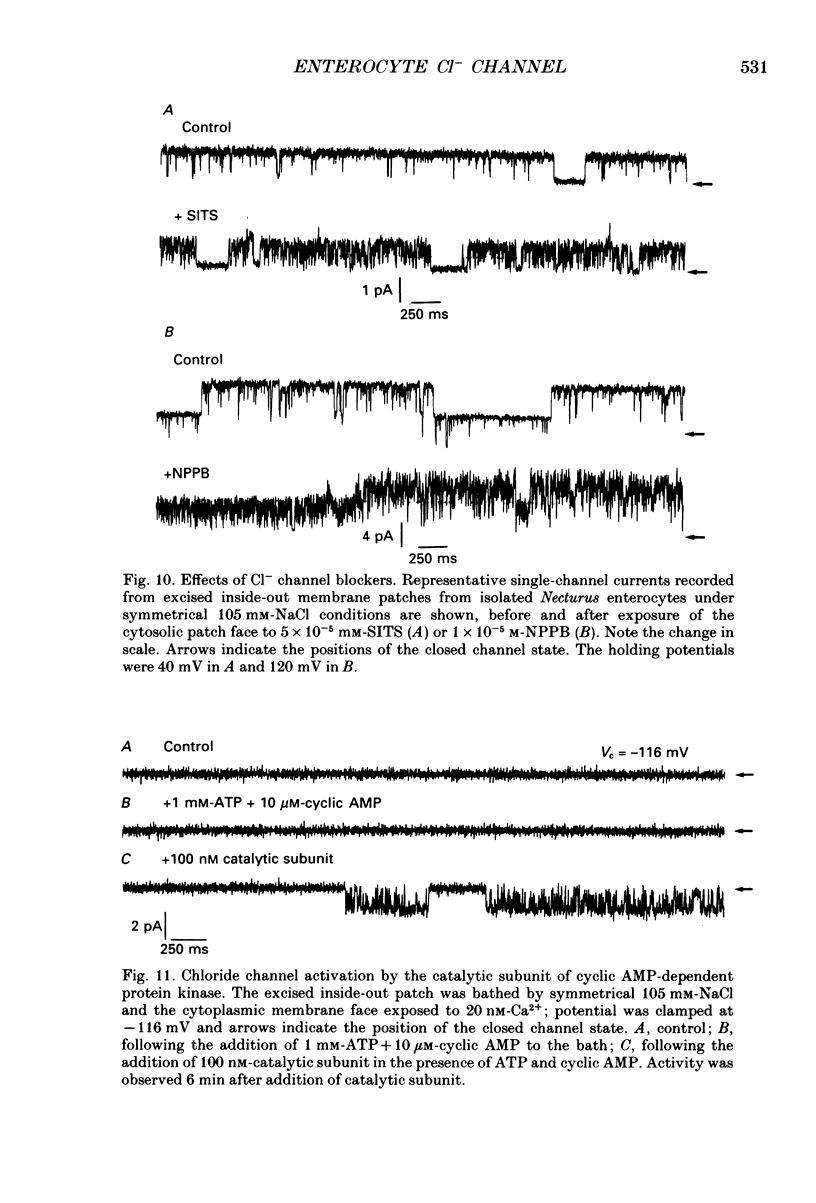
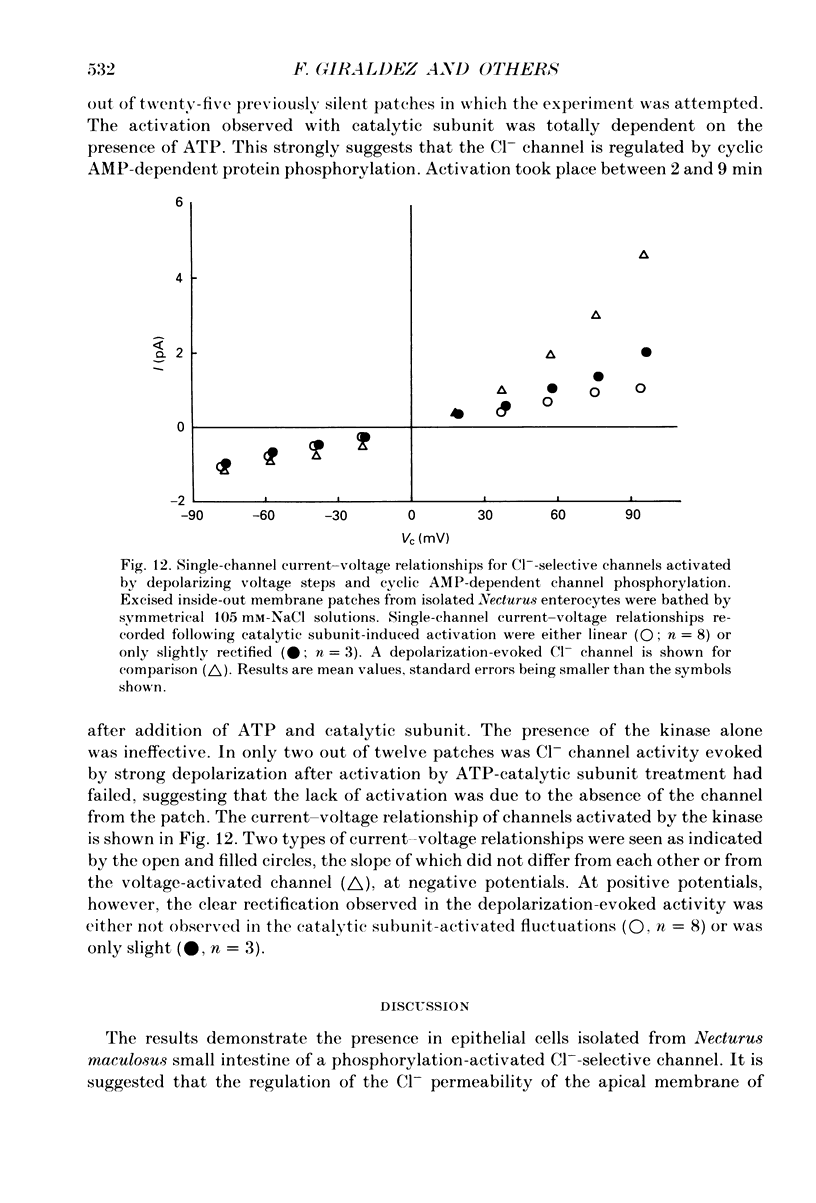
1. The cell-attached and excised inside-out configurations of the patch-clamp technique were employed to probe isolated enterocytes of Necturus maculosus for the presence of Cl(-)-selective channels. 2. Chloride-selective channels were rarely observed unless cells were previously stimulated by agonists that raise cyclic AMP. In cell-attached patches forskolin (20 microM) or dibutyryl cyclic AMP 2 mM) evoked single-channel activity that reversed, depending on the cell, between 9 and 27 mV positive to the spontaneous membrane potential. This is close to the Cl- equilibrium potential in those cells; the single-channel current-voltage relationship was linear with a unitary slope conductance between 17 and 25 pS (pipettes filled with 100 mM-NaCl). 3. Large depolarizing voltage steps also activated Cl- channels in excised inside-out membrane patches that were previously quiescent. This mode of activation produced a distinctive single-channel current-voltage relationship with strong outward rectification at depolarizing membrane potentials. Single-channel cord conductance at negative potentials was 15-18 pS and increased to 45 pS at + 100 mV. 4. Altering the Cl- concentration in the bathing solution of excised inside-out patches displaced the observed reversal potential (Erev) to values predicted for Cl- equilibrium potential. Replacement of K+ for Na+ was without effect. 5. The effect of different anions upon Erev was used to determine the channel anion selectivity in excised inside-out patches. The permeability sequence was SCN- greater than I- greater than Br- greater than Cl- greater than F- greater than HCO3- greater than gluconate which corresponds to Eisenman's sequence 1. Neither ionic size nor diffusion rates determine the permeation of ions through the channel. 