Abstract
Background
Mucormycosis, commonly known as the “black fungus,” is a severe infection affecting multiple organ systems, including the skin, brain, lungs, and gastrointestinal tract. This case report is focused on pulmonary mucormycosis, which is frequently observed in organ transplant recipients. Diagnostic challenges arise from nonspecific symptoms.
Case Presentation
Our case involved a 61-year-old male with a history of renal transplantation. The patient initially presented with a persistent cough and bloody sputum and was treated unsuccessfully for a presumed fungal infection. A lung biopsy confirmed pulmonary mucormycosis, emphasizing the heightened vulnerability of immunocompromised individuals.
Conclusion
This case highlights the intricate nature of mucormycosis diagnosis and management, particularly in post-organ transplantation patients. It underscores the importance of awareness and collaboration among medical specialists, including infectious disease experts, pulmonologists, and transplant teams, to optimize outcomes in the face of this life-threatening infection.
Keywords: mucormycosis, bacterial infections and mycoses, immunocompromised host, case report, adult, respiratory tract infections, organ transplantation
Introduction
Mucormycosis, a severe infection caused by molds belonging to the family Mucoromycotina, is predominantly environmental, with transmission occurring through inhalation of the spores they produce.
Immunocompromised individuals, particularly those who have undergone organ transplantation, face an increased risk of developing mucormycosis.1,2 Despite not being a reportable disease, a study conducted in the San Francisco region revealed that out of 1 000 000 people, 1.7 individuals were diagnosed with mucormycosis. This incidence is on the rise, as substantiated by current medical literature.3 Diagnosing mucormycosis poses significant challenges due to the nonspecific nature of its symptoms. Notably, suspicion should be raised in cases where individuals diagnosed with a fungal infection fail to respond to antifungal medications typically effective against aspergillosis.3 This failure to respond underscores the importance of considering mucormycosis as a differential diagnosis in such cases, highlighting the need for heightened clinical awareness and vigilance in the diagnostic process.
As the incidence of mucormycosis continues to draw attention, comprehensive understanding and awareness of its risk factors, transmission mechanisms, and diagnostic nuances become pivotal in formulating effective preventive measures and timely interventions.
Case Presentation
This case involves a 61-year-old man with a complex medical history marked by end-stage renal disease post-renal transplant in 2022, who was under immunosuppressive therapy. Additionally, he suffered from diabetes mellitus type 2 and essential hypertension. The patient's chief complaints upon presentation were right-sided chest pain and a productive cough yielding bloody sputum, with a similar episode occurring a month prior. During the earlier episode, he received a diagnosis of an “unknown” pulmonary fungal infection at another facility and commenced on voriconazole at a dosage of 400 mg twice daily. Despite that antifungal intervention, the resolution of symptoms remained elusive.
Upon admission, his vital signs indicated a temperature of 98.7 degrees Fahrenheit, blood pressure of 172/72 mmHg, a respiratory rate of 18 breaths per minute, and oxygen saturation at 99% on room air. The physical examination was largely unremarkable.
Given the clinical presentation, the initial suspicion centered around tuberculosis and a pulmonary embolism. A thorough diagnostic workup yielded unremarkable results, including a computed tomography (CT) angiogram and a 3-day sputum culture for acid-fast bacilli (AFB). A subsequent chest CT revealed a notable 5.1 × 4.9 × 5.5 cm cavitary or centrally necrotic mass in the right upper lobe. This mass was accompanied by hilar adenopathy and multiple nodules within the right lung, raising concerns for pulmonary carcinoma with intrapulmonary metastasis (Figure 1).
Figure 1.
A computed chest tomography showed a 5.1 × 4.9 × 5.5 cm cavitary or centrally necrotic mass in the right upper lobe.
The interventional radiology team conducted a diagnostic lung biopsy to establish a definitive diagnosis. The biopsy results unveiled acute pneumonitis with necrosis and fungal organisms consistent with Mucor species (Figure 2). The patient was discharged before the biopsy results became available; however, he was contacted and recommended to initiate treatment with liposomal amphotericin to address the confirmed mucormycosis.
