Abstract
Nucleoside deoxyribosyl transferase (NDT) is an enzyme that catalyzes the transfer of purine and pyrimidine bases between 2'-deoxyribonucleosides and is widely used for synthesizing nucleoside analogs in various biotechnological applications. While NDT exhibits high activity toward natural nucleosides, its activity toward unnatural nucleoside analogs is significantly lower. Previously, the NDT mutant named fNDT(L59Q) was identified displaying 4.4-fold higher activity toward 2'-fluoro-2'-deoxyuridine (2FDU). In this study, molecular evolution strategies using error-prone PCR were employed to further generate mutant enzymes with enhanced activity toward 2FDU. After two rounds of mutational screening, two mutant clones that exhibited high activity against 2FDU were identified as fNDT-i1 (V52A) and fNDT-i2 (L28I), respectively. A double mutant, fNDT-i4, was subsequently constructed by combining the V52A and L28I mutations. Whole-cell-based activity measurements showed that fNDT-i4 exhibited 4.0- and 20.6-fold higher activity at 40°C and 50°C, respectively, compared to the wild-type NDT. The detailed characterization of the purified enzymes conducted under various conditions, including temperature, pH, thermal stability, and enzyme kinetics experiments, showed that fNDT-i1 and fNDT-i4 exhibited 3.1- and 3.7-fold higher catalytic efficiency, respectively than wild-type NDT. The L59Q mutation was identified as a key factor in improving the thermal stability, whereas the V52A and L28I mutations were critical for improving substrate affinity and reaction efficiency. These findings provide the potential of fNDT-i1 and fNDT-i4 as highly efficient biocatalysts for developing industrially relevant nucleoside analog synthesis.
One-Sentence Summary
The nucleoside deoxyribosyl transferase mutant were engineered to enhance biological activity and physical resistance for production of fluorinated deoxynucleoside as a raw material of oligonucleotide therapeutics.
Keywords: Error-prone PCR, Nucleoside deoxyribosyl transferase, Nucleoside analog, Enzyme engineering
Graphical Abstract
Graphical Abstract.

Introduction
Nucleotides, the fundamental units of nucleic acids, play vital roles in many biochemical processes, including storing genetic information, signal transduction, and energy transfer (Kepp et al., 2017; Pérez et al., 2018). In addition to these functions, nucleotides and nucleosides have a range of biological activities, including antitumor, antiviral, and immunosuppressive properties (Galmarini et al., 2002; Konkina et al., 2023). Since the Food and Drug Administration (FDA) approval of an oligonucleoside drug named fomivirsen in 1998 for the treatment of cytomegalovirus retinitis (Marwick, 1998), a variety of oligonucleotide drugs have been approved and used. Recently, the FDA approval of remdesivir as a treatment for COVID-19 has increased interest in nucleoside therapeutics (Spinner et al., 2020). Oligonucleotide-based medicines with therapeutic potential can act through various mechanisms such as antisense oligonucleotides, siRNAs, and aptamers (Oberthuer et al., 2006; Burnett & Rossi, 2012; Thakur et al., 2022). Their ability to inhibit gene or protein expression enables the treatment of diseases, including neurodegenerative disorders, cancer, and diabetic retinopathy, that frequently exhibit resistance to small-molecule therapies (Hnik et al., 2009; Tanaka & Nyce, 2000; Sah & Aronin, 2011; Xiong et al., 2021).
Nevertheless, their susceptibility to nuclease degradation is a significant challenge in their delivery (Gagliardi & Ashizawa, 2021). This issue is frequently addressed by chemical modifications such as phosphorothioate backbones and 2ʹ-modifications (e.g. 2ʹ-F, 2ʹ-O-Me) (Shukla et al., 2010). These modifications enhance nuclease resistance, prolong the half-life of the drug, and improve target specificity (Corey, 2007). Among them, the 2ʹ-F modification is particularly noteworthy for enhancing stability and RNA-targeting specificity (Roberts et al., 2020). Although these modifications are effective, their chemical synthesis is frequently expensive, energy-intensive, and environmentally unfriendly. In addition, they constrain scalability (Fresco-Taboada et al., 2013).
Enzymatic synthesis using nucleoside deoxyribosyltransferase (NDT, EC 2.4.2.6) is a promising alternative approach (Okuyama et al., 2003; Bosch et al., 2006; Miyamoto et al., 2007; Fernández-Lucas et al., 2011, 2021; Del Arco et al., 2021). NDT facilitates the exchange of purine and pyrimidine bases between nucleosides (Kaminski, 2002). Type I is specific to purines, while Type II can act on both purines and pyrimidines (Crespo et al., 2017). Macnutt first found NDT in Lactobacillus helveticus in 1952 (Macnutt, 1952). Since then, it has been identified in several other organisms, including Lactobacillus leichmannii, Bacillus psychrosaccharolyticus, Trypanosoma brucei, and Streptococcus species (Chawdhri et al., 1991; Armstrong et al., 1996; Bosch et al., 2006; Fresco-Taboada et al., 2018). NDT accepts a diverse range of nucleobases as substrates and displays enhanced regioselectivity compared to proteins such as purine nucleoside phosphorylase (PNP) (Vichier‐Guerre et al., 2020). On the other hand, its activity toward fluorinated nucleosides, including 2ʹ-F derivatives, remains constrained.