6. In channels activated by depolarization the open probability (Po) was insensitive to changes in the Ca2+ concentration (less than 10(-8)-10(-3) M) bathing the cytoplasmic face of excised inside-out patches. Depolarization was also without marked effect on Po. 7. Chloride channels in excised inside-out patches were inhibited by stilbene and diphenylamine-2-carboxylate derivatives. 4-Acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid (SITS, 5 x 10(-5) M) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB, 1 x 10(-5) M) caused an irreversible 'flickery' blockade without altering single-channel current. 3'5-Dichlorodiphenylamine-2-carboxylic acid (DDPC, 5 x 10(-5) M) reduced the currents at every voltage without apparent effects on gating properties of the channel.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Dreinhöfer J., Gögelein H., Greger R. Blocking kinetics of Cl- channels in colonic carcinoma cells (HT29) as revealed by 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB). Biochim Biophys Acta. 1988 Dec 8;946(1):135–142. doi: 10.1016/0005-2736(88)90466-x. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium. J Membr Biol. 1985;83(1-2):169–175. doi: 10.1007/BF01868748. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Sepúlveda F. V., Sheppard D. N. A chloride conductance activated by adenosine 3',5'-cyclic monophosphate in the apical membrane of Necturus enterocytes. J Physiol. 1988 Jan;395:597–623. doi: 10.1113/jphysiol.1988.sp016937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giráldez F., Sepúlveda F. V. Changes in the apparent chloride permeability of Necturus enterocytes during the sodium-coupled transport of alanine. Biochim Biophys Acta. 1987 Apr 9;898(2):248–252. doi: 10.1016/0005-2736(87)90044-7. