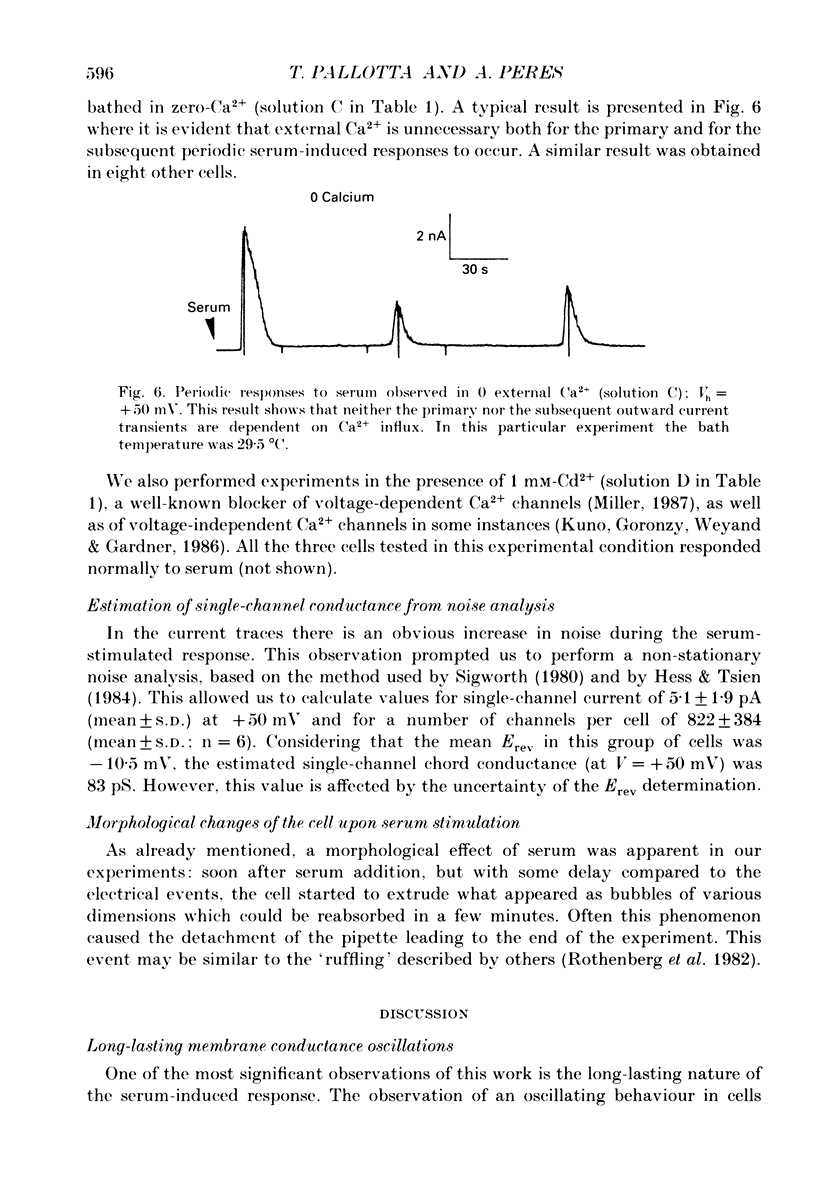
Abstract
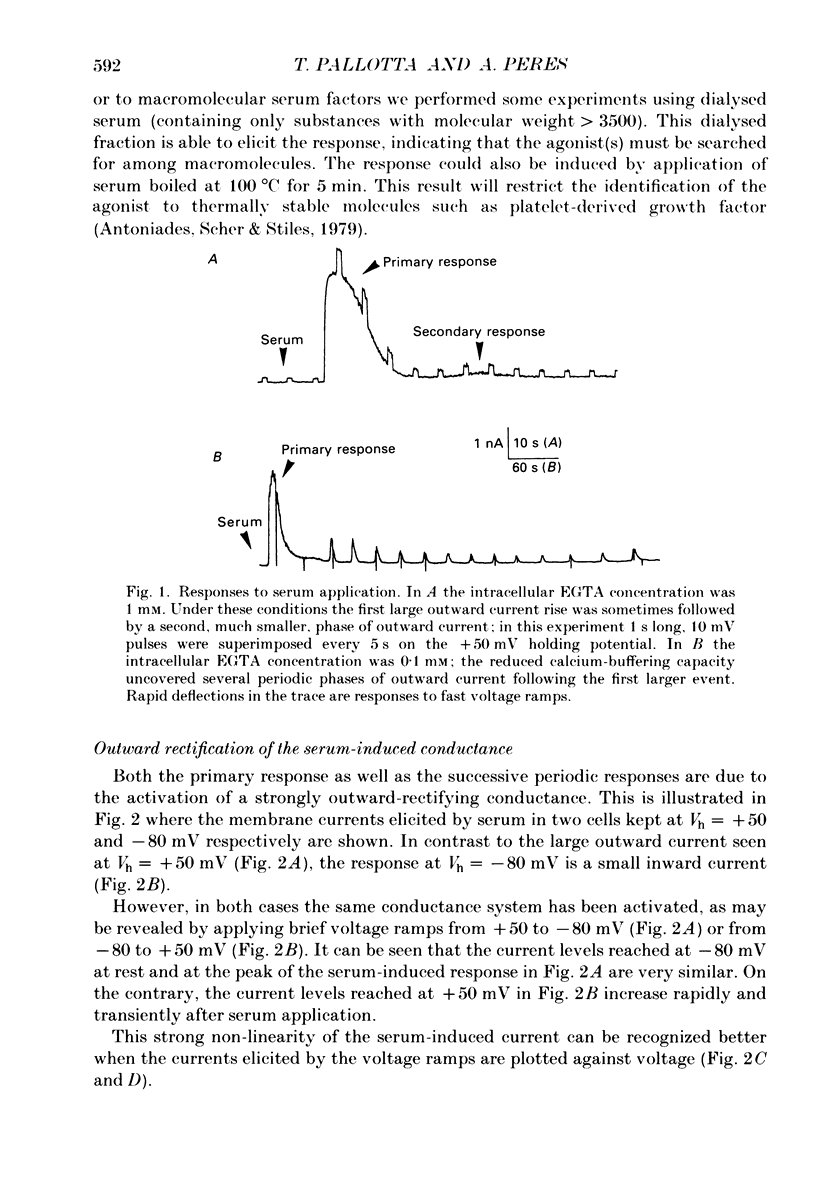
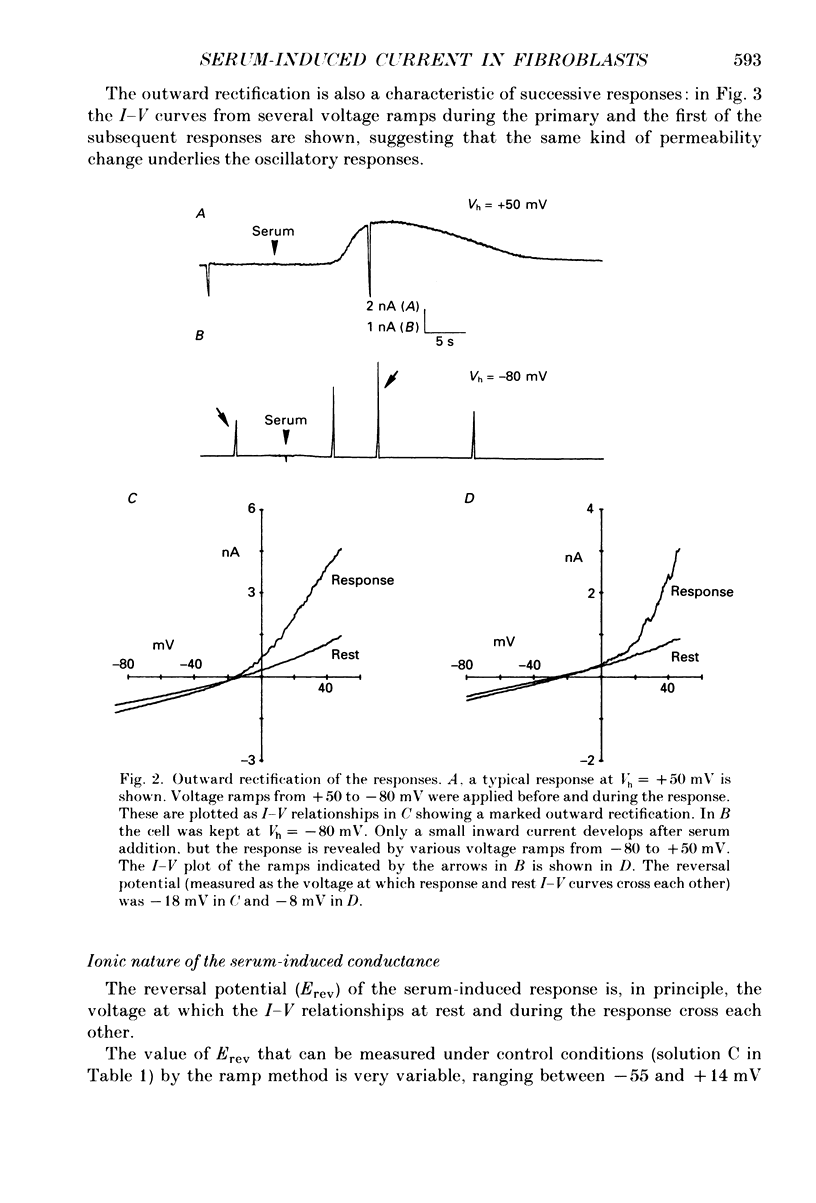
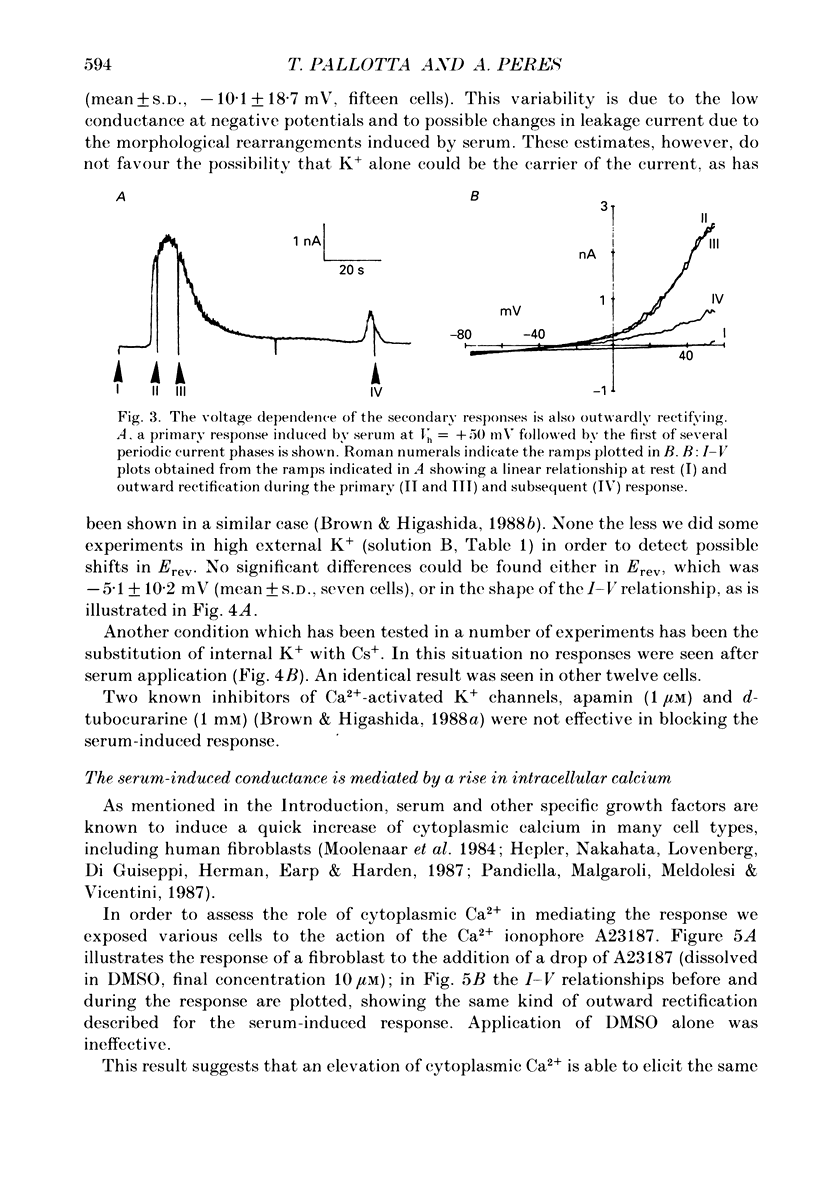
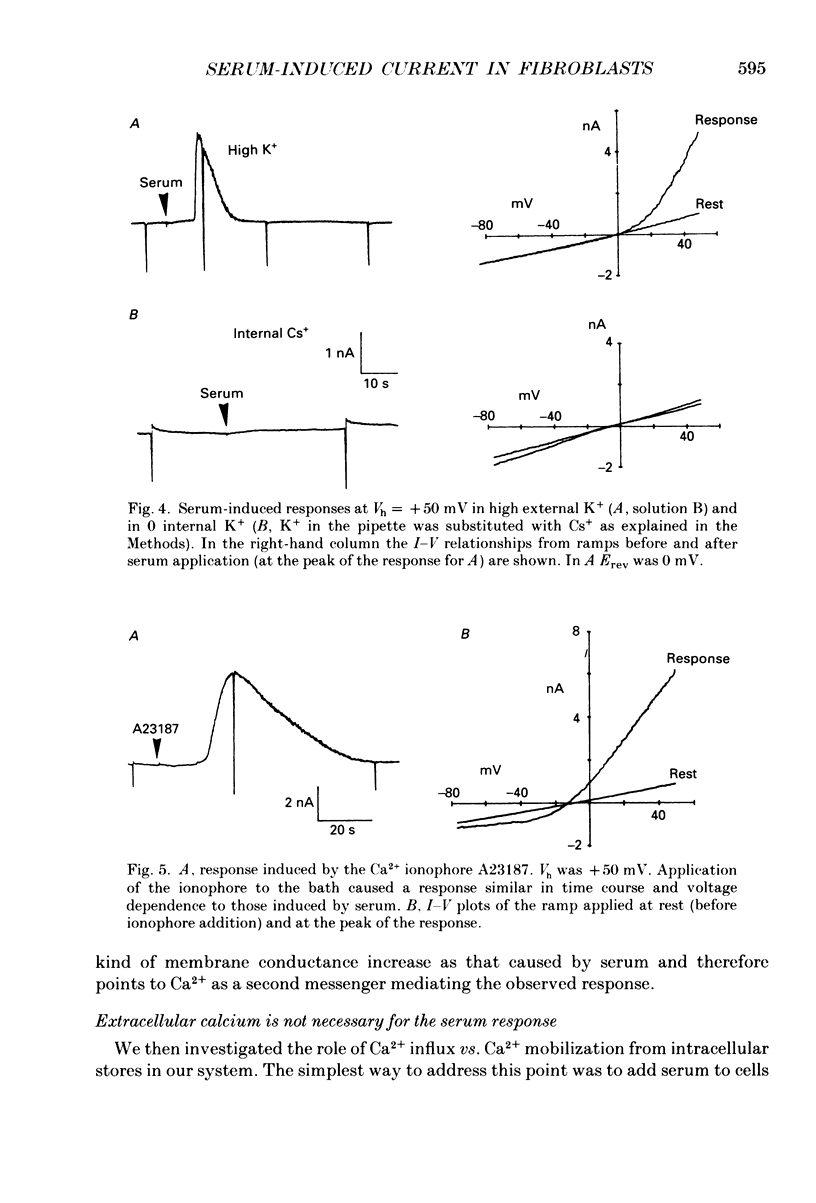
1. Application of fetal calf serum to quiescent human fibroblasts, kept under whole-cell voltage clamp at positive potentials, induced a series of transient rises in membrane conductance. 2. The first transient increase in conductance developed with very short time lag (2-10 s) after serum addition, while the period between successive transients was 30-90 s, being remarkably constant in each particular cell. 3. Raising the Ca2(+)-buffering capacity of the intracellular solution with 1 mM-EGTA suppressed the appearance of the sustained oscillations. 4. The conductance increase was strongly voltage dependent: voltage ramps applied before, during and after the transients revealed the activation of an outwardly rectifying conductance with variable reversal potentials (between +14 and -55 mV). 