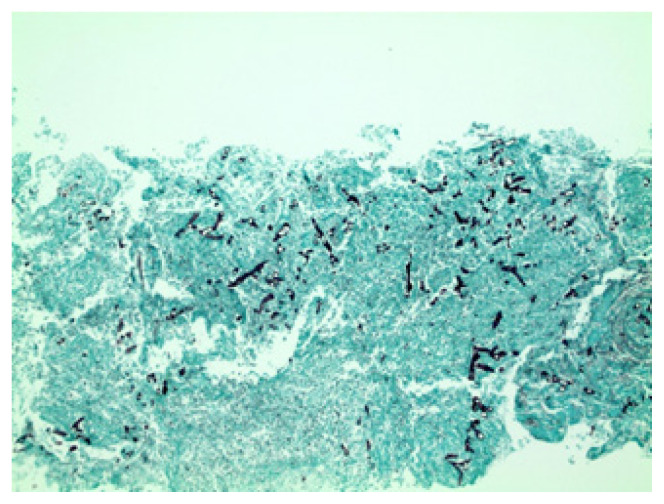
Figure 2.
The pathology report showed necrosis and fungal organisms consistent with the Mucor species.
Discussion
We discuss a patient who presented with right-sided chest pain and bloody sputum in the setting of diabetes mellitus type 2 and renal transplantation while on immunosuppressants. The patient presented with these symptoms 1 month prior and was diagnosed with an “unknown” pulmonary fungal infection. He was started on treatment with voriconazole 400 mg twice a day with no resolution in symptoms.
In cases of pulmonary mucormycosis, patients typically exhibit pneumonia symptoms and hemoptysis, as in our patient, which makes it essential for the provider to have a high clinical suspicion when evaluating the patient. Major risk factors for pulmonary mucormycosis include hematological malignancies and solid organ transplantation, whereas rhino-orbit-cerebral mucormycosis is classically-associated with diabetes mellitus.4 Lately, individuals with diabetes have been linked with mucormycosis post-coronavirus infection.5,6 In this case, the patient had both diabetes mellitus type 2 and a renal transplantation while on immunosuppression, putting him at risk for developing a mucormycosis infection.
Diagnostic workup for pulmonary mucormycosis consists of CT scans, cultures, polymerase chain reaction tests, and histology. The reversed halo sign on a chest CT scan is an early but very suggestive sign of pulmonary mucormycosis in neutropenic patients.4 In our case, however, a chest CT scan and acid-fast sputum test were inconclusive. Definitive diagnosis was established through a diagnostic lung biopsy revealing acute pneumonitis with necrosis and fungal organisms consistent with Mucor species.
Prompt diagnosis and treatment are crucial in cases of pulmonary mucormycosis due to its high mortality rate. Because of its rapid local progression and prominent angioinvasion, this infection has a high mortality rate (40–76%) and carries substantial morbidity in some cases.7 Another study of case reports showed that the in-hospital mortality was 65% for patients with isolated pulmonary mucormycosis, 96% for those with disseminated disease, and 80% overall.8
Medical and surgical treatment is necessary for pulmonary mucormycosis to improve survival. Several studies have shown that combining early surgical resection and antifungal therapy significantly improves survival compared to antifungal treatment alone.7 However, there are concerns about the limited penetration of antifungals into the affected tissues due to the substantial necrosis accompanying this infection.8 Hence, it is essential to consider surgical debridement in addition to medical treatment. In addition, surgical resection may provide additional benefits to patients with pulmonary mucormycosis confined to 1 lung.8
An analysis based on case reports showed an 11% mortality rate for surgical treatment with or without antifungal treatment compared to the 68% mortality for those who received medical treatment alone (P = .0004).8 Currently, the first-line therapy and drug of choice is liposomal amphotericin B.4,9,10 After a stable or partial response, step-down treatment involves oral isavuconazole or posaconazole delayed-release tablets until a complete response is achieved. When there is a risk of relapse, such as in patients with persistent neutropenia or the prolonged use of high-dose immunosuppressive therapy, secondary prophylaxis should be considered.4 Given the high mortality rate of this infection, future research should focus on new antifungal therapies and the outcomes of combination therapy involving antifungals and surgery.