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Chloride channels in the luminal membrane of the rectal gland of the dogfish (Squalus acanthias). Properties of the "larger" conductance channel. Pflugers Arch. 1987 Jun;409(1-2):114–121. doi: 10.1007/BF00584757. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P., Gögelein H., Kunzelmann K., Greger R. Characteristics of apical chloride channels in human colon cells (HT29). Pflugers Arch. 1987 Nov;410(4-5):487–494. doi: 10.1007/BF00586530. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouse M. E., Schneider G. T., Gage P. W. A large anion-selective channel has seven conductance levels. Nature. 1986 Jan 2;319(6048):58–60. doi: 10.1038/319058a0. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Lee J. A. A large conductance, voltage- and calcium-activated K+ channel in the basolateral membrane of rat enterocytes. FEBS Lett. 1986 Sep 29;206(1):87–92. doi: 10.1016/0014-5793(86)81346-1. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Mason W. T. Single channel recordings obtained from basolateral membranes of isolated rabbit enterocytes. FEBS Lett. 1985 Oct 21;191(1):87–91. doi: 10.1016/0014-5793(85)80999-6. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Sheppard D. N., Giraldez F. Possible target for cystic fibrosis in the small intestinal epithelium. Gut. 1989 Jan;30(1):142–144. doi: 10.1136/gut.30.1.142-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Giraldez F., Sepúlveda F. V. K+ channels activated by L-alanine transport in isolated Necturus enterocytes. FEBS Lett. 1988 Jul 18;234(2):446–448. doi: 10.1016/0014-5793(88)80134-0. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Giraldez F., Sepúlveda F. V. Kinetics of voltage- and Ca2+ activation and Ba2+ blockade of a large-conductance K+ channel from Necturus enterocytes. J Membr Biol. 1988 Oct;105(1):65–75. doi: 10.1007/BF01871107. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988 Jul;29(7):957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]