A previous study addressed this limitation by developing a thermostable NDT mutant (NDTL59Q or fNDT) with enhanced activity toward 2FDU (Yoo et al., 2022). Building on this foundation, this study used molecular evolution to identify the enzymes with improved catalytic efficiency for fluorinated deoxynucleosides. The newly identified enzymes were subjected to comprehensive enzymatic and kinetic analyses, which provided insights into their potential applications in synthesizing modified nucleosides for therapeutic use.
Materials and Methods
Error-Prone PCR for NDT Mutant Library
The fNDT gene, previously characterized in previous research (Yoo et al., 2022), was used to generate a mutant library using error-prone PCR with a Diversify® PCR Random Mutagenesis Kit (TaKaRa, Japan); Supplementary Table 1 lists the primers used. The mutation frequency was controlled by adjusting the Mn²⁺ and dGTP concentrations. PCR reactions were prepared with 33 µL of water, 5 µL of 10× TITANIUM Taq Buffer, 4 µL of 2 mM dGTP, 4 µL of 8 mM MnSO4, 1 µL of 50× Diversify dNTP Mix, 1 µL of 10 µM primer mix (Infusion_28a_F and Infusion_28a_R, Supplementary Table 1), 10 ng of pUC19_fNDT template DNA, and 1 µL of TITANIUM Taq polymerase. The PCR conditions included an initial denaturation at 94°C for 30 s, followed by 25 cycles of 94°C for 30 s and 68°C for 1 min, with a final extension at 68°C for 1 min. The products were resolved on a 0.7% agarose gel, and the mutated genes were extracted using a Universal DNA Purification Kit (TIANGEN, China). The cloning vector was prepared by PCR using Q5® High-Fidelity DNA Polymerase (New England Biolabs, USA) with pET28a_PCR_F and pET28a_PCR_R primers. The insert and vector were ligated using the In-Fusion® HD Cloning Kit (Takara, Japan) and transformed into Escherichia coli BL21 (DE3) by electroporation.
High-Throughput Screening of NDT Mutant Library
The NDT mutant library was screened using a two-round process (Fig. 1B). In the primary screening, the mutants were cultured in 24-well plates containing LB broth with kanamycin. After overnight incubation at 37°C with shaking (220 rpm), 10 µL of the culture was transferred to fresh LB medium with kanamycin and grown under the same conditions. Protein expression was induced with 2 µL of 0.5 M IPTG for 20 hr. The cells were harvested and resuspended in distilled water. Subsequently, 10 µL of the suspension was added to a reaction mixture containing 1 mM 2FDU, adenine, and potassium phosphate buffer (pH 6.0). The reactions were conducted at 40°C with shaking at 200 rpm for 20 hr.
Fig. 1.
Screening and activity assessment of NDT mutants for enhanced 2’-fluoro-2’-deoxyuridine (2FDU) conversion. (A) Reaction scheme for converting 2FDU to 2FDA, catalyzed by nucleoside deoxyribosyl transferase. (B) Schematic diagram of the two-round screening process used to identify NDT mutants with enhanced activity. (C) HPLC analysis of 2FDU to 2FDA conversion. The a line represents the retention time of 2FDU, the b line corresponds to 2FDA, the c line to adenine, and the d line shows a reaction mixture of fNDT with 2FDU as substrate.
Secondary screening focused on mutants showing activity in the primary round. The mutants were cultured in 20 mL LB broth supplemented with kanamycin until an OD600 of 0.7–0.8 was reached, followed by IPTG induction (50 µL of 0.5 M) for 4 hr. The cells were harvested and adjusted to 0.3 OD600 for substrate reactions, which were conducted identically to the primary screening. The reactions were quenched by dilution with 0.1 N NaOH, filtered through 0.45 µm membranes, and stored at 4°C for high-performance liquid chromatography (HPLC) analysis.
High-Performance Liquid Chromatography (HPLC) Analysis
HPLC was conducted in isocratic elution mode with a mobile phase comprising 0.1% acetic acid and methanol in a 9:1 ratio. The flow rate was established at 1.0 mL/min. An ODS-3 column (4.6 × 150 mm, 5 μm; GL Science, Japan) was used, with detection at 254 nm. The column temperature was maintained at 30°C, with a total run time of 20 min and an injection volume of 20 µL. Under these conditions, the retention times were as follows: adenine at 2.6 min, 2FDU at 5.9 min, and 2FDA (2′-fluoro-2′-deoxyadenosine) at 10.8 min (Fig. 1C).
NDT Enzyme Expression and Purification
Colonies of NDT, fNDT, fNDT-i1, and fNDT-i4 were cultured overnight at 37°C in 5 mL of LB broth supplemented with kanamycin by shaking at 220 rpm. A 1 mL aliquot of the overnight culture was transferred to 50 mL of fresh LB broth containing kanamycin and grown under the same conditions until OD600 = 0.6–0.8. Protein expression was induced with 0.8 mM IPTG and incubated for 3 hr at 37°C. The cells were harvested and resuspended in NPI-10 buffer. The cells were lysed by sonication. The lysate was centrifuged at 2600× g for 20 min at 4°C. The supernatant was applied to Ni-NTA agarose (Qiagen, Germany) for affinity purification. The proteins were washed with NPI-20 buffer and eluted using the NPI-300 buffer. The eluted proteins were concentrated and exchanged into a storage buffer (50 mM NaH2PO4·2H2O, 1 mM EDTA, 0.2 mM DTT, 1% glycerol, pH 7.0) using an Amicon® Ultra-4 Centrifugal Filter (Merck, Germany). The protein purity was assessed by SDS-PAGE (Bio-Rad, Hercules, CA, USA), and the protein concentrations were determined using the Bradford assay (Sigma, Korea). The purified proteins were stored at 4°C until further use.