More recently, as an added threat, COVID-19 has been linked with various infections, including fungal ones like mucormycosis.11 Several case reports have linked pulmonary mucormycosis acquisition before and after COVID-19 infection.12 A significant number of these cases have occurred in diabetic patients. Possible etiologies hypothesized are: (1) using steroids leads to hyperglycemia, (2) intubation in critically-ill patients creates avenues for spore inhalation, (3) cytokine release increases serum iron levels, and immune cell dysregulation creating an optimal environment for Mucor growth.12 As COVID-19 emerges as a possible long-term health concern and becomes linked with mucormycosis, the escalating occurrence of fungal infection is now facing an even greater risk of advancement.
Conclusion
While still considered rare, instances of pulmonary mucormycosis are on the rise. Recognizing it as the primary causative agent is becoming increasingly crucial, and a distinct treatment approach is necessary compared to other fungal infections. This is particularly evident in cases such as the one detailed here involving an organ transplant recipient who, despite a confirmed fungal infection diagnosis and targeted treatment for aspergillosis, experiences persistent symptoms without improvement.
As the medical community grapples with the surge in mucormycosis cases, the ability to differentiate it from other fungal infections and initiate prompt, tailored interventions become paramount in the ongoing battle against this potentially life-threatening pathogen.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity.
Footnotes
Conflicts of Interest: The authors declare they have no conflicts of interest.
The authors are employees of HCA Florida Northwest Hospital, a hospital affiliated with the journal's publisher.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1.About Mucormycosis. Centers for Disease Control and Prevention; [Accessed January 30, 2024]. Updated August 9, 2021. www.cdc.gov/fungal/diseases/mucormycosis/definition.html . [Google Scholar]
- 2.Hernández JL, Buckley CJ. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Jan, [Accessed January 30, 2024]. Mucormycosis. Updated June 12, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544364/ [Google Scholar]
- 3.Kontoyiannis DP, Hickey RC. Mucormycosis National Organization for Rare Disorders. [Accessed January 30. 2024]. https://rarediseases.org/rare-diseases/mucormycosis/
- 4.Danion F, Coste A, Le Hyaric C, et al. What is new in pulmonary mucormycosis? J Fungi (Basel) 2023;9(3):307. doi: 10.3390/jof9030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponnaiyan D, Anitha CM, Prakash PSG, et al. Mucormycosis diagnosis revisited: current and emerging diagnostic methodologies for the invasive fungal infection (review) Exp Ther Med. 2022;25(1):47. doi: 10.3892/etm.2022.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahalaxmi I, Jayaramayya K, Venkatesan D, et al. Mucormycosis: an opportunistic pathogen during COVID-19. Environ Res. 2021;201:111643. doi: 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez JF, Maselli DJ, Simpson T, Restrepo MI. Pulmonary mucormycosis: what is the best strategy for therapy? Respir Care. 2013;58(5):e60–e63. doi: 10.4187/respcare.02106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–1050. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Lee SC. Current treatments against mucormycosis and future directions. PLoS Pathog. 2022;18(10):e1010858. doi: 10.1371/journal.ppat.1010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain MK, Ahmed S, Khan A, Siddiqui AJ, Khatoon S, Jahan S. Mucormycosis: a hidden mystery of fungal infection, possible diagnosis, treatment and development of new therapeutic agents. Eur J Med Chem. 2023;246:115010. doi: 10.1016/j.ejmech.2022.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis K, Almog R, Peleg Y, Spiegelman L. A case report of invasive mucormycosis in a COVID-19 positive and newly-diagnosed diabetic patient. J Educ Teach Emerg Med. 2023;8(3):V10–V13. doi: 10.21980/J81M1G. [DOI] [PMC free article] [PubMed] [Google Scholar]