5. No significant shifts of the reversal potential were observed when the extracellular K+ concentration was increased to 126 mM. Substitution of K+ with Cs+ as intracellular cation eliminated the outward current in response to serum. 6. External application of the Ca2+ ionophore A23187 elicited currents which were very similar in voltage dependence and time course to those triggered by serum. 7. The serum-induced response persisted unaffected by the absence of external Ca2+. The response was also seen in the presence of 1 mM-Cd2+ in the external solution. 8. Serum addition caused a rapid morphological rearrangement of the cells. 9. It is concluded that serum triggers a mobilization of Ca2+ from intracellular stores which in turn activates cationic channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Membrane current responses of NG108-15 mouse neuroblastoma x rat glioma hybrid cells to bradykinin. J Physiol. 1988 Mar;397:167–184. doi: 10.1113/jphysiol.1988.sp016994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiod T., Field A. C., Ogden D. C., Sandford C. A. Internal perfusion of guinea-pig hepatocytes with buffered Ca2+ or inositol 1,4,5-trisphosphate mimics noradrenaline activation of K+ and Cl- conductances. FEBS Lett. 1987 Jun 15;217(2):247–252. doi: 10.1016/0014-5793(87)80672-5. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Gillo B., Lass Y. Role of calcium mobilization in mediation of acetylcholine-evoked chloride currents in Xenopus laevis oocytes. J Physiol. 1985 Sep;366:299–313. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Periodic increase of cytoplasmic free calcium in fertilized hamster eggs measured with calcium-sensitive electrodes. J Physiol. 1986 Aug;377:193–205. doi: 10.1113/jphysiol.1986.sp016181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Goronzy J., Weyand C. M., Gardner P. Single-channel and whole-cell recordings of mitogen-regulated inward currents in human cloned helper T lymphocytes. Nature. 1986 Sep 18;323(6085):269–273. doi: 10.1038/323269a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Moolenaar W. H., de Laat S. W., van der Saag P. T. Serum triggers a sequence of rapid ionic conductance changes in quiescent neuroblastoma cells. Nature. 