Characterization and Kinetics of NDT Mutant Enzyme
The activity of the purified enzymes (NDT, fNDT, fNDT-i1, and fNDT-i4) was assessed under varying conditions. For the time-dependent activity, 50 µg of each enzyme was incubated in 100 µL of a solution containing 1 mM 2FDU, 1 mM adenine, and 1 mM potassium phosphate buffer (pH 6.0) at 40°C and 200 rpm for 2, 4, 10, 24, 48, and 96 hr. The temperature-dependent activity was measured by incubating the same reaction mixture at temperatures ranging from 30°C to 60°C for 20 hr. The pH-dependent activity was evaluated using a 50 mM sodium citrate buffer (pH 4.0–6.0) and a 50 mM potassium phosphate buffer (pH 6.0–8.0) under similar conditions. The thermal stability was analyzed by preheating the enzymes at 40–80°C for 5 min, followed by activity measurements at 40°C. The kinetic parameters were determined by varying the substrate concentrations (2–20 mM) in 100 µL reaction volumes, using 10 µg of enzyme per reaction. The reactions were incubated at 40°C and 200 rpm for 20 hr. After incubation, the samples were diluted tenfold with 0.1 N NaOH and analyzed by HPLC. Each condition was tested in six replicates, with the highest and lowest values excluded, and the mean values plotted. The kinetic parameters (Vmax, Km) were calculated using Lineweaver–Burk plots.
Substitutions of the 59th Amino Acid
Site-directed mutagenesis was performed to substitute the 59th amino acid of the ndt gene with various residues. The primers used (Q59X-F and Q59X-R) are detailed in Supplementary Table 1. PCR was conducted with Q5® High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA). The amplified products were ligated using the In-Fusion® HD Cloning Kit (Clontech, Japan). The ligation mixture was desalted with an agarose cone for 1 hr and then transformed into E. coli BL21 (DE3) by electroporation.
Results
Screening of NDT Mutant Library
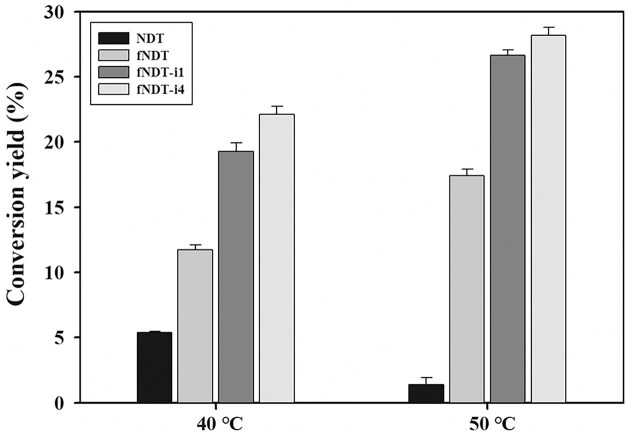
A mutant library was constructed using error-prone PCR based on the fNDT sequence as a template. The NDT mutants with enhanced activity converting to 2FDA were selected through two-round screening, which performed qualitative and quantitative analysis using whole-cell conversion (Fig. 1B). In the initial screening round, 24.4% of the total 570 mutants exhibited conversion activity from 2FDU to 2FDA, and 6% of mutants showed a higher conversion rate compared to fNDT (Supplementary Fig. S1A). In the secondary screening, fNDT-i1 and fNDT-i2 were identified, which improved the conversion yield by 1.70- and 1.73-fold compared to fNDT, respectively (Supplementary Fig. S1B). Sequence analysis showed that fNDT-i1 had a single amino acid substitution from valine to alanine at the 52nd position, even though it contained three more nucleotide changes as T155C, T180C and T264C. fNDT-i2 also had a single amino acid substitution from leucine to isoleucine at the 28th position and a nucleotide change at C82A (Table 1). Based on these results, fNDT-i4, harboring two amino acid substitutions at L28I and V52A, was constructed. The enzymatic activity was evaluated by whole-cell conversion at 40°C and 50°C using fNDT-i1-, fNDT-i2-, and fNDT-i4-expressing E. coli strains. As a result, fNDT-i4 showed the highest conversion yield, demonstrating a 1.88- and 1.62-fold increase at 40°C and 50°C, respectively, compared to fNDT (Fig. 2). The L59Q harboring mutants, such as fNDT, fNDT-i1, and fNDT-i4, had higher conversion yields at 50°C than at 40°C.
Table 1.
2FDU to 2FDA conversion titer of NDT colonies identified through screening
| Colony | Nucleotide change | Amino acid change | Yield (Ratio of 2FDA to fNDT 2FDA area) |
|---|---|---|---|
| NDT | – | – | 0.42 |
| fNDT | T176A | L59Q | 1 |
| fNDT-i1 | T155C, T176A, T180C, T264C | V52A, L59Q | 1.88 |
| fNDT-i2 | C82A, T176A | L28I, L59Q | 1.24 |
| fNDT-i4 | C82A, T155C, T176A | L28I, V52A, L59Q | 1.96 |
Fig. 2.
Conversion yield by E. coli containing NDT and mutants. The reaction was conducted at 40°C and 50°C for 20 hr. The values are the means of three replications ± standard deviation.
Purification and Characterization of NDT Mutants
NDT, fNDT, fNDT-i1, and fNDT-i4 were expressed in an E. coli-harboring pET28a expression system and purified via immobilized metal affinity chromatography (Fig. 3). The enzymatic activities of purified NDT wild type and mutants were evaluated under various parameters, including the reaction times, temperature, pH, and thermal stability (Fig. 4). In the time-dependent profiles of the conversion yield, fNDT-i1 and fNDT-i4 achieved over 80% conversion at 96 hrs compared to only 30% for wild type NDT (Fig. 4A). In particular, fNDT-i1 and fNDT-i4 achieved approximately 75% conversion yield within 24 hrs. In contrast, fNDT only exhibited a conversion rate of less than 60%. The temperature profiles of fNDT-i1 and fNDT-i4 showed optimal catalytic activity at 40°C, similar to wild-type NDT. On the other hand, the enzymatic activity decreased significantly above 50°C (Fig. 4B). In contrast, fNDT showed the highest activity at 50°C, which is consistent with previous findings (Yoo et al., 2022). The pH-dependent activity assays showed that all enzymes had similar conversion yields in sodium citrate and potassium phosphate buffers at pH 6 (Fig. 4C). Most enzymes had maximal activity between pH 5 and 6, but fNDT-i1 and fNDT-i4 still exhibited sustained activity at pH 7. Furthermore, all enzymes exhibited a significant decrease in activity at pH 8. The thermal stability assays indicated that the conversion yields of NDT, fNDT, and fNDT-i1 were retained up to 60°C, while the activity of fNDT-i4 showed a more than 50% decrease at the same temperature (Fig. 4D). All enzymes showed a significant decrease in activity at over 70°C.
Fig. 3.
SDS-PAGE analysis of purified NDT and mutants. Lane M: AccuLadder™ 3-color prestained protein size marker; Lane 1: E. coli BL21(DE3) pET28a-NDT soluble protein; Lane 2: purified NDT protein; Lane 3: E. coli BL21(DE3) pET28a-fNDT soluble protein; Lane 4: purified fNDT protein; Lane 5: E. coli BL21(DE3) pET28a-fNDT-i1 soluble protein; Lane 6: purified fNDT-i1 protein; Lane 7: E. coli BL21(DE3) pET28a-fNDT-i4 soluble protein; Lane 8: purified fNDT-i4 protein.
Fig. 4.
Effect of various conditions on the conversion yield NDT and mutants. The data shown represent the average values from four independent experiments. The error bars indicate standard deviations. Reactions were performed with 50 µg of each enzyme in 100 µL of a solution containing 1 mM 2FDU, 1 mM adenine, and 1 mM potassium phosphate buffer (pH 6.0) at 40°C and 200 rpm for 20 hr with only the tested parameter varied: (A) time, (B) temperature, (C) pH, and (D) heat stability (preheating).
Enzyme Kinetics of NDT Mutants
The enhanced catalytic performance of the mutants was evaluated by determining the kinetic parameters at 40°C (Table 2, Fig. 5). fNDT exhibited enhanced kinetic parameters compared to wild-type NDT: a 1.17-fold increase in Vmax and a reduced Km at 1.783 µM. Consequently, the catalytic efficiency (Kcat/Km) of fNDT improved 1.22-fold. fNDT-i1 and fNDT-i4 showed more significant improvements in catalytic efficiency compared to the wild type and fNDT. The Vmax of fNDT-i1 and fNDT-i4 were approximately double that of the wild-type NDT. Moreover, the substrate affinities for 2FDU were enhanced significantly, and the Km values of fNDT-i1 and fNDT-i4 were 0.69- and 0.57-fold lower than the wild-type NDT, respectively. The increased substrate affinities by mutation contributed to a 3.07- and 3.69-fold increase in catalytic efficiency (kcat/Km) of fNDT-i1 and fNDT-i4, respectively. Consequently, the catalytic activity of fNDT-i1 and fNDT-4 was significantly improved compared to the wild-type NDT.
Table 2.
Kinetics parameters of NDT and mutants
| Kinetics value | NDT | fNDT | fNDT-i1 | fNDT-i4 |
|---|---|---|---|---|
| Vmax (µM • hr−1) | 14.75 ± 0.03 | 17.28 ± 0.17 | 31.28 ± 0.4 | 29.57 ± 0.39 |
| Km (µM) | 1.865 ± 0.02 | 1.783 ± 0.06 | 1.289 ± 0.07 | 1.056 ± 0.21 |
| Kcat (hr−1) | 2.950 ± 0.006 | 3.456 ± 0.03 | 6.256 ± 0.08 | 5.914 ± 0.08 |
| Kcat/Km (hr−1 • µM−1) | 1.582 ± 0.02 | 1.940 ± 0.05 | 4.861 ± 0.18 | 5.842 ± 1.2 |
Fig. 5.