1979 Jun 21;279(5715):721–723. doi: 10.1038/279721a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Almers W. Fast calcium transients in rat peritoneal mast cells are not sufficient to trigger exocytosis. EMBO J. 1986 Jan;5(1):51–53. doi: 10.1002/j.1460-2075.1986.tb04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Henkart M. P. Oscillatory membrane potential changes in cells of mesenchymal origin: the role of an intracellular calcium regulating system. J Exp Biol. 1979 Aug;81:49–61. doi: 10.1242/jeb.81.1.49. [DOI] [PubMed] [Google Scholar]
- Okada Y., Doida Y., Roy G., Tsuchiya W., Inouye K., Inouye A. Oscillations of membrane potential in L cells. I. Basic characteristics. J Membr Biol. 1977 Aug 4;35(4):319–335. doi: 10.1007/BF01869957. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Peres A., Sturani E., Zippel R. Properties of the voltage-dependent calcium channel of mouse Swiss 3T3 fibroblasts. J Physiol. 1988 Jul;401:639–655. doi: 10.1113/jphysiol.1988.sp017184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A., Zippel R., Sturani E. Serum induces the immediate opening of Ca2+-activated channels in quiescent human fibroblasts. FEBS Lett. 1988 Dec 5;241(1-2):164–168. doi: 10.1016/0014-5793(88)81052-4. [DOI] [PubMed] [Google Scholar]
- Rothenberg P., Reuss L., Glaser L. Serum and epidermal growth factor transiently depolarize quiescent BSC-1 epithelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7783–7787. doi: 10.1073/pnas.79.24.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. M., Proctor J., Grant M., Thomas C. Epidermal growth factor stimulates phosphatidylinositol turnover for ten hours in A431 cells without activation of protein kinase C. Biochem Biophys Res Commun. 1988 Sep 15;155(2):877–881. doi: 10.1016/s0006-291x(88)80577-1. [DOI] [PubMed] [Google Scholar]
- Ueda S., Oiki S., Okada Y. Oscillations of cytoplasmic concentrations of Ca2+ and K+ in fused L cells. J Membr Biol. 1986;91(1):65–72. doi: 10.1007/BF01870215. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Yada T., Oiki S., Ueda S., Okada Y. Synchronous oscillation of the cytoplasmic Ca2+ concentration and membrane potential in cultured epithelial cells (Intestine 407). Biochim Biophys Acta. 1986 Jun 16;887(1):105–112. doi: 10.1016/0167-4889(86)90129-1. [DOI] [PubMed] [Google Scholar]