Kinetic analysis of NDT and mutants. (A) Michaelis–Menten kinetics plot, (B) Lineweaver–Burk plot. The experiments were conducted with six replicates, and the error bars represent the standard deviations of these measurements.
Substitutions of 59th Leucine to Other Amino Acids
Previous experiments confirmed that the activities of fNDT, fNDT-i1, and fNDT-i4 were enhanced for 2FDU. The common L59Q mutation in fNDT, fNDT-i1, and fNDT-i4 plays a significant role in this activity enhancement. The effect of the 59th leucine was examined further by substituting it with several amino acids with distinct properties (Supplementary Fig. S3). These included phenylalanine with a hydrophobic side chain, glutamic acid with a negatively charged side chain, serine with a polar uncharged side chain, alanine with a small hydrophobic side chain, and lysine with a positively charged side chain. As a result, five distinct mutations were introduced into the ndt gene. The L59F and L59K mutants exhibited lower activity than wild-type NDT, while the L59E, L59S, and L59A mutants showed higher activity, albeit lower than fNDT.
Discussion
2'-Fluoro-modified nucleosides are essential for producing various nucleic acid-based therapeutic agents, and their economic production is crucial (Meng et al., 2024). Although traditional chemical synthesis methods have been studied extensively, these approaches require multiple steps, leading to excessive energy consumption and time demands. Furthermore, using organic solvents and generating isomers increases the complexity of separation and purification processes (Liu et al., 2008). In contrast, enzyme-catalyzed synthesis of nucleoside analogs is a promising alternative because of its simplified process and high regioselectivity (Del Arco et al., 2021).
This study engineered an NDT enzyme with broad substrate specificity for purine and pyrimidine analogs to enhance its activity toward 2′-fluoronucleosides through error-prone PCR. Screening was performed in two rounds. The primary screening was conducted without considering cell growth to solely confirm the presence or absence of mutant enzyme conversion activity. As a result, 24.4% of the colonies exhibited conversion activity (Supplementary Fig. S1A). Additionally, 6% of the total colonies exhibited higher activity than fNDT, and these colonies were selected for secondary screening. In the second screening, two colonies showing the highest activity were designated fNDT-i1 and fNDT-i2. fNDT-i1 and fNDT-i2 contained the V52A and L28I mutations, respectively. fNDT-i4 was constructed by combining these two mutations. Whole-cell conversion experiments showed that fNDT, fNDT-i1, and fNDT-i4, containing the L59Q mutation, exhibited higher activity at 50°C than at 40°C (Fig. 2). Hence, the L59Q mutation enhances enzymatic activity at elevated temperatures. Various characterization experiments were conducted to evaluate the properties of the mutant proteins, including reaction time, temperature, pH, and thermal stability. fNDT-i1 and fNDT-i4 achieved approximately 80% conversion yield within 24 hr, demonstrating high efficiency in a short time. These results suggest the potential for improving industrial efficiency by shortening the production process time and reducing costs (Hassan et al., 2019). The optimal temperature experiments showed that, in contrast to whole-cell conversion results, the activities of fNDT-i1 and fNDT-i4 were reduced significantly at 50°C (Figs. 2 and 4B). This discrepancy can be attributed to the differences between whole-cell conversion conditions and the purified enzyme activity measurements. In whole-cell experiments, the intracellular environment contains factors such as cations, substrates, and coenzymes that stabilize proteins at higher temperatures (Ward et al., 1988). On the other hand, in purified enzyme assays, proteins are exposed directly to temperature in aqueous solutions, which can lead to conformational change or partial denaturation, thereby reducing their activity at elevated temperatures (García et al., 2012). The optimal temperature for NDT, fNDT-i1, and fNDT-i4 was 40°C, whereas fNDT exhibited optimal activity at 50°C. This result is consistent with previous studies and, along with the whole-cell conversion results, indicates that the L59Q mutation enhances the optimal temperature of the enzyme (Yoo et al., 2022). The pH results showed that fNDT-i1 and fNDT-i4 maintained enzyme activity across a broader pH range, from pH 4 to 7 (Fig. 4C). Hence, the introduction of the L59Q, V52A, and L28I mutations improved the stability of the enzyme structure under varying pH conditions. Thermal stability assays showed that fNDT-i4 exhibited a sharp decrease in activity at 60°C (Fig. 4D), most likely due to changes in hydrophobic interactions caused by L28I mutations. Hydrophobic interactions play a key role in protein thermal stability, particularly in the packing of the hydrophobic core (Gromiha et al., 2013; Koudelakova et al., 2013). In particular, hydrophobic interactions at the α-helix/β-sheet interface are particularly important (Kellis et al., 1988). The L28I mutation shifted the hydrophobic interaction from phenylalanine at position 8 of the β-sheet to leucine at position 139 of the helix, reducing the overall thermal stability of the protein (Supplementary Fig. S2B, S2C).
Kinetic analysis revealed improved enzyme efficiency for fNDT-i1 and fNDT-i4 (Table 2). In particular, the Km of fNDT-i1 and fNDT-i4, which contain the V52A mutation, were reduced, indicating an increased affinity for the substrate. The 52nd amino acid, positioned at the entrance of the binding pocket, is not part of the active site but plays a role in the substrate accessibility (Armstrong et al., 1996; Supplementary Fig. S2D). The valine-to-alanine substitution reduces the size of the side chain, improving the substrate accessibility. This change likely contributed to enhanced activity, particularly with bulky substrates like fluoro-substituted nucleosides (Yin et al., 2018).
Overall, the whole-cell conversion and temperature optimization experiments confirmed that the L59Q mutation contributed significantly to increased activity against 2FDU. The L59Q mutation is particularly notable for altering the side chain properties, unlike the V52A and L28I mutations. This effect was analyzed more thoroughly by performing substitution experiments using amino acids with varying properties (Supplementary Fig. S3). Among the six mutations, L59Q exhibited the highest activity. This mutation replaces the hydrophobic leucine side chain with the polar, uncharged glutamine. This change alters the protein surface interactions and helps stabilize the enzyme structure at high temperatures.
Structural analysis of NDT and fNDT-i4 using AlphaFold and AutoDock Vina revealed that the L28I, V52A, and L59Q mutations were located outside the active site (Supplementary Fig. S2A). The L28I mutation was distant from the active site, while the V52A and L59Q mutations were positioned at the entrance of the binding pocket without directly interacting with 2FDU. The L28I mutation is presumed to alter hydrophobic interactions, potentially influencing the overall protein structure and substrate binding. The V52A mutation likely affects substrate entry and exit due to changes in side chain size. Additionally, as shown in Supplementary Fig. S3, the L59Q mutation appears to enhance protein activity at high temperatures by altering side chain properties.In conclusion, this study introduced and characterized mutations to enhance the activity and stability of NDT, highlighting their potential for cost-effective production of 2'-fluoro nucleosides. In particular, the L59Q mutation was identified as a key factor in improving the thermal stability, while the V52A and L28I mutations enhanced the substrate affinity and reaction efficiency. These findings provide a foundation for developing industrially relevant nucleoside analog synthesis. Future research should focus on in-depth structural analyses of the reaction mechanisms of these variants and validate their stability and productivity in large-scale processes. In addition, investigating their potential for broader applications in synthesizing various nucleic acid derivatives could further expand their industrial utility.
Supplementary Material
Contributor Information
Su-Been Yang, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Korea.
Yeon-Jin Yoo, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Korea.
Kanghyun Choi, R&D Center, ST Pharm Co., Ltd. Seoul 06170, Korea.
Byungkyun Kim, R&D Center, ST Pharm Co., Ltd. Seoul 06170, Korea.
Si-Sun Choi, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Korea.
Seung-Hoon Kang, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Korea; Department of Biopharmaceutical Engineering, Inha University, Incheon 22212, Korea.
Eung-Soo Kim, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Korea; Department of Biopharmaceutical Engineering, Inha University, Incheon 22212, Korea.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2021R1A2C2012203).
Conflict of Interest
The authors declare no conflict of interest.
References
- Armstrong S., Cook W. J., Short S. A., Ealick S. E. (1996). Crystal structures of nucleoside 2-deoxyribosyltransferase in native and ligand-bound forms reveal architecture of the active site. Structure, 4(1), 97–107.. 10.1016/s0969-2126(96)00013-5 [DOI] [PubMed] [Google Scholar]
- Bosch J., Robien M. A., Mehlin C., Boni E., Riechers A., Buckner F. S., Van Voorhis W. C., Myler P. J., Worthey E. A., DeTitta G., Luft J. R., Lauricella A., Gulde S., Anderson L. A., Kalyuzhniy O., Neely H. M., Ross J., Earnest T. N., Soltis M., Hol W. G. (2006). Using fragment cocktail crystallography to assist inhibitor design of trypanosoma brucei nucleoside 2-deoxyribosyltransferase. Journal of Medicinal Chemistry, 49(20), 5939–5946.. 10.1021/jm060429m [DOI] [PubMed] [Google Scholar]
- Burnett J. C., Rossi J. J. (2012). RNA-based therapeutics: Current progress and future prospects. Chemistry & Biology, 19(1), 60–71.. 10.1016/j.chembiol.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawdhri R. F., Hutchinson D. W., Richards A. O. (1991). Nucleoside deoxyribosyltransferase and inosine phosphorylase activity in lactic acid bacteria. Archives of Microbiology, 155(4), 409–411.. 10.1007/bf00243463 [DOI] [Google Scholar]
- Corey D. R. (2007). Chemical modification: The key to clinical application of RNA interference?. Journal of Clinical Investigation, 117(12), 3615–3622.. 10.1172/jci33483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo N., Sánchez-Murcia P. A., Gago F., Cejudo-Sanches J., Galmes M. A., Fernández-Lucas J., Mancheño J. M. (2017). 2′-deoxyribosyltransferase from Leishmania mexicana, an efficient biocatalyst for one-pot, one-step synthesis of nucleosides from poorly soluble purine bases. Applied Microbiology and Biotechnology, 101(19), 7187–7200.. 10.1007/s00253-017-8450-y [DOI] [PubMed] [Google Scholar]
- Del Arco J., Acosta J., Fernández-Lucas J. (2021). New trends in the biocatalytic production of nucleosidic active pharmaceutical ingredients using 2′-deoxyribosyltransferases. Biotechnology Advances, 51, 107701. 10.1016/j.biotechadv.2021.107701 [DOI] [PubMed] [Google Scholar]
- Fernández-Lucas J., Acebrón I., Wu R. Y., Alfaro Y., Acosta J., Kaminski P. A., Arroyo M., Joachimiak A., Nocek B. P., De la Mata I., Mancheño J. M. (2021). Biochemical and structural studies of two tetrameric nucleoside 2′-deoxyribosyltransferases from psychrophilic and mesophilic bacteria: Insights into cold-adaptation. International Journal of Biological Macromolecules, 192, 138–150.. 10.1016/j.ijbiomac.2021.09.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Lucas J., Fresco-Taboada A., Acebal C., de la Mata I., Arroyo M. (2011). Enzymatic synthesis of nucleoside analogues using immobilized 2′-deoxyribosyltransferase from Lactobacillus reuteri. Applied Microbiology and Biotechnology, 91(2), 317–327.. 10.1007/s00253-011-3221-7 [DOI] [PubMed] [Google Scholar]
- Fresco-Taboada A., de la Mata I., Arroyo M., Fernández-Lucas J. (2013). New insights on Nucleoside 2′-deoxyribosyltransferases: A versatile biocatalyst for one-pot one-step synthesis of nucleoside analogs. Applied Microbiology and Biotechnology, 97(9), 3773–3785.. 10.1007/s00253-013-4816-y [DOI] [PubMed] [Google Scholar]
- Fresco-Taboada A., Fernández-Lucas J., Acebal C., Arroyo M., Ramón F., De la Mata I., Mancheño J. (2018). 2′-deoxyribosyltransferase from Bacillus psychrosaccharolyticus: A mesophilic-like biocatalyst for the synthesis of modified nucleosides from a psychrotolerant bacterium. Catalysts, 8(1), 8. 10.3390/catal8010008 [DOI] [Google Scholar]
- Gagliardi M., Ashizawa A. T. (2021). The challenges and strategies of antisense oligonucleotide drug delivery. Biomedicines, 9(4), 433. 10.3390/biomedicines9040433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmarini C. M., Mackey J. R., Dumontet C. (2002). Nucleoside analogues and nucleobases in cancer treatment. The Lancet Oncology, 3(7), 415–424.. 10.1016/s1470-2045(02)00788-x [DOI] [PubMed] [Google Scholar]
- García-Contreras R., Vos P., Westerhoff H. V., Boogerd F. C. (2012). Why in vivo may not equal in vitro—new effectors revealed by measurement of enzymatic activities under the same in vivo-like assay conditions. The FEBS journal, 279(22), 4145–4159.. 10.1111/febs.12007 [DOI] [PubMed] [Google Scholar]
- Gromiha M. M., Pathak M. C., Saraboji K., Ortlund E. A., Gaucher E. A. (2013). Hydrophobic environment is a key factor for the stability of thermophilic proteins. Proteins: Structure, Function, and Bioinformatics, 81(4), 715–721.. 10.1002/prot.24232 [DOI] [PubMed] [Google Scholar]
- Hassan M. E., Yang Q., Xiao Z., Liu L., Wang N., Cui X., Yang L. (2019). Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech, 9(12), 440. 10.1007/s13205-019-1969-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnik P., Boyer D. S., Grillone L. R., Clement J. G., Henry S. P., Green E. A. (2009). Antisense oligonucleotide therapy in diabetic retinopathy. Journal of Diabetes Science and Technology, 3(4), 924–930.. 10.1177/193229680900300440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski P. A. (2002). Functional cloning, heterologous expression, and purification of two different N-deoxyribosyltransferases from Lactobacillus helveticus. Journal of Biological Chemistry, 277(17), 14400–14407.. 10.1074/jbc.m111995200 [DOI] [PubMed] [Google Scholar]
- Kellis J. T. Jr, Nyberg K., Sali D., Fersht A. R. (1988). Contribution of hydrophobic interactions to protein stability. Nature, 333(6175), 784–786.. 10.1038/333784a0 [DOI] [PubMed] [Google Scholar]
- Kepp O., Loos F., Liu P., Kroemer G. (2017). Extracellular nucleosides and nucleotides as immunomodulators. Immunological Reviews, 280(1), 83–92.. 10.1111/imr.12571 [DOI] [PubMed] [Google Scholar]
- Konkina M. A., Drenichev M. S., Nasyrova D. I., Porozov Y. B., Alexeev C. S. (2023). Studies on enzymatic transglycosylation catalyzed by bacterial nucleoside deoxyribosyltransferase II and nucleoside phosphorylase for the synthesis of pyrimidine 2′-deoxyribonucleosides containing modified heterocyclic base. Sustainable Chemistry and Pharmacy, 32, 101011. 10.1016/j.scp.2023.101011 [DOI] [Google Scholar]
- Koudelakova T., Chaloupkova R., Brezovsky J., Prokop Z., Sebestova E., Hesseler M., Khabiri M., Plevaka M., Kulik D., Kuta Smatanova I., Rezacova P., Ettrich R., Bornscheuer U. T., Damborsky J. (2013). Engineering enzyme stability and resistance to an organic cosolvent by modification of residues in the access tunnel. Angewandte Chemie International Edition, 52(7), 1959–1963.. 10.1002/anie.201206708 [DOI] [PubMed] [Google Scholar]
- Liu P., Sharon A., Chu C. K. (2008). Fluorinated nucleosides: Synthesis and biological implication. Journal of Fluorine Chemistry, 129(9), 743–766.. 10.1016/j.jfluchem.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnutt W. S. (1952). The enzymically catalysed transfer of the deoxyribosyl group from one purine or pyrimidine to another. Biochemical Journal, 50(3), 384–397.. 10.1042/bj0500384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick C. (1998). First “antisense” drug will treat CMV retinitis. Jama, 280(10), 871. 10.1001/jama.280.10.871-jmn0909-6-1 [DOI] [PubMed] [Google Scholar]
- Meng Y., Sun N., Liang L., Yu B., Chang J. (2024). 2'-Fluorinated nucleoside chemistry for new drug discovery: Achievements and prospects. National Science Review, 11(10), nwae331. 10.1093/nsr/nwae331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Masaki T., Chohnan S. (2007). Characterization of N-deoxyribosyltransferase from Lactococcus lactis subsp. lactis. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1774(10), 1323–1330.. 10.1016/j.bbapap.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Oberthuer A., Berthold F., Warnat P., Hero B., Kahlert Y., Spitz R., Ernestus K., König R., Haas S., Eils R., Schwab M., Brors B., Westermann F., Fischer M. (2006). Customized oligonucleotide microarray gene expression–based classification of neuroblastoma patients outperforms current clinical risk stratification. Journal of Clinical Oncology, 24(31), 5070–5078.. 10.1200/jco.2006.06.1879 [DOI] [PubMed] [Google Scholar]
- Okuyama K., Shibuya S., Hamamoto T., Noguchi T. (2003). Enzymatic synthesis of 2′-deoxyguanosine with nucleoside deoxyribosyltransferase-II. Bioscience, Biotechnology, and Biochemistry, 67(5), 989–995.. 10.1271/bbb.67.989 [DOI] [PubMed] [Google Scholar]
- Pérez E., Sánchez-Murcia P. A., Jordaan J., Blanco M. D., Mancheño J. M., Gago F., Fernández-Lucas J. (2018). Enzymatic synthesis of therapeutic nucleosides using a highly versatile purine nucleoside 2’-deoxyribosyltransferase from Trypanosoma brucei. Chemcatchem, 10(19), 4406–4416.. 10.1002/cctc.201800775 [DOI] [Google Scholar]
- Roberts T. C., Langer R., Wood M. J. (2020). Advances in oligonucleotide drug delivery. Nature Reviews Drug Discovery, 19(10), 673–694.. 10.1038/s41573-020-0075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah D. W. Y., Aronin N. (2011). Oligonucleotide therapeutic approaches for Huntington disease. Journal of Clinical Investigation, 121(2), 500–507.. 10.1172/jci45130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Sumaria C. S., Pradeepkumar P. I. (2010). Exploring chemical modifications for Sirna Therapeutics: A structural and Functional Outlook. Chemmedchem, 5(3), 328–349.. 10.1002/cmdc.200900444 [DOI] [PubMed] [Google Scholar]
- Spinner C. D., Gottlieb R. L., Criner G. J., Arribas López J. R., Cattelan A. M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K. M., Castagna A., Chai L. Y., Roestenberg M., Tsang O. T., Bernasconi E., Le Turnier P., Chang S.-C., SenGupta D., Hyland R. H., Osinusi A. O., Marty F. M. (2020). Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. Jama, 324(11), 1048. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Nyce J. W. (2000). Respirable antisense oligonucleotides: A new drug class for respiratory disease. Respiratory Research, 2(1),5–9.. 10.1186/rr32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Sinhari A., Jain P., Jadhav H. R. (2022). A perspective on oligonucleotide therapy: Approaches to patient customization. Frontiers in Pharmacology, 13, 1006304. 10.3389/fphar.2022.1006304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichier-Guerre S., Ku T. C., Pochet S., Seley-Radtke K. L. (2020). An expedient synthesis of flexible nucleosides through enzymatic glycosylation of proximal and distal fleximer bases. Chembiochem, 21(10), 1412–1417.. 10.1002/cbic.201900714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward O. P., Moo-Young M. (1988). Thermostable enzymes. Biotechnology Advances, 6(1), 39–69.. 10.1016/0734-9750(88)90573-3 [DOI] [PubMed] [Google Scholar]
- Xiong H., Veedu R. N., Diermeier S. D. (2021). Recent advances in oligonucleotide therapeutics in oncology. International Journal of Molecular Sciences, 22(7), 3295. 10.3390/ijms22073295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Liu Y., Meng L., Zhou H., Wu J., Yang L. (2018). Rational molecular engineering of glutamate dehydrogenases for enhancing asymmetric reductive amination of bulky α-keto acids. Advanced Synthesis & Catalysis, 361(4), 803–812.. 10.1002/adsc.201801251 [DOI] [Google Scholar]
- Yoo Y. J., Choi K. H., Kim B. K., Choi S. S., Kim E. S. (2022). Isolation and characterization of engineered nucleoside deoxyribosyltransferase with enhanced activity toward 2'-fluoro-2'-deoxynucleoside. Journal of Microbiology and Biotechnology, 32(8), 1041–1046.. 10.4014/jmb.2204.04